Abstract
Electrical stimulation is one of the methods to stimulate skin sensation, and can provide sensations such as vibration and pressure by changing the polarity of the stimulus. These stimuli can be combined to design a variety of tactile sensations. However, there is a major problem with electrical stimulation: As the amount of electric current is increased, itching or pain sensation is elicited. This study aims to suppress the itching and pain caused by electrical stimulation, and to present strong, clear, and stable, pressure and vibration sensations. We applied an anesthetic cream containing lidocaine, which is one of the most used local anesthetics, to reduce the induced pain and itching. Therefore, we specifically examine the applicability of lidocaine toward a desirable situation, in which pain thresholds are increased and tactile thresholds are not significantly affected. The results showed a significant relationship between the application of the cream and the dynamic range of stimulating current, and subsequently the quality of experience by human participants.
S. Kaneko—JSPS Research Fellow.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The methods of stimulating cutaneous sensations can be approximately classified into two categories: mechanical stimulation by physical deformation of the skin, and electrical stimulation through the direct generation of nerve activity. The mechanical stimulation can generate a sense of texture [1] and unevenness [2] by vibrating the skin or presenting a spatial skin distortion pattern, whereas electrical stimulation [3] directly stimulates the nerve axons extending from mechanoreceptors using electrodes placed on the skin surface. Electrical stimulation exhibits the following advantages over mechanical stimulation: low thickness and weight, low power consumption, and the absence of mechanical moving parts. Devices such as visual-tactile conversion devices that use these advantages of electrical stimulation have been previously proposed [4], for the visually impaired and for large area tactile displays [5] that present tactile sensations of virtual object surfaces to the entire palm.
There are two types of electrical stimulations: anodic stimulation, in which current flows from a single electrode to a group of surrounding electrodes; and cathodic stimulation, in which current flows from a group of surrounding electrodes to a single electrode. There are differences in thresholds and sensations between these two modes [6], with cathodic stimulation characteristically producing pressure sensations, believed to originate primarily from Merkel cells; and anodic stimulation characteristically producing vibration sensations, believed to originate primarily from Meissner corpuscles [7]. These stimuli can be combined to design a variety of tactile sensations [8].
However, there is a major problem with electrical stimulation: As the amount of electric current is increased, itching or pain sensation is elicited. This problem is exaggerated in the case of multi-point stimulation, in which each electrode induces pain under different thresholds, and pain from just a single electrode degrades the entire experience.
This study aims to suppress the itching and pain caused by electrical stimulation, and to present strong, clear, and stable, pressure and vibration sensations. We applied an anesthetic cream containing lidocaine, which is one of the most used local anesthetics, to reduce the induced pain and itching. However, it is undesirable for lidocaine to reduce the target sensations such as vibration and pain. Therefore, we specifically examine the applicability of lidocaine toward a desirable situation, in which pain thresholds are increased (pain becomes less perceptible) and tactile thresholds are not significantly affected (tactile sensation is fully perceptible). Dynamic range was used to investigate the expansion of the electric stimulus presentation range.
In recent years, many efforts have been made to use chemical substances for tactile displays. Lu et al. [9] proposed a method of providing numbness and other sensations in VR space, through a series of efforts called Chemical Haptics by applying solutions such as sansho, capsaicin, and lidocaine. Based on their study, the current method can be considered as an attempt to combine electrical stimulation with Chemical Haptics.
2 Experiments and Result
We conducted three experiments: Experiment 1, in which we applied electrical stimulation to three locations (fingertip, forearm, and forehead), with different skin thicknesses, under three conditions direct contact (C1), castor oil application (C2), and anesthetic cream application (C3); Experiment 2, in which we performed an experiment similar to Experiment 1, under a different polarity of the electrical stimuli; and Experiment 3, in which we focused on the intensity of subjective tactile sensation, and examined whether the anesthetic cream affected the perceived intensity of target tactile sensation.
All experiments were approved by the Ethics Committee of the University of Electro-Communications, Japan.
2.1 Experiment 1
Conditions. The anesthetic cream commercially available in Japan (Daiichi Sankyo Healthcare, lidocaine concentration 2\(\mathrm {\%}\)) was used. As a control condition, castor oil cream (Casoda, Heritage Products, USA), which comprises the same base material as the anesthetic cream, was used.
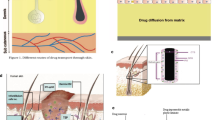
The forearm was chosen imitating a wristwatch-type wearable device, and the forehead was chosen assuming a HMD-embedded device (Fig. 1a).
Apparatus. The electrical stimulation device consists of nine electrodes with a diameter of 1.5 \(\mathrm {mm}\) and a center-to-center distance of 2.5 \(\mathrm {mm}\) (Fig. 1b). The central electrode was used as the stimulating electrode, and the 8 surrounding electrodes operated as the returning current electrodes. In anodic stimulation, the center electrode becomes the anode, and in cathodic stimulation, the center electrode becomes the cathode. In this experiment, we monitored the current flowing through the skin by measuring the voltage across a series-connected 1 \(\mathrm {k\Omega }\) resistor using an oscilloscope.
Procedure. The order of the experiments was counterbalanced among the subjects to overcome the effect of the order of application substances. Six male participants between 21–27 years of age were tested. The experiment was conducted over 3 days, with one condition at each location measured each day.
We applied 1.0 \(\mathrm {g}\) of the ointment per 10 \(\mathrm {cm^2}\), on the skin of the fingers, forearm, and forehead, sealing the area with plastic wrap and masking tape, for 1 h to allow adequate penetration of the ointment. This procedure was skipped for condition C1. The areas under cream application were wiped off with gauze and we started the measurement. Anodic current stimulation with a pulse width of 200 \(\mathrm {us}\) was applied at 30 pulses per sec (pps), and the participants adjusted the amplitude by interacting with the up and down keys of the keyboard, to find the threshold value for the slight perception of the stimulus (herein referred to as the “tactile threshold”), and the threshold amplitude for the perception of pain (herein referred to as the “pain threshold”). Three trials were performed for each condition and location, at an interval of 30 s between each trial.
Results. The tactile and pain thresholds, recorded under each condition, are shown in Fig. 2. The variance of the values can be observed to be large and the individual differences were large, especially for the pain threshold. This agrees with the results of a previous research [10].
Subsequently, we focused on the ratio of pain threshold to tactile threshold. This ratio is believed to indicate the ease of adjustment to the stimulated tactile sensation without pain, and can be referred to as a dynamic range in electrotactile sensation. The dynamic range was calculated and normalized through Condition C1 (Fig. 3). A two-way ANOVA with correspondence was performed on these results. The main effect was observed only for the change in application condition (F = 12.995, p < 0.05), and not for that of the application location (F = 2.412, n.s.). No interaction was observed between the application conditions and locations (F = 2.703, n.s.). The Bonferroni corrected t-test for the results of the change of application condition showed a significant difference between the Conditions C2 and C3 (p < 0.05), and a marginally significant trend between Conditions C1 and C3 (p < 0.1). Therefore, the application Condition C3 can be concluded to possess the widest dynamic range. The dynamic range change in the arms appear to be larger and that in the fingers was smaller, however with no significant difference.
2.2 Experiment 2
Experiment 2 was based on the variation of electrical stimulation. We measured and compared the tactile and pain thresholds of anodic and cathodic stimuli for Conditions C1 and C3.
Conditions. The conditions of anodic and cathodic stimulations were applied under Conditions C1 and C3. The forearm was chosen as the stimulation location, because in experiment 1, some participants did not fell pain at the maximum current at the fingers. Moreover, the use of forehead for electrical stimulation is less common than other locations.
Participants. There were 12 male participants between 21–27 years of age. The experiment was conducted over 2 days, with one condition measured each day. The same procedure as Experiment 1 was adopted.
Results. Similar to Experiment 1, the dynamic range was calculated for each participant, normalized through Condition C1, and a two-way ANOVA with correspondence was performed (Fig. 4a). The main effect was observed under application conditions (F = 10.39, p < 0.01), and not for the variation of stimulus type (F = 0.014, n.s.). No interaction was detected between application conditions and stimulus type (F = 0.014, n.s.). The dynamic range was shown to be significantly higher under Condition C3, than under Condition C1.
2.3 Experiment 3
In Experiments 1 and 2, we confirmed the ratio between the pain and tactile thresholds (dynamic range) to be increased by the application of lidocaine. However, it is also possible that pressure and vibration sensations may also be suppressed owing to the effects of the anesthetic cream, and this would be detrimental to study objective of the application of lidocaine. Experiment 3 was conducted to confirm this, in which we compared the subjective intensity of two types of stimuli with and without application of the local anesthetic cream.
Conditions. The same stimulation and application conditions as Experiment 2 were adopted.
Procedure. Fourteen participants (13 males and 1 female) between 21–27 years of age were included in the study. The experiment was conducted over 2 days, with one condition measured per day.
The same procedure as Experiments 1 and 2 were followed until the electrical stimulation. First, the pain threshold was measured by adjusting the current across the electrode, after which the participants rated the subjective intensity of the stimulus at the threshold on a 7-point Likert scale from 0–6 (0: “very weak stimulus”, 6: “very strong stimulus”). At the pain threshold, pain is just barely perceived, and the strength of the sensation is significantly related to vibratory and pressure sensations.
Results. The responses to the Likert scale were analyzed through ART-ANOVA (Fig. 4b). Similar to Experiment 2, the main effect was observed under the application condition (F = 23.119, p < 0.01), and not under the stimulus conditions (F = 2.323, n.s). No interaction was observed between the application conditions and stimuli (F = 10.39, n.s). The responses obtained for Condition C3 were significantly higher than those under Condition C1, suggesting that the application of anesthetic cream allowed a strong electrical current, and enabled a strong perception of target tactile sensation.
After the experiment, positive comments such as “the stimulus was stronger than in the previous application” and “I felt pure vibration and pressure sensation”, were obtained. Comments such as “I did not feel any significant change”, “I could observe that the range of stimulation became wider, but I did not feel any change in the intensity of stimulation” were also obtained.
3 Discussion
The experiments confirmed that the application of an anesthetic cream prior to electrical stimulation increased the dynamic range, both with respect to the magnitude of electrical current and the subjective intensity of stimulation. The comparison of the results of conditions C2 and C3 with those of castor oil (control condition) attributed this phenomenon to the anesthetic effect, and eliminated the influence of sweating from the application of the cream. Through these experiments, the effectiveness of the proposed method of applying local anesthetic cream was demonstrated.
When local anesthetics are applied, C fibers, which are unmyelinated fibers that control pain and itching, are anesthetized, followed by the anesthetization of thin myelinated fibers (sensory nerves: A\(\mathrm {\delta }\) fibers control warmth and pain, A\(\mathrm {\gamma }\) fibers control intrinsic sensation and muscle tone, and A\(\mathrm {\beta }\) fibers control touch and pressure), and finally A\(\mathrm {\alpha }\) fibers (motor nerves), which are thick myelinated fibers [11]. The results of the current experiments are with existing literature.
Although the results of Experiment 1 did not show the main effect under changing application locations, the effect of lidocaine on fingers were observed to be marginally smaller than that of the other two locations. The transdermal absorption of the chemicals in the palm of the hand was approximately 0.83 times lower than that on the back of the forearm [12], suggesting the anesthetic effect to be weaker in the fingertips. In contrast, although the transdermal absorption rate of the forehead was approximately 6 times greater than that of the back of the forearm, the dynamic range was approximately equal to that of the forearm. Since electrical stimulation is easily affected by skin conditions such as sweat, and as the forehead comprises concentrated sweat glands, the anesthetic effect might be underestimated owing to sweat. The details of the effect of sweat and transdermal absorption rate require to be focused in future studies.
In Experiment 3, there was a significant difference between the subjective perception intensity of the subjects with and without the application of local anesthetic cream, but it was not a dramatic change. Furthermore, the increase of dynamic range with respect to the increase in the magnitude of current, as shown in experiments 1 and 2, the volume adjustment of stimulation current to become easy.
The limitation of the proposed method in this experiment is that it requires an application time of 1 h, prior to the main stimulation. Increased concentration of the cream might shorten the time, which can be a separate research topic in the future. However, since we do not need to turn off and on the effect quickly, we consider it is not a very significant practical limitation.
4 Conclusion
In this study, we proposed a method to expand the dynamic range of electrical stimulation for tactile display by applying a local anesthetic cream containing lidocaine to reduce itching and pain. The results suggested that the application of local anesthetic creams can increase the dynamic range with respect to the magnitude of electrical current and subjective perceptional intensity, enabling the perception of strong stimuli. The experimental results, conducted based on multiple locations, anodic stimulation, and cathodic stimulation, revealed no significant difference caused by the difference in locations, and the dynamic range of the electrical stimulation to be expanded by the local anesthetic cream, independent of the type of stimulation.
Although this experiment was conducted with a single electrode, the problem of pain perception under electrical stimulation is exaggerated with multi-point electrodes (during electrical stimulation through multiple points, if even single electrode causes pain, it will be an unpleasant experience). In the future, we will verify the results under multi-point electrical stimulation. We will also conduct study to stimulate more immediate and vivid electrical stimuli by changing the concentration of lidocaine, to elucidate the relationship between anesthetic effects and electrical stimuli.
References
Tanaka, Y., Nguyen, D.P., Fukuda, T., Sano, A.: Wearable skin vibration sensor using a PVDF film. In: 2015 IEEE World Haptics Conference (WHC), pp. 146–151 (2015)
Hayward, V., Terekhov, A.V., Wong, S.C., Geborek, P., Bengtsson, F., Jörntell, H.: Spatio-temporal skin strain distributions evoke low variability spike responses in cuneate neurons. J. Roy. Soc. Interface 11(93), 20131015 (2014)
Kaczmarek, K.A., Webster, J.G., Bach-y-Rita, P., Tompkins, W.J.: Electrotactile and vibrotactile displays for sensory substitution systems. IEEE Trans. Biomed. Eng. 38(1), 1–16 (1991)
Bach-y-Rita, P., Kaczmarek, K.A., Tyler, M., Garcia-Lara, J.: Form perception with a 49-point electrotactile stimulus array on the tongue: a technical note. J. Rehabili. Res. Dev. 35, 427–430 (1998)
Kajimoto, H.: Design of cylindrical whole-hand haptic interface using electrocutaneous display. In: Isokoski, P., Springare, J. (eds.) EuroHaptics 2012. LNCS, vol. 7283, pp. 67–72. Springer, Heidelberg (2012). https://doi.org/10.1007/978-3-642-31404-9_12
Kaczmarek, K.A., Tyler, M.E., Bach-Y-Rita, P.: Electrotactile haptic display on the fingertips: preliminary results. In: Proceedings of 16th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, vol. 2, pp. 940–941 (1994)
Yem, V., Kajimoto, H.: Comparative evaluation of tactile sensation by electrical and mechanical stimulation. IEEE Trans. Haptics 10(1), 130–134 (2017)
Sato, K., Tachi, S.: Design of electrotactile stimulation to represent distribution of force vectors. In: 2010 IEEE Haptics Symposium, pp. 121–128 (2010)
Lu, J., Liu, Z., Brooks, J., Lopes, P.: Chemical haptics: rendering haptic sensations via topical stimulants, pp. 239–257 (2021)
Mason, J.L., MacKay, N.A.M.: Pain sensations associated with electrocutaneous stimulation. IEEE Trans. Biomed. Eng. BME 23(5), 405–409 (1976)
Liu, S., Kopacz, D.J., Carpenter, R.L.: Quantitative assessment of differential sensory nerve block after lidocaine spinal anesthesia. J. Am. Soc. Anesthesiol. 82(1), 60–63 (1995)
Feldman, R.J.: Regional variation in percutaneous penetration of 14C cortisol in man. J. Inves. Dermatol. 48, 181–183 (1967)
Acknowledgement
This research was supported by JSPS KAKENHI Grant Number JP20H05957.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this paper
Cite this paper
Hamazaki, T., Saito, T., Kaneko, S., Kajimoto, H. (2022). Expanding Dynamic Range of Electrical Stimulation Using Anesthetic Cream. In: Seifi, H., et al. Haptics: Science, Technology, Applications. EuroHaptics 2022. Lecture Notes in Computer Science, vol 13235. Springer, Cham. https://doi.org/10.1007/978-3-031-06249-0_21
Download citation
DOI: https://doi.org/10.1007/978-3-031-06249-0_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06248-3
Online ISBN: 978-3-031-06249-0
eBook Packages: Computer ScienceComputer Science (R0)