Abstract
In the flash lag effect (FLE), a moving object is seen to be ahead of a brief flash that is presented at the same spatial location; a haptic analogue of the FLE has also been observed [1, 2]. Some accounts of the FLE relate the effect to temporal delays in the processing of the stationary stimulus as compared to that of the moving stimulus [3–5]. We tested for movement-related processing effects in haptics. People judged the temporal order of two vibrotactile stimuli at the two hands: One hand was stationary, the other hand was executing a fast, medium, or slow hand movement. Stimuli at the moving hand had to be presented around 36 ms later, to be perceived to be simultaneous with stimuli at the stationary hand. In a control condition, where both hands were stationary, perceived simultaneity corresponded to physical simultaneity. We conclude that the processing of haptic stimuli at moving hands is accelerated as compared to stationary ones–in line with assumptions derived from the FLE.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Perception is subject to a manifold of spatial and temporal distortions, which can be highly informative for our understanding of basic perceptual processes. One of these distortions is the flash-lag effect (FLE). In the original visual FLE a moving stimulus is shown; at some point in time a brief stationary visual stimulus (the flash) is presented next to its trajectory. If the flash is presented spatially aligned with the moving stimulus, the moving stimulus is perceived ahead in direction of movement [1,2,3,4,5,6]. Similar effects were reported for auditory, visual-auditory and haptic stimuli [2, 7,8,9]: In the haptic FLE, participants swayed one of their hands from right to left and vice versa, while they kept the other hand stationary below that trajectory. They judged whether the moving hand was left or right to the stationary one when a brief tactile stimulus (“flash”) was applied to the moving finger. If the two fingers were spatially aligned the moving finger was perceived to be ahead. Potentially linked to this haptic effect are mislocalizations of tactile stimuli during unseen hand movement in direction of movement [10, 11].
Some explanations for the FLE, and for haptic mislocalization relate the positional effects to temporal delays in the processing of the flash relative to that of the moving stimulus. Differential latency theory assumes that processing time for stationary stimuli (i.e., the flash) is longer than for moving stimuli ([3], cf. [10, 11]): Hence, when the stationary flash becomes aware, the moving stimulus is perceived at a time point after the onset of the flash, and thus it is perceived shifted in direction of movement. In turn, a moving stimulus should be perceived to be temporally simultaneous with a stationary stimulus, when the moving stimulus is physically delayed. Such a hypothetical delay has been computed (from the positional FLE and stimulus velocity) to be around 50 ms for the visual FLE, and around 56 ms for the haptic analogue [2, 12]. The attention-shift account of the FLE [4] makes a similar prediction: It assumes that attention is initially focused on the moving stimulus, and that it takes time to shift attention to the stationary flash, when it appears. During this time, the moving stimulus continues to move, and so the flash is perceived as lagging the moving stimulus. Again, a stationary stimulus should be perceived temporally delayed relative to an attended moving stimulus, because the attention shift takes time. Finally, the temporal-sampling account also assumes that the magnitude of FLE depends on differential processing times—even if these differences are not the main reason for the FLE according to this theory [5].
In the present study, we studied movement-related processing time differences in haptics. We investigate delays in the perception of the time point of a stimulus that is applied to a stationary hand as compared to a moving hand. FLE studies computed hypothetical delays between stationary and movement-related stimuli from positional judgments. Here, we directly measure the delay avoiding potential problems with localizing unseen hands. Participants performed a temporal order judgments task for vibrotactile stimuli presented at the two hands (TOJ task, “Which hand was stimulated first?”): one hand was always stationary, for the other hand we varied movement speed (fast, medium, slow, stationary control). The hands never crossed in order to avoid known confounds [13]. The task included a wide range of stimulus onset asynchronies (SOAs). We analyzed the point of subjective simultaneity (PSS) and the just noticeable differences (JNDs). We predicted that stimuli at the moving hand, must be given later than at the stationary hand to be perceived to be simultaneous—but of course not in the stationary control condition. We also checked for an effect of movement speed, but note that accounts for the FLE do not predict speed effects on temporal delay [2, 3].
2 Methods
2.1 Participants
We collected data from 10 students from Giessen (20‒24 years, average 22, 6 females, right-handed). 8 participated for course credit, 2 for pay (8€/hour). All were naïve to the purpose of the study, reported no tenosynovitis in the past and showed no motor or cutaneous impairments. Data from one participant were excluded from analysis due to imprecise judgments (2 JNDs > mean + 2 SD, average JND 251 ms). Participants provided written informed consent, the experiment was approved by the local ethics committee LEK FB06 and conducted in accordance with 2013 Declaration of Helsinki.
2.2 Stimuli and Setup
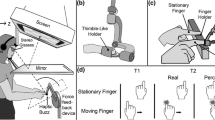
Participants sat at a visuo-haptic workbench (Fig. 1) including a PHANToM 1.5A force feedback device (resolution 0.003 cm, 1000 Hz; 38 × 27 × 20 cm3 workspace), a 22ʺ LCD screen (Samsung, 120 Hz), wireless stereo glasses (Nvidia 3D Vision kit), two tactile actuators (Haptuator Mark II, Tactile Labs) and headphones (Sony MDR-XD100). Tactile actuators were embedded in thimble-like holders, in which participants inserted the distal phalanx of their left and right index fingers. One finger holder was fixed to the PHANToM device, the other was on the table, 38 cm left of the center of the PHANToM’s workspace. The position of the left holder warranted that the right finger never crossed the left one when moving along the x-axis (=sideways, see Fig. 1). The screen was viewed through a mirror via stereo glasses (40-cm viewing distance; head stabilized by chinrest). The mirror occluded vision from the hands. The visual display served to guide participants through the experiment, but turned blacked during the movements. All devices were connected to a PC which controlled the experiment, collected responses and recorded finger positions from the PHANToM.
During the experiment, the left index finger was held stationary, the right index finger moved periodically back and forth along the x-axis (Fig. 1). In line with previous literature [13] we call each unidirectional movement segment a “stroke”. Participants produced 3 strokes, and in the last/3rd stroke one vibrotactile stimulus (sine-wave 50 Hz, 10 ms duration) was presented to each index finger at various SOAs. Auditory metronome signals (sine-wave 698 Hz, 20 ms duration) set the different paces for the moving index finger by defining time points for reversing movement direction. Additional auditory feedback (sine-wave 500 Hz, 20 ms duration) was used to inform participants about reversing points (14 cm left & right to center). As a result, during stimulation in the 3rd stroke participants perform active movements that are temporally and spatially well defined [2]. White noise masked the sounds of actuators and PHANToM. The PHANToM confined movements by a virtual corridor of 0.5 cm depth (z) and 35 cm length (x). The corridor was 4 cm above (y) the stationary finger.
2.3 Design and Procedure
In an experimental trial, participants performed 3 strokes with the right index finger, and during the 3rd stroke two vibrotactile stimuli were presented, to the moving right and the stationary left index finger, respectively. The task was to judge which of the two stimuli was presented first (temporal order judgment task, TOJ). We varied the movement speed of the right hand (within participants): The metronome was set to 1.80 Hz (fast), 1.16 Hz (medium), or 0.75 Hz (slow), corresponding to stroke durations of 556 ms, 862 ms and 1333 ms, respectively. In half of the trials, movement started on the left side (1st stroke to right), in the other half on the right side (1st stroke to left). In addition, we presented a stationary control condition, where the “moving finger” was fixed by PHANToM forces in one of two positions (for sake of consistency the finger connected to the PHANToM is still referred to as “moving”). In all conditions including the stationary one, the standard stimulus was delivered to the right finger either when it was 2 cm left to the center of the movement path or 2 cm right to the center. The comparison was delivered to the stationary left index finger with stimulus onset asynchronies (SOAs) of ±10 ms, ±20 ms, ±40 ms, ±80 ms, ±140 ms or ±180 ms with respect to the standard (negative sign indicating that stationary comparison was pre-sented first). Using the method of constant stimuli we determined relative frequencies of the response that the moving finger was stimulated first as a function of SOA.
Each block of the experiment comprised trials from only one of the four movement speed conditions (fast, medium, slow, control)–namely two trials per each combination of SOA, starting position and position of the standard stimulus in random order (2 × 12 × 2 × 2 = 96 trials); each session included one block per movement speed condition, the order of which was balanced across participants according to a Latin square design. There were four different sessions on different days (96 × 4 × 4 = 1536 trials overall, 384 per condition), lasting overall 8 h. Session 1 started with movement training. Initially, participants trained only the movement in synchrony with the slow, medium and fast metronome–without the TOJ task. Further, before each experimental block in this session, participants performed a corresponding movement training including the TOJ task. Each training ended when the movement was performed with maximally two movement errors in 20 successive trials. There was no training in sessions 2 to 4.
Each experimental trial started with a voice saying “left” or “right” and a visual landmark, which both indicate the starting position of the right moving finger (14 cm left/ right to center). Once the participants reached the starting position, in the stationary control condition another landmark appeared (−2, 2 cm from center), participants moved the finger to that position, and after about 1000 ms the vibrotactile stimuli were presented. In the other conditions, metronome and white noise started, participants were instructed to wait for two metronome signals and afterwards to move forth and back along the x-axis in synchrony with the metronome; auditory feedback signals were given when the moving finger reached reversing points. The screen went black during movement. In the 3rd stroke the vibrotactile stimuli were presented. In all conditions, participants responded by moving the right finger to the extreme right and then up to respond that the first stimulus was presented at the right index finger, and to the extreme left and up to respond that the first stimulus was at the left index finger. In case of a “movement error”, participants obtained feedback about their error without responding, and the trial was repeated later. Movement errors were defined by a root-mean-squared error >45% in stroke duration or length (targets 1000 ms and 28 cm). If the comparison stimulus at the stationary finger had to be given prior to the standard stimulus at the moving finger (negative SOA), we had to predict when the moving finger would reach the target position for the vibrotactile stimulus (−2, 2 cm). We modeled finger movement using the following function (data from [2, 13]).
xt: position of moving finger; T: period of 2 strokes; t: time from stroke onset; AM: movement amplitude (31 cm). We calculated at which position the moving finger would be, when it required the time span specified in the SOA to move to its target position. When the moving finger was at that position, we delivered the stimulus at the stationary finger. The stimulus at the moving finger was given after the exact SOA, leading to some jitter of presentation around the proper target position. We mimicked the positional jitter when the stimulus at the moving finger was delivered first. Jitter also reduced spatial predictability of the standard stimulus.
2.4 Data Analysis
We determined condition-wise individual psychometric functions as percentages of trials in which the moving stimulus was perceived first. We fitted cumulative Gaussians using Bayesian methods in psignifit 4 [14]; µ assessed the point of subjective simultaneity (PSS, SOA at which the standard is equally often perceived first and second); σ assessed just noticeable differences (JND = 84% discrimination threshold). PSSs and JNDs were analysed by paired-sample t-tests and repeated-measures ANOVAs.
3 Results
Figure 2a depicts psychometric functions; Fig. 2b and c depict average PSSs and JNDs, respectively. Because we had clear hypotheses on the PSSs, we directly tested these using specific planned t-tests, and no unspecific preceding omnibus analysis was required. As expected, PSSs in the actual movement conditions were significantly below 0, slow: t(8) = 2.2, p = .030, PSS = −31 ms; medium: t(8) = 2.4, p = .021, PSS = −29 ms; fast: t(8) = 2.0, p = .041, PSS = −49 ms (one-sided), indicating that the stimulus at the stationary finger needs to be delivered before the stimulus at the moving finger to be perceived to be simultaneous. Also as expected, the PSS in the control condition (both fingers stationary), did not deviate from 0, t(8) = 0.3, p = .790 (two-sided), PSS = −2 ms. Further planned t-tests confirmed that PSSs were larger in the stationary condition as compared to the actual movement conditions, slow: t(8) = 2.4, p = .023; medium t(8) = 2.7, p = .013; fast t(8) = 2.3, p = .024 (one-sided; Fig. 2b). PSSs did not differ between actual movement conditions; slow-medium: t(8) = 0.4, p = .688; medium-fast: t(8) = 1.1, p = .291; slow-fast: t(8) = 1.1, p = .293 (two-sided). Results suggest that movement per se affects processing time, whereas the movement’s speed did not have a significant effect—as expected [2].
We analysed JNDs, for which we did not have hypotheses, first in an ANOVA with the variable Movement Speed (control, slow, medium, fast). JNDs differed significantly, F(3, 24) = 20.0, p < .001. Bonferroni-corrected t-tests between each two conditions (overall-α < 5%) revealed significant differences between the stationary and each actual movement condition, and between the fast and the slow conditions, suggesting that JNDs were lower in the control condition, and higher in the fast movement condition.
4 Discussion
We tested whether tactile stimuli at a moving hand are perceived to occur earlier than those presented at a stationary hand. The results confirm this assumption: Stimuli at a moving hand had to be given around 36 ms later, on average, to be perceived to be simultaneous with stimuli at a stationary hand. Movement speed did not have a significant influence on the PSSs, but it had a large and clear effect on JNDs. When both hands were stationary physical and perceived simultaneity coincided. We conclude that movement per se speeds up processing of associated haptic signals, as expected.
We had derived our hypotheses from theories accounting for the flash-lag effect: Differential latency theory claims that processing time is shorter for moving as compared to stationary stimuli [3]. In our case, processing time was shorter for a stimulus applied to a moving hand as compared to when applied to a stationary hand. The tactile stimulus at the moving hand can be considered a moving stimulus in that it moves in reference to the environment (but not relative to the observer). Thus, our results are in line with this view. In [4] it has been assumed that time is required for an attentional shift from the moving towards the stationary stimulus, which also fits with our finding that stimuli at the stationary hand are perceived later than stimuli at the moving hand (assuming attention is at the moving hand). Thus, findings are in line with differential-latency, attention-shift and temporal-sampling accounts [3,4,5]. They though cannot unequivocally be applied to the situations under which FLEs actually have occurred in the previous studies due to a number of differences. Most studies used visual stimuli. In most visual studies, the moving stimulus was continuously visible, moved in reference to the observer and the stationary stimulus suddenly appeared. Here, both stimuli suddenly appeared, and the moving stimulus did not move in reference to the observer. There are also differences to the situation for that the haptic FLE was observed [2, 9]: There, participants were asked for the position of the moving hand at the moment of a tactile signal at that hand. Here, we found slower processing of tactile signals from a stationary as compared to a moving hand, but it is not clear whether this can be extended to delays between sudden tactile as compared to motion signals from a moving hand.
Independent of the FLE, results demonstrate that stimuli at a moving hand are processed faster, and perceived to occur earlier than stimuli at a stationary hand. This is in line with previous observations in tactile-auditory TOJ tasks: When tactile stimuli were presented to a stationary limb, they were perceived later than physically simultaneous auditory stimuli, but these differences were reduced when the tactile stimuli were presented to a moving limb [15]. However, a similar reduction was also observed in an auditory-tactile TOJ task, when one limb was voluntarily moved, but the tactile stimulus was given to the other limb [16]. The present study unequivocally confirms that in the intrasensory haptic comparison moving stimuli are processed faster. With respect to the JND effects, we observed that judgment precision decreased during voluntary movements, the more so the faster the movement was. Previous studies typically did not report a corresponding effect in JNDs [15, 16]. However, in these previous studies typically only a single movement was performed. Here, we asked participants to perform three strokes in synchrony with a metronome, in order to present stimuli during active movements that are temporally and spatially well defined [2]. Probably, it is these additional timing demands that costed precision in the TOJ task.
Why did we observe a processing difference between stimuli on the moving as compared to the stationary hand? Note first that there was no processing difference between left and right hand in the stationary condition, so effects cannot be explained by the hands being used. Secondly, PSSs effects of movement are independent of JND effects of movement, in that JNDs significantly increase with movement speed, but PSSs do not. Also, in the previous study on audio-tactile TOJs similar effects of movement on PSSs were found, but not on JNDs [15]. Another candidate for speeding up could be temporal anticipation of signals on the moving limb, but this also appears unlikely: Signal presentation was spatially defined, and the time point of signals was obscured by noise in motor system and signal position. However, efference copies from voluntary movement could play a role for PSS effects, because they modulate sensory processing of movement-related skin areas and might have enhanced stimulus processing on the moving hand (sensory attenuation can also occur, but would have slowed down processing [15, 17]). Alternatively, information on the moving hand may be processed faster, because the moving hand receives more attention, e.g., to allow for precise movement control. Future experiments will distinguish between these options.
Our finding of a temporal bias during hand movement fits into a number of findings showing that time perception is subject to perturbations during the observation of moving/changing stimuli [18]. Technologically, such findings can be used to modulate time perception when required, for instance to expand time in stressful situations [19]. Knowing that certain stimuli are processed faster than others can, e.g., be used to subjectively lengthen intervals between events marked by the stimuli.
References
Nijhawan, R.: Motion extrapolation in catching. Nature 370, 256–257 (1994)
Cellini, C., Scocchia, L., Drewing, K.: The buzz-lag effect. Exp. Brain Res. 234(10), 2849–2857 (2016). https://doi.org/10.1007/s00221-016-4687-4
Whitney, D., Murakami, I., Cavanagh, P.: Illusory spatial offset of a flash relative to a moving stimulus is caused by differential latencies for moving and flashed stimuli. Vis. Res. 40, 137–149 (2000)
Baldo, M.V., Klein, S.A.: Extrapolation or attention shift? Nature 378, 565–566 (1995)
Brenner, E., van Beers, E.R., Rotman, G., Smeets, J.B.: The role of uncertainty in the systematic spatial mislocalization of moving objects. J. Exp. Psychol.: HPP 32, 811–825 (2006)
Hubbard, T.L.: The flash-lag effect and related mislocalizations: findings, properties, and theories. Psychol. Bull. 140, 308–338 (2006)
Alais, D., Burr, D.: The “Flash-Lag” effect occurs in audition and cross-modally. Curr. Biol. 13, 59–63 (2003)
Vroomen, J., de Gelder, B.: Temporal ventriloquism: sound modulates the flash-lag effect. J. Exp. Psychol.: HPP 30, 513–518 (2004)
Drewing, K., Hitzel, E., Scocchia, L.: The haptic and the visual flash-lag effect and the role of flash characteristics. PLoS ONE 13(1), e0189291 (2018)
Watanabe, J., Nakatani, M., Ando, H., Tachi, S.: Haptic localizations for onset and offset of vibro-tactile stimuli are dissociated. Exp. Brain Res. 193, 483–489 (2009)
Maij, F., Wing, A.M., Medendorp, W.P.: Spatiotemporal integration for tactile localization during arm movements: a probabilistic approach. J. Neurophysiol. 110, 2661–2669 (2013)
Lopez-Moliner, J., Linares, D.: The flash-lag effect is reduced when the flash is perceived as a sensory consequence of our action. Vis. Res. 46(13), 2122–2129 (2006)
Drewing, K., Hartmann, F., Vroomen, J.H.: The crossed-hands deficit in temporal order judgments occurs for present, future, and past hand postures. In: 2019 IEEE World Haptics Conference (WHC), pp. 145–150. IEEE (2019)
Schütt, H.H., Harmeling, S., Macke, J.H., Wichmann, F.A.: Painfree and accurate Bayesian estimation of psychometric functions for (potentially) overdispersed data. Vis. Res. 122, 105–123 (2016)
Hao, Q., Ogata, T., Ogawa, K.I., Kwon, J., Miyake, Y.: The simultaneous perception of auditory–tactile stimuli in voluntary movement. Front. Psychol. 6, 1429 (2015)
Hao, Q., Ora, H., Ogawa, K.I., Ogata, T., Miyake, Y.: Voluntary movement affects simultaneous perception of auditory and tactile stimuli presented to a non-moving body part. Sci. Rep. 6(1), 1–8 (2016)
Brown, H., Adams, R.A., Parees, I., Edwards, M., Friston, K.: Active inference, sensory attenuation and illusions. Cogn. Process. 14(4), 411–427 (2013). https://doi.org/10.1007/s10339-013-0571-3
Matthews, W.J.: How do changes in speed affect the perception of duration? J. Exp. Psychol.: HPP 37, 1617–1627 (2011)
Botev, J., Drewing, K., Hamann, H., Khaluf, Y., Simoens, P., Vatakis, A.: ChronoPilot – Modulating time Perception. In: IEEE AIVR 2021, the 4th International Conference on Artificial Intelligence and Virtual Reality (AIVR), pp. 1–4. IEEE (2021)
Acknowledgment
Research was supported by EU (ChronoPilot, Horizon 2020 FET Open Programme, grant agreement 964464) and by DFG (SFB/TRR135/1-3, A05, project 222641018). Thanks to Leandra Ruloff for conducting the experiment as part of her thesis work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this paper
Cite this paper
Drewing, K., Vroomen, J. (2022). Moving Hands Feel Stimuli Before Stationary Hands. In: Seifi, H., et al. Haptics: Science, Technology, Applications. EuroHaptics 2022. Lecture Notes in Computer Science, vol 13235. Springer, Cham. https://doi.org/10.1007/978-3-031-06249-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-06249-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06248-3
Online ISBN: 978-3-031-06249-0
eBook Packages: Computer ScienceComputer Science (R0)