Abstract
This chapter reviews the diversity, evolutionary relationships, ecology, and conservation of the Gulf of Guinea oceanic islands’ endemic caecilian and anuran fauna. A total of nine amphibian species (representing five families) are known from São Tomé and Príncipe islands, all of which are endemic. No amphibians have been reported from Annobón. Taxonomic research on this group of animals began in the second half of the nineteenth century with subsequent refinement following the advent of molecular techniques. The presence of several amphibians from distinct evolutionary lineages is unexpected for oceanic islands and has motivated several biogeographic studies to reconstruct the evolutionary histories of these enigmatic species. Yet, the continental source for many of the islands’ amphibians remains unknown. The amphibians of São Tomé and Príncipe also exhibit intriguing phenotypic diversity for addressing long-standing hypotheses in evolutionary biology, including body size evolution and gigantism on islands, intraspecific variation and interspecific divergence in coloration, and reproductive and dietary niche partitioning. Recent studies have confirmed the presence of the fungal pathogen Batrachochytrium dendrobatidis in amphibian communities on both São Tomé and Príncipe, but it is unclear whether this pathogen is negatively impacting local populations. Most of the Gulf of Guinea oceanic island endemic amphibians are incredibly abundant and widespread, occurring in primary forest, secondary forest, and agricultural habitats across the islands. Three anuran species (Hyperolius thomensis, Leptopelis palmatus, Ptychadena newtoni) have more limited distributions and/or more specialized ecologies; consequently, additional land-use change poses a threat to the long-term persistence of these taxa.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Island faunas have inspired evolutionary biologists for centuries, and the enigmatic history of insular amphibians is particularly captivating. Amphibians are considered poor dispersers across saltwater barriers and are thus naturally absent from most oceanic islands (Darwin 1859; Vitt and Caldwell 2014). Yet overseas dispersal and in situ diversification have contributed to the accumulation of a surprisingly diverse amphibian fauna in the Gulf of Guinea oceanic islands, which are located 250–300 km from the western coast of Central Africa. The presence of a combined nine amphibian species on São Tomé and Príncipe islands, all of which are endemic, presents an intriguing biogeographic anomaly within which to explore the potential pathways and timing of overseas dispersal events. In addition, some lineages have further diversified within and between islands in the archipelago, presenting the opportunity to investigate the tempo and mechanisms underlying in situ diversification. This chapter presents an updated taxonomic overview of the amphibians on São Tomé and Príncipe islands (no amphibians occur on Annobón), highlighting the biogeographic patterns, organismal biology, and conservation threats for each species. The amphibian fauna of Bioko Island, a land-bridge island that is part of the Gulf of Guinea archipelago, is entirely distinct from that of the oceanic islands in the archipelago (see Sánchez-Vialas et al. 2020). We also provide a brief history of the research on amphibians in the archipelago and highlight important avenues for future work.
History of Amphibian Research
The history of amphibian research in the Gulf of Guinea oceanic islands is closely linked to the history of reptile research, as most authors have worked with both groups. Ceríaco et al. (2022) provide a comprehensive summary of the herpetological surveys and studies carried out on the islands. Hence, we focus on the primarily amphibian-focused studies and refer to Ceríaco et al. (2022) for more general studies.
The first herpetological studies in the Gulf of Guinea oceanic islands were based on opportunistic collections by European medical staff and colonial officers who visited or worked in the region. The first published record of an amphibian from São Tomé dates to 1868, when the German zoologist Wilhelm C. H. Peters (1815–1883) described the Príncipe Giant Tree Frog Hylambates (currently Leptopelis) palmatus based on three female specimens (holotype ZMB 6067) collected by German explorer Heinrich Wolfgang Ludwig Dhorn (1838–1913) on Príncipe (Peters 1868). In 1870, Peters described the Príncipe Puddle Frog Arthroleptis dispar (later transferred to Phrynobatrachus by Laurent 1941) based on a single specimen from Príncipe (holotype ZMB 6133; Peters 1870), also collected by Dhorn. In 1873, the Portuguese zoologist José Vicente Barboza du Bocage (1823–1907), director of the National Museum of Lisbon (also known as Museu Bocage, now Museu Nacional de História Natural e da Ciência, Lisbon, Portugal; MUHNAC), described the São Tomé caecilian Siphonops (currently Schistometopum) thomense based on two preserved specimens donated to the museum by Pedro Carlos de Aguiar Craveiro Lopes (1834–?), Portuguese governor of São Tomé and Príncipe at the time (Bocage 1873). In 1874, Peters described Siphonops brevirostris based on a single specimen with imprecise locality information (“Westküste Afrikas [Guinea]”) that he acquired from an animal dealer (Peters 1874). The type locality for S. brevirostris has since been restricted to Ilhéu das Rolas (Gorham 1962), but the justification for this restriction is doubtful (Nussbaum and Pfrender 1998). This specimen is extant at the Museum für Naturkunde (Berlin, Germany; ZMB) and Nussbaum and Pfrender (1998) indicate the holotype is ZMB 4911 rather than ZMB 4711 as reported in the description. In his major revision of caecilians, Peters subsequently placed both S. thomense and S. brevirostris in the primarily neotropical genus Dermophis based on a combination of shared morphological features (Peters 1879). Upon examining specimens of S. thomense, Peters later determined that his S. brevirostris was the same as Bocage’s S. thomense (Peters 1880), and this synonymy continues to be recognized by most authors. Finally, Parker (1941) placed the species in the genus Schistometopum where it remains today.
The German zoologist Richard Greeff (1829–1892) explored São Tomé and Ilhéu das Rolas (a small islet ~2 km off the southern tip of São Tomé) from 1879 to 1880 and provided one of the first reports of its herpetofauna (Greeff 1884a). Greeff was particularly interested in São Tomé caecilians and published a brief study on their biology (Greeff 1884b). Greeff’s specimens are still extant in the collections of the Museum für Naturkunde (Berlin, Germany; ZMB), Zoologische Staatssammlung München (München, Germany; ZSM), and Zoologisches Museum Hamburg (Hamburg, Germany; ZMH).
In 1885, the Botanical Gardens of the University of Coimbra sent their chief gardener Adolfo Frederico Möller (1842–1920) to São Tomé to explore and collect natural history specimens for the Botanical Gardens and the university museum. Most of the zoological specimens collected by Möller were sent to the University of Coimbra (now part of the Museu da Ciência da Universidade de Coimbra; Coimbra, Portugal; MCUC) and Vieira (1886) published a brief inventory of these specimens. Almost all of this material was examined and identified by José Vicente Barbosa du Bocage and is still extant in the collections of MCUC (Themido 1941; LMPC pers. obs.). Some amphibian and reptile specimens, however, were likely sent by Möller to the Russian zoologist Jacques von Bedriaga (1854–1906) who was a scholar at the University of Coimbra. Bedriaga published a thorough revision of the amphibians and reptiles of São Tomé (Bedriaga 1892), where he described Moller’s Reed Frog, Rappia (currently Hyperolius) molleri, endemic to São Tomé (Bedriaga 1892). There are no further records of the specimens sent by Möller to Bedriaga, and they are presumably lost; however, one syntype of H. molleri is extant at the Natural History Museum (London, UK; NHMUK).
Also in 1885, Francisco Xavier Oakley de Aguiar Newton (1864–1909) was hired by the Museu Bocage to conduct zoological surveys in the Gulf of Guinea. From 1885 to 1895, Newton explored all the Gulf of Guinea islands, as well as Benin, and his specimens were ultimately deposited in the Museu Bocage. The amphibians were studied by Barbosa du Bocage and based on this collection, he described the São Tomé Giant Reed frog Hyperolius thomensis and Newton’s Grassland frog Rana (currently Ptychadena) newtoni, both from São Tomé (Bocage 1886). Unfortunately, the entirety of Newton’s collections was lost in the fire that destroyed the Museu Bocage in 1978.
The Italian explorer Leonardo Fea (1852–1903) explored the four principal islands of the Gulf of Guinea from 1901 to 1902 under the sponsorship of the Museo Civico di Storia Naturale of Genoa (currently known as Museo Civico di Storia Naturale “Giacomo Doria;” Genoa, Italy; MSNG). Fea’s collections were initially studied by George Albert Boulenger (1858–1937; Boulenger 1906) and are still extant in the MSNG with a small subset in the Natural History Museum of London (NHMUK). Based on material Fea collected on Príncipe, Boulenger (1906) described Phrynobatrachus feae, which was later placed in synonymy with P. dispar by Schätti and Loumont (1992).
During the 1950s and 1960s, the Portuguese Zoology Center of the Overseas Research Committee (Centro de Zoologia da Junta de Investigações do Ultramar; Lisbon, Portugal; CZL) conducted zoological surveys on São Tomé and Príncipe. Several herpetological specimens were collected by different researchers associated with the colonial enterprise over the course of multiple scientific surveys. The material collected during these surveys was studied by the Portuguese herpetologist Sara Maria Bárbara Marques Manaças (1896–?), resulting in two publications (Manaças 1958, 1973). Most of the specimens were housed in the collections of the Instituto de Investigação Científica Tropical (Lisbon, Portugal; IICT), but in 2016 they were incorporated into the MUHNAC collections.
Throughout the 1960s and 1970s, several authors used existing specimens in various collections for taxonomic revisions of different genera. For instance, the Swiss herpetologist Jean-Luc Perret (b. 1925) reviewed the taxonomic position and status of São Tomé and Príncipe anurans (Perret 1962, 1966, 1973, 1976, 1988). Perret placed both reed frog species in a new genus Nesionixalus based on several shared morphological features (Perret 1976); however, subsequent morphological and genetic analyses have found strong support for both species belonging to the genus Hyperolius (Drewes 1984; Drewes and Wilkinson 2004; Portik et al. 2019). The North American herpetologist Edward Harrison Taylor (1889–1978) described Schistometopum ephele based on material collected from São Tomé by the Leonardo Fea Expedition and deposited in the MSNG (MSNG 8773; Taylor 1965).
Following the independence of São Tomé and Príncipe from Portugal in 1975, several teams undertook expeditions to the islands to document biodiversity. In 1984 a team from the zoology and anthropology department of the Faculty of Sciences of the University of Lisbon and the Museu Bocage, Lisbon, Portugal, led by Luis Mendes (b. 1946), conducted a 1-month zoological expedition to São Tomé (Mendes et al. 1988). Although the expedition did not have a dedicated herpetologist, some amphibian specimens were collected, and these are extant in MUHNAC collections.
Ronald Nussbaum (b. 1942) from the University of Michigan and Michael Pfrender (b. 1960) visited the islands of São Tomé and Príncipe in 1988 and placed S. ephele in synonymy with S. thomense (Nussbaum and Pfrender 1998). The specimens they collected are in the University of Michigan Museum of Zoology (Ann Arbor, United States of America; UMMZ). From 1989 to 1991, expeditions to São Tomé and Príncipe led by Catherine Loumont (b. 1942), Tillman Nill (dates unknown) Jakob Fahr (dates unknown) and Jan Haft (b. 1967), resulted in reviews of the herpetofauna of these islands, including important natural history data on amphibians (Loumont 1992; Schätti and Loumont 1992; Fahr 1993; Haft and Franzen 1996). Some of these specimens are housed in the ZMB and the collections of the Musée d’Histoire Naturelle de la Ville de Genéve, Switzerland (MHNG).
The beginning of the twenty-first century marked a new period for the study of the amphibians of the Gulf of Guinea oceanic islands. Robert C. Drewes (b. 1942), herpetology curator of the California Academy of Sciences (San Francisco, United States of America; CAS), and his team have made 12 expeditions to the islands since 2001. The amphibian collections resulting from these expeditions are deposited at CAS and are currently the largest in the world (Table 18.1). Drewes and his colleagues have published several studies, including the description of the São Tomé Puddle Frog, Phrynobatrachus leveleve (Uyeda et al. 2007), taxonomic reviews and updates (Drewes and Stoelting 2004; Drewes and Wilkinson 2004), biogeographic history (Measey et al. 2007), and population genetics (Stoelting et al. 2014). During this same period, John Measey (b. 1968) and colleagues conducted evolutionary, ecological, and biomechanics studies of the São Tomé caecilian, making important contributions to our understanding of these secretive animals (Delêtre and Measey 2004; Measey and Herrel 2006; Measey and Van Dongen 2006; Wollenberg and Measey 2009; Herrel and Measey 2010, 2012). More recently, Rayna C. Bell (b. 1985), herpetology curator of the CAS, has contributed studies on the biogeographic and evolutionary history of Hyperolius (Bell et al. 2015a, b, 2017), hybridization of H. molleri and H. thomensis on São Tomé (Bell et al. 2015b; Bell and Irian 2019), the description of H. drewesi, endemic to Príncipe, and the evolutionary history of the São Tomé caecilians (O’Connell et al. 2021). As the type material of H. thomensis was lost in the 1978 fire at the Museu Bocage in Lisbon, Portugal (Drewes and Wilkinson 2004), and the original type localities of H. thomensis and H. molleri were vague and may have included individuals with hybrid ancestry, Bell (2016) designated neotypes for both H. thomensis and H. molleri. Bell participated in several expeditions led by Drewes, and most of the specimens are at CAS with a smaller subset at the Smithsonian Institution’s National Museum of Natural History (Washington DC, United States of America; USNM). Since 2013, a team from MUHNAC led by Luis M. P. Ceríaco (b. 1987) completed four herpetological surveys in São Tomé and Príncipe. A few amphibian specimens were collected and are now housed in MUHNAC.
Diversity and Endemism
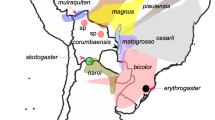
Although the islands of São Tomé and Príncipe have never been physically connected to the African continent, they host a remarkable nine endemic species of amphibians, each of which is restricted to just one of the islands (Table 18.2, Fig. 18.1). We provide a brief summary of the taxonomic status of each species, its biogeographic history (when known), and notes on distribution, ecology, and behavior.
Gulf of Guinea oceanic island amphibians: (1) São Tomé caecilians, Schistometopum thomense (top) and Schistometopum ephele (bottom), from São Tomé Island; (2) Príncipe Giant Tree Frog, Leptopelis palmatus, from Príncipe Island; (3) Drewes’ Reed Frog, Hyperolius drewesi, from Príncipe Island; (4) Moller’s Reed Frog, Hyperolius molleri, from São Tomé Island; (5) São Tomé Giant Reed Frog, Hyperolius thomensis, from São Tomé Island; (6) Leveleve Puddle Frog, Phrynobatrachus leveleve, from São Tomé Island; (7) Príncipe Puddle Frog, Phrynobatrachus dispar, from Príncipe Island; (8) Newton’s Grass Frog, Ptychadena newtoni, from São Tomé Island. Photo credits: Andrew Stanbridge
Gymnophiona
Caecilian diversity in the Gulf of Guinea oceanic islands includes two endemic species (family Dermophiidae) that are distributed across São Tomé Island, even though caecilians were reported from Príncipe Island in error (Taylor 1968). The presence of these enigmatic and secretive amphibians on an oceanic island is especially captivating given their presumed low vagility and dispersal potential (Taylor 1968). Caecilians are not the only fossorial vertebrates that have reached the archipelago, however, as there are also several endemic fossorial squamates on both São Tomé and Príncipe (Ceríaco et al. 2022). This high level of endemic diversity among vertebrates with low dispersal potential provides strong support for the overseas rafting hypothesis proposed by Measey et al. (2007) and described in further detail in Melo et al. (2022).
Family Dermophiidae
The São Tomé caecilian S. thomense was initially described by Bocage (1873), and though he did not observe the species in life, he noted the two preserved specimens were uniform light yellow and olive in coloration, respectively. Unfortunately, a fire at the Lisbon Museum in 1978 destroyed this material and reports of a possible extant syntype at the Berlin Museum (ZMB 8738) are doubtful because the size of the specimen does not match Bocage’s description (Nussbaum and Pfrender 1998). Several decades later, Taylor described S. ephele and recognized S. ephele as distinct from S. thomense based on its smaller, pointier head and prominent brown flecking pattern (Taylor 1965). The provenance of this material is reported as “Agua Izé, 400–700 m, Ilha São Thomé,” a locality that is likely between Agua Izé (a coastal community on the eastern side of the island) and the community of Java that is directly inland of Agua Izé at ~600 m elevation (pers comm G. Doria, MSNG). In the description, Taylor also noted that two other individuals under this number were uniform yellow (unflecked) and thus referred to S. thomense. Nussbaum and Pfrender (1998) quantified coloration, morphometric, and meristic variation of Schistometopum specimens collected from ten sites across São Tomé (including Ilhéu das Rolas). Although they found strong separation in a multivariate comparison of northern and southern populations, Nussbaum and Pfrender (1998) interpreted this variation as a phenotypic cline in a widespread species, placing S. ephele in synonymy with S. thomense. Stoelting et al. (2014) revisited this hypothesis with mtDNA (16s and ND4) sequence data and sampling from more than 20 sites across the island. These authors found deep genetic divergence between lineages that they proposed may correspond to S. thomense and S. ephele; however, the distributions of these mtDNA lineages overlapped in the center of the island. Consequently, Stoelting et al. (2014) proposed that the lineages diverged in allopatry but refrained from making taxonomic recommendations solely based on these maternally inherited loci. A more recent study examining genome-wide variation found strong support for distinct lineages corresponding to S. thomense and S. ephele, and inferred a history of divergence in allopatry with a narrow hybrid zone where the ranges presently overlap in the center of São Tomé Island (O’Connell et al. 2021). Based on this evolutionary history, and the revised interpretation of the apparent phenotypic cline in the Nussbaum and Pfrender (1998) study, O’Connell et al. (2021) removed S. ephele from synonymy with S. thomense.
The only other species in the genus Schistometopum is the East African S. gregorii, which presents an intriguing biogeographic scenario for the island endemic species (Wilkinson et al. 2003; San Mauro et al. 2014). Depending on the method and/or mutation rate used, estimates for divergence between S. thomense and S. gregorii based on mtDNA loci range from 0.6 to 3.2 Myr (Loader et al. 2007), indicating that dispersal to São Tomé occurred relatively recently in the islands’ 13 Myr history. Using genome-wide markers, divergence between S. thomense and S. ephele on São Tomé was estimated at 281–326 kya with secondary contact between the lineages occurring ~100 kya (O’Connell et al. 2021), highlighting that São Tomé is accumulating endemic diversity via both overseas dispersal and in situ diversification even in the more recent period of its long geologic history. On São Tomé, caecilians occur in a wide variety of habitats ranging from sea level to over 1400 m elevation, including agricultural fields, modified landscapes, and the ~2 km2 islet Ilhéu das Rolas (Bocage 1886; Loumont 1992; Fahr 1993; Haft and Franzen 1996; Nussbaum and Pfrender 1998; Drewes and Stoelting 2004; Measey and Van Dongen 2006; Stoelting et al. 2014). Due to this broad distribution and high abundance (estimated density 0.3 per m2; Measey 2006), caecilians are well known to São Toméans, who refer to them as “Cobra-bobô” (i.e., yellow snake).
At collection sites with harder, mineral soils, animals may be found under leaf litter or rotten logs, whereas at sites with softer soils, including agricultural fields, animals are found within the soil (Haft 1992; Haft and Franzen 1996; Delêtre and Measey 2004). Likewise, in drier habitats, caecilians occur deeper in the soil (Nussbaum and Pfrender 1998) whereas, after heavy rains and in the evening, they can be observed moving aboveground (Fahr 1993; Haft and Franzen 1996). Correspondingly, laboratory studies quantifying the behavior and biomechanics of burrowing in São Tomé caecilians indicated that they did not construct tunnels in soils with high compaction and that even intermediate levels of soil compaction can deter or prevent burrowing (Ducey et al. 1993). In addition, in laboratory settings, caecilians opted to use existing tunnels rather than construct new ones (Ducey et al. 1993). These behaviors are consistent with the more terminal mouth position of São Tomé caecilians relative to the subterminal (countersunk lower jaw) mouths of caecilians that are dedicated burrowers (Sherratt et al. 2014). Like all caecilians, São Tomé caecilians show skin-vertebral independence and use internal and whole-body concertina locomotion while burrowing or moving through narrow tunnels (Herrel and Measey 2010). When moving across high friction substrates (i.e., a moist towel) São Tomé caecilians switch to lateral undulating locomotion (Herrel and Measey 2010). An extensive survey of caecilian body size variation across São Tomé found that individuals at higher elevation sites (where soil temperatures are cooler) were longer and heavier than individuals at lower elevations (Measey and Van Dongen 2006). This trend has been noted in many endothermic vertebrates with the explanation that larger body sizes result in lower heat loss via larger surface-area-to-volume ratios (Bergmann 1847); however, the potential mechanisms to explain this pattern in an ectotherm are less clear.
São Tomé caecilians are sexually dimorphic in head size (but not in body size), which could indicate ecological divergence between the sexes (e.g., differences in diet; Nussbaum and Pfrender 1998) or antagonistic behaviors among males (Delêtre and Measey 2004). Delêtre and Measey (2004) tested this first hypothesis with a diet study of males, females, and juveniles from both natural forest and agricultural sites. Earthworms (including epigeic [surface-active] and endogeic [deeper soil-dwelling] species) accounted for over 98% of identified prey items, with the remaining contents comprising centipedes, ants, mites, and unidentified larvae (Delêtre and Measey 2004). Counter to expectations, the authors did not find significant differences in prey mass or prey size between the sexes (though prey size did correlate with gape diameter among females), suggesting that larger head size in males is not related to capturing larger prey. A subsequent study of caecilian diets reexamined the Delêtre and Measey (2004) earthworm morphospecies dataset and found that adults fed on both epigeic and endogeic earthworms in equal proportions, whereas juveniles appeared to only feed on endogeic species (Jones et al. 2006). Future research investigating the extent of dietary specialization in São Tomé caecilians (across habitats and throughout the year) as well as potential ontogenetic shifts in diet will provide important insight into the role caecilians play in mediating population dynamics of soil ecosystem engineers (earthworms, termites and ants) and soil ecology more broadly (Lavelle et al. 1997; Jones et al. 2006).
Observations in captivity suggest that São Tomé caecilians are predominantly nocturnal, sit-and-wait predators, extending their heads beyond their burrows and waiting for prey to come within reach (Haft and Franzen 1996; Hofer 1998). To our knowledge, predation behaviors in field settings have not yet been documented in the scientific literature; however, in laboratory settings São Tomé caecilians feeding on earthworms exerted strong bite forces and used long-axis body rotations to subdue and shred their prey (Measey and Herrel 2006; Herrel and Measey 2012). Laboratory measurements of resting metabolism and aerobic capacity of São Tomé caecilians indicated they have very low resting metabolic rates with a high capacity for aerobic metabolism that is consistent with a largely sedentary, sit-and-wait predatory lifestyle (Smits and Flanagin 1994). The same study also revealed surprisingly high cutaneous gas exchange in caecilians despite their thickened skin, suggesting that cutaneous respiration is likely sufficient to support resting metabolic rates (Smits and Flanagin 1994).
Based on the current understanding of reproductive biology in caecilians, all species have internal fertilization via an intromittent organ formed by an eversible portion of the male’s cloaca (the phallodaeum) that may vary in shape and ornamentation among species (Gower and Wilkinson 2002). The phallodaeum of São Tomé caecilians is quite similar to that of S. gregorii from Tanzania, but Tanzanian and Kenyan S. gregorii differ from one another, and these populations have previously been hypothesized to be distinct species (Taylor 1968; Gower and Wilkinson 2002). A better understanding of intraspecific and interspecific variation in phallodaeum morphology would provide deeper insights as to the potential significance of this trait in reproductive isolation. Several authors have noted bite marks on the heads of male and female São Tomé caecilians from both field-caught and laboratory individuals (e.g., Nussbaum and Pfrender 1998; Teodecki et al. 1998). Biting among conspecific males in territorial disputes and males biting females during copulation have been proposed as alternative hypotheses for larger head size in males (Delêtre and Measey 2004). The role of biting in communication and/or sexual selection in São Tomé caecilians would be a compelling future avenue of behavioral research in these curious organisms.
Viviparity has evolved independently in several lineages of caecilians, including the family Dermophiidae, which are all viviparous (Gower et al. 2008; San Mauro et al. 2014). Observations of São Tomé caecilians in captivity suggest clutch sizes typically range from 2 to 7 young that are fully formed at birth with no signs of gill scars (Nussbaum and Pfrender 1998). The energetic demands of reproduction are likely high as young caecilians are born at up to 50% the length of their mothers (Wake 1977; Nussbaum and Pfrender 1998), and females reproduce biennially (Teodecki et al. 1998). In addition, developing fetuses have specialized dentition with which to scrape the epithelium of the oviduct and stimulate the secretion of nutrient-rich “uterine milk” from their mothers during gestation (Parker 1956; Parker and Dunn 1964; Wake 1977). Captive-born young reached adult size after 2 years (Haft and Franzen 1996), and the adult coloration and pattern were present at birth (Nussbaum and Pfrender 1998). It is tempting to consider that the prominent yellow coloration of São Tomé caecilians may be aposematic, and a study of yellow coloration across all caecilians indicates that this conspicuous coloration has evolved multiple times in species that are surface-active (Wollenberg and Measey 2009). Anecdotal evidence suggests São Tomé caecilians are distasteful (Hofer 1998; Teodecki et al. 1998); however, their chemical defenses have not yet been characterized. Likewise, the dominant predators of São Tomé caecilians are also unknown; consequently, much additional foundational research is needed to understand whether this yellow coloration is cryptic or aposematic. While a role in intraspecific communication and/or sexual selection is also a possible explanation for this bright coloration, São Tomé caecilians likely have poor eyesight (Mohun et al. 2010) and like all caecilians are considered to rely primarily on olfactory cues to sense their environments (Himstedt and Simon 1995).
Anura
Anuran diversity in the Gulf of Guinea oceanic islands includes seven endemic species from four families: Arthroleptidae, Hyperoliidae, Phrynobatrachidae, and Ptychadenidae (Table 18.2).
Family Arthroleptidae
The Príncipe Giant Tree Frog Leptopelis palmatus has historically been confused with another large-bodied species from continental Africa, L. rufus, with several authors placing L. rufus in synonymy with L. palmatus (Anderson 1909; Parker 1936; Witte 1941; Perret 1962). Throughout this period of nearly a century of taxonomic confusion, L. palmatus was reported from Cameroon, Equatorial Guinea (including Bioko Island), Gabon, and Nigeria (Boulenger 1882; Mocquard 1902; Boulenger 1906; Nieden 1910; Ahl 1931; Schiøtz 1963; Mertens 1965). Perret (1973) resurrected L. rufus after comparing a large series of males and females with the sole female holotype of L. palmatus available for study and clarified that L. palmatus was an insular species. Upon this close examination, Perret (1973) confirmed that the two species differed in tympanum size and several additional morphological features, concluding that the two species may not even be closely related. Male specimens were finally collected and formally described following an expedition to the islands in 2002 (Drewes and Stoelting 2004). Loumont (1992) characterized the karyotype of this species, reporting 24 chromosomes.
The phylogenetic relationships within the African genus Leptopelis are poorly understood, and consequently, the biogeographic history of L. palmatus remains unclear. Previous studies have hypothesized that L. palmatus is closely related to a group of large-bodied species in West and Central Africa (L. macrotis, L. millsoni, and L. rufus) based on a combination of mtDNA and morphological data (Idris 2004). A more recent mtDNA phylogeny with expanded taxonomic sampling does not support this relationship (Jaynes et al. 2021), and a more robust phylogenetic inference is sorely needed. The distribution of L. palmatus ranges from sea level to over 600 m elevation on Príncipe, primarily in forested habitats (Loumont 1992; Drewes and Stoelting 2004). Males and females can be observed at night perched one meter or higher off the ground on branches or leaves, especially near small flowing streams (Loumont 1992; Drewes and Stoelting 2004; RCB and LAS pers. obs). By contrast, large females have been encountered on or near the ground both in the evening and during the day (Drewes and Stoelting 2004; RCB and LAS pers obs). Although males lack vocal sacs (Drewes and Stoelting 2004), they produce advertisement calls at breeding sites (characterized in Jaynes et al. 2021). Both male and female dorsal coloration is variable, ranging from dark green/black with or without small white spots to bright green and even bright yellow (Manaças 1958; Loumont 1992; Drewes and Stoelting 2004; Jaynes et al. 2021). It does not appear that this variation is sexually dimorphic or related to ontogeny, as has been described in many species of the African genus Hyperolius (Schiøtz 1967; Portik et al. 2019). Manaças (1958) reported Orthoptera (crickets), Blattodea (cockroaches and termites), and Coleoptera (beetles) in the stomach contents of specimens they examined.
Sexual size dimorphism is quite pronounced in L. palmatus, with male snout–vent length less than half that of females (Drewes and Stoelting 2004). In addition, the largest measured female was 110 mm snout–vent length (Loumont 1992), which remains the largest reported size of any female specimen in the entire genus Leptopelis by more than 20 mm (Channing and Rödel 2019). Despite these large adult body sizes, post-metamorphic individuals are quite small (10–11 mm; Drewes and Stoelting 2004). This combination of extreme sexual size dimorphism and exceptionally large body size in females may indicate selection for increased fecundity (e.g., Darwin 1874). Unfortunately, the reproductive biology of L. palmatus is entirely unknown. Other species in the genus Leptopelis bury their eggs in humid soil from which larvae hatch and complete their development within streams or ponds (Portik and Blackburn 2016). One species, L. brevirostris, is thought to reproduce by direct development because females produce large eggs that are buried far from water (Perret 1966; Amiet and Schiøtz 1974; Schiøtz 1999). Documenting this important aspect of its biology will be critical to understanding the habitats L. palmatus relies upon throughout its lifecycle.
Family Hyperoliidae
Three species of reed frogs in the genus Hyperolius are endemic to the islands of São Tomé and Príncipe: the São Tomé Giant Reed Frog Hyperolius thomensis and Moller’s Reed Frog H. molleri (both endemic to São Tomé), and H. drewesi (endemic to Príncipe). Prior to the recognition of H. drewesi as a distinct species, H. molleri was reported from both São Tomé and Príncipe islands (Loumont 1992; Fahr 1993; Drewes and Wilkinson 2004). Loumont (1992) characterized the karyotypes of H. thomensis and H. molleri (sensu stricto), reporting 24 chromosomes.
Phylogenetic analyses indicate that the three island species form a monophyletic group and are part of the H. cinnamomeoventris species complex (Drewes and Wilkinson 2004; Schick et al. 2010; Bell et al. 2015a, 2017; Portik et al. 2019). Within the H. cinnamomeoventris species complex, the island endemics are most closely related to H. olivaceus, a species distributed throughout the Lower Guinean forests of Gabon and the Republic of Congo (Bell et al. 2017). The distribution of H. olivaceus encompasses the Ogooué River and the mouth of the Congo River, suggesting that either river drainage could have served as a source for a vegetation raft that ferried reed frogs to the archipelago. Divergence time estimates indicate the island endemics and H. olivaceus shared a most recent common ancestor in the Late-Miocene to Pliocene (Bell et al. 2015a, 2017; Portik et al. 2019). The island endemics, however, shared a most recent common ancestor within the last 1.7–0.5 Ma, with divergence between H. molleri and H. drewesi estimated at 1.1 Ma to 270 ka (Bell et al. 2015a). As with the São Tomé caecilians, the timing of colonization and in situ diversification of the reed frogs are quite recent in the islands’ long geological histories. The pattern of divergence among the three species is consistent with a single dispersal event to the islands and suggests that reed frogs first colonized São Tomé Island, diversified in situ, and then dispersed to Príncipe Island (Bell et al. 2015a, b). The pattern of lower genetic diversity in H. drewesi relative to H. molleri is also consistent with this colonization history (Bell et al. 2015b). Although it appears that dispersal between São Tomé and Príncipe occurred at some point in the past, analyses of mtDNA and genome-wide variation indicated that gene flow between the islands is not ongoing (Bell et al. 2015b).
H. thomensis inhabits closed-canopy, primary forest habitats from 300 to 1300 m elevation, which are primarily on the wetter southern half of São Tomé (Loumont 1992; Drewes and Stoelting 2004; Gilbert and Bell 2018; Bell and Irian 2019). By contrast, H. molleri occurs in a wide range of habitats across the island from sea level to ~1400 m elevation, including swampy areas in the drier habitats on the northern side of São Tomé, agricultural areas, secondary and primary forests (Loumont 1992; Fahr 1993; Bell et al. 2015b; Gilbert and Bell 2018; Bell and Irian 2019). H. drewesi is ecologically similar to H. molleri, occurring in a wide range of habitats across Príncipe Island from sea level to ~600 m elevation (Loumont 1992; Drewes and Stoelting 2004; Bell 2016). Observations in the field and in captivity suggest that all three species are primarily nocturnal (Fahr 1993); however, like most anurans, these species are primarily observed during their reproductive periods, and thus less is known about their activities during other times of day or throughout the rest of the year.
The forest specialist H. thomensis differs from the other two species in its reproductive biology. First, males of H. thomensis produce advertisement calls from high up in the canopy (from 1 to >5 m), whereas males of H. molleri and H. drewesi call from perches 30–200 cm above ground on leaves and thin branches overhanging slow-moving streams and pools of standing water (Fahr 1993; Gilbert and Bell 2018). Furthermore, the abundance of individuals at breeding sites varies between species, with only a single to several H. thomensis calling at a given site versus upwards of 50 individuals of H. molleri congregating along a 15 m long stretch of stream (Fahr 1993; Gilbert and Bell 2018). These differences in calling site and breeding aggregation size may be associated with the specialized microhabitats H. thomensis select to deposit their eggs. While H. molleri and H. drewesi deposit their egg masses on leaves overhanging water (Fahr 1993; Drewes and Stoelting 2004; Bell 2016), which is the typical reproductive mode for Hyperolius (Portik and Blackburn 2016), H. thomensis deposit their eggs on the walls of water-filled cavities in trees, rotting logs, and bamboo (Drewes and Stoelting 2004; Gilbert and Bell 2018). These specialized breeding microhabitats are the only standing water available for anuran reproduction in some landscapes and may provide shelter to vulnerable eggs and larvae from potential predators (Drewes and Stoelting 2004; Lehtinen et al. 2004); however, they also present some unique challenges (lower dissolved oxygen, lower nutrient availability; Guimarães-Souza et al. 2006; Ferreira et al. 2019). Large egg size (2–2.5 mm; Perret 1976) and small clutch size in H. thomensis (20–40; Drewes and Stoelting 2004) relative to most species of Hyperolius (Channing and Rödel 2019) may be adaptations to the specialized reproduction in this species.
All three Hyperolius endemic to São Tomé and Príncipe are sexually dimorphic in size, with females displaying larger body sizes than males (Bell 2016; Bell and Irian 2019). The forest specialist H. thomensis is also substantially larger than H. molleri, H. drewesi, and H. olivaceus and is among the largest of the ~150 described species in the genus (Portik et al. 2020). The selective mechanisms underlying body size evolution in Hyperolius and in anurans more broadly are still poorly understood (Womack and Bell 2020); consequently, investigating ecological differences among these closely related species may provide some important insights. For instance, although both H. thomensis and H. molleri are insectivorous (Perret 1976), they may consume different sizes or types of prey, as demonstrated in other sympatric reed frog species that differ in body size (Luiselli et al. 2004). Males of all three species possess dorsal epidermal asperities (fine projections from the skin; Perret 1988), which are pigmented in H. thomensis and H. molleri but not in H. drewesi (Bell 2016). The potential functions of these sexually dimorphic features, which are also present in several continental species of Hyperolius, are poorly understood. Although the island reed frogs do not exhibit sexual dichromatism, as exhibited by a large proportion of the genus Hyperolius (Portik et al. 2019), H. molleri and H. drewesi exhibit differences in juvenile and adult coloration (Schiøtz 1967) with metamorphic and juvenile individuals of both species displaying light brown coloration with thin, white dorsolateral lines (Loumont 1992; Fahr 1993; Bell 2016). In addition, H. thomensis exhibit bright orange and black ventral coloration that is often associated with aposematism and chemical defense in amphibians (e.g., Kang et al. 2017), but this hypothesis has not yet been tested in H. thomensis. A handful of colorful Hyperolius from Cameroon was screened for defensive alkaloids, and none were found (Portik et al. 2015). Similar to L. palmatus, H. thomensis also exhibit extensive variation in dorsal coloration ranging from dark or bright green to turquoise to golden with dark spots, whereas both H. molleri and H. drewesi are consistently bright green (RCB and LAS pers. obs).
Despite differences in body size, coloration, and breeding biology, H. thomensis and H. molleri hybridize where their ranges are sympatric (Bell et al. 2015b). Males of the two species produce advertisement calls that differ in dominant frequency, and these differences are strongly correlated with body size (Gilbert and Bell 2018). Correspondingly, hybrid males are intermediate in body size and produce advertisement calls with intermediate peak frequencies (Gilbert and Bell 2018). Variation in both size and ventral coloration among hybrid frogs overlaps with that of H. molleri (Bell and Irian 2019); consequently, hybrids cannot reliably be identified without genetic analysis. Several sites with high proportions of hybrid individuals are at the boundary of primary forest and agricultural development, where breeding frogs congregate around artificial bodies of water (e.g., cisterns; Bell and Irian 2019). Hybrids can also be found at the crater lake Lagoa Amélia, which is within 1 km of the forest edge (Bell and Irian 2019). Although adult H. thomensis can be found in anthropogenically modified habitats and reproduce at these sites (Strauss et al. 2018), these environments may be population sinks if larvae and juveniles experience lower survival than in forested sites. In addition, the geographic extent of hybridization between H. thomensis and H. molleri across the island is unknown as are the potential consequences of hybridization, both of which warrant further attention.
Family Phrynobatrachidae
Two species of puddle frogs in the genus Phrynobatrachus are endemic to São Tomé and Príncipe: P. dispar on Príncipe and P. leveleve on São Tomé and Ilhéu das Rolas. Prior to the recognition of P. dispar and P. leveleve as a distinct species, P. dispar was reported from both Príncipe and São Tomé (including Ilhéu das Rolas; Boulenger 1906; Loumont 1992; Fahr 1993; Drewes and Stoelting 2004). Loumont (1992) characterized the karyotypes of Phrynobatrachus on both São Tomé and Príncipe, reporting 16 chromosomes.
Unlike the island endemic reed frogs, phylogenetic analyses of the genus Phrynobatrachus indicate that divergence between P. dispar on Príncipe and P. leveleve on São Tomé is not recent (Uyeda et al. 2007; Zimkus et al. 2010). Genetic divergence at mtDNA (cytochrome b) between the two is ~19% (Uyeda et al. 2007), and the island endemics form a monophyletic group with P. mababiensis (Zimkus et al. 2010), a southern African species that ranges from Angola to Tanzania and Mozambique (Channing and Rödel 2019). This pattern suggests that Phrynobatrachus may have colonized the archipelago twice, though estimates of continental species diversity and phylogenetic relationships in this genus are still in a state of flux. Genetic diversity within species on each island (based on mtDNA) is quite low, further supporting the existence of only a single species on each island and Schätti and Loumont’s (1992) proposed synonymy of Boulenger’s P. feae (Uyeda et al. 2007).
Puddle frogs are abundant and widespread on both islands (and on Ilhéu das Rolas), occurring from sea level to ~1400 m on São Tomé and from sea level to ~950 m on Príncipe in primary forest, secondary forest, agricultural fields and residential areas (Loumont 1992; Fahr 1993; Drewes and Stoelting 2004; Uyeda et al. 2007). They can be found perched low to the ground in grasses or shrubs, in crevices, and on the ground, especially near small bodies of water (Loumont 1992; Fahr 1993). Like many species of Phrynobatrachus, P. dispar and P. leveleve are active during the day and at night but are most often encountered at twilight (Fahr 1993). Both species use a wide range of water bodies for reproduction, including small temporary puddles at higher elevations (Fahr 1993), which P. leveleve sometimes share with Ptychadena newtoni (Drewes and Stoelting 2004). On Príncipe, P. dispar shares breeding sites with H. drewesi and heterospecific amplexus between the species has been observed (Bell and Scheinberg 2016). Relatively small clutch size (15–30 eggs) and rapid larval development (14–20 days) suggest the puddle frogs’ reproductive biology is well suited for breeding in ephemeral puddles, which may enable P. dispar and P. leveleve to occupy higher elevations than the other island endemic anurans (Fahr 1993). Male advertisement calls have been described, however, these studies were conducted prior to two species being recognized, and the calls likely correspond only to P. leveleve (Loumont 1992; Fahr 1993). Likewise, Fahr (1993) reports that adults in captivity live up to 2 years, but it is not clear whether these observations correspond to P. dispar or to P. leveleve or both.
Like many frogs, P. dispar and P. leveleve exhibit sexual size dimorphism with females being slightly larger than males (Uyeda et al. 2007). These diminutive frogs fall within the typical size range of Phrynobatrachus on the continent (Channing and Rödel 2019); however, similar to the São Tomé caecilians, patterns of intraspecific body size variation on each island are consistent with Bergmann’s Rule with larger individuals occupying higher elevations (Uyeda et al. 2007). Males of both species have dorsal epidermal asperities, and these are also present in females of P. dispar but apparently absent in female P. leveleve (Uyeda et al. 2007). Both species are also highly variable in coloration but are generally of various shades and patterns of brown (Uyeda et al. 2007).
Family Ptychadenidae
Newton’s Grassland Frog P. newtoni is endemic to São Tomé, but like many of the Gulf of Guinea amphibians, this species has a convoluted taxonomic history. First, P. newtoni was placed in synonymy of P. oxyrhynchus by Andersson (1937), and then in synonymy of P. mascareniensis by Guibé and Lamotte (1957). Perret (1976) removed P. newtoni from synonymy with P. mascareniensis and noted that although the species resembled P. oxyrhynchus, it was likely unique given the high level of endemism on the Gulf of Guinea islands. A phylogenetic study of mitochondrial DNA indicated that P. newtoni was indeed unique and that it was part of the P. mascareniensis species complex (Measey et al. 2007). Loumont (1992) characterized the karyotype of P. newtoni, reporting 24 chromosomes.
The P. mascareniensis complex consists of a dozen named and candidate taxa with a distribution that covers much of continental Africa, Madagascar, the Seychelles, and the Mascarenes (Zimkus et al. 2017). Within the P. mascareniensis species complex, P. newtoni appears to be most closely related to the Nile Grass frog, P. nilotica, which occurs along the Nile Basin and into eastern Africa (Zimkus et al. 2017). This relationship is somewhat surprising considering that lineages within the P. mascareniensis complex occur throughout West and Central Africa, and are much more proximate to the Gulf of Guinea. Previous authors have interpreted this pattern as evidence for rafting along the Congo River drainage as a dispersal route to the islands (Measey et al. 2007). Divergence time estimates suggest P. newtoni and P. nilotica shared a most recent common ancestor in the Mid- to Late-Miocene (Zimkus et al. 2017); however, a more robust phylogeny of the P. mascareniensis species complex will provide a better understanding of the evolutionary history of P. newtoni.
On São Tomé, P. newtoni occurs in lower elevation habitats (sea level to ~600 m) including plains, agricultural fields, and around human-built structures (Loumont 1992; Fahr 1993; Drewes and Stoelting 2004). Most of the remaining habitat at these lower elevations is heavily impacted by human activities, and thus while P. newtoni appears to be somewhat resilient to these landscape changes, there is some concern that very little of its original habitat remains (Fahr 1993; Drewes and Stoelting 2004). Not much is known of the biology of P. newtoni. Throughout its distribution, P. newtoni occurs with both H. molleri and P. leveleve, and the three species may use the same temporary water bodies for reproduction (Fahr 1993; Drewes and Stoelting 2004). As with most anurans, this species is sexually size-dimorphic and is the largest species in the genus Ptychadena with females reaching up to 76 mm snout–vent length (Loumont 1992; Channing and Rödel 2019). Its advertisement call is described (Loumont 1992; Fahr 1993) and it is mostly a nocturnal species but can be found during the day if it is raining (Fahr 1993). Like most Ptychadena, P. newtoni is primarily a ground-dwelling species and is a very accomplished jumper (Fahr 1993). As documented in P. mascareniensis on Madagascar (Tolojanahary et al. 2011), the diet of P. newtoni likely consists primarily of arthropods.
Conservation
Most of the Gulf of Guinea oceanic island endemic amphibians are incredibly abundant and widespread, occurring in primary forest, secondary forest, and agricultural habitats across the islands (e.g., S. ephele, S. thomense, H. molleri, H. drewesi, P. dispar, P. leveleve). By contrast, H. thomensis, L. palmatus and P. newtoni appear to have more specialized habitat requirements, and these habitats are under considerable anthropogenic pressure. Consequently, these species are considered Endangered according to the most recent IUCN assessments (Table 18.2; IUCN 2021). In the case of H. thomensis and L. palmatus, these species appear to prefer closed-canopy forest habitats and have more specialized reproduction; thus, they may be more susceptible to deforestation. For H. thomensis in particular, deforestation may directly limit the availability of suitable breeding sites as cavities that collect water typically occur in large mature trees. Furthermore, if deforestation is associated with hybridization between H. thomensis and H. molleri (as suggested in Bell and Irian 2019), the rarer H. thomensis may be at risk of extinction by hybridization (Rhymer and Simberloff 1996). In the case of P. newtoni, although this species occurs around the São Tomé capital and other heavily modified landscapes (e.g., the Agripalma oil palm plantation), very little of its original habitat remains unchanged and the impacts of land use on recruitment and adult survival are unknown. Although the São Tomé caecilian also appears quite resilient to land-use change, reports that it may be extirpated from the heavily developed Ilhéu das Rolas are concerning (Loumont 1992).
The amphibian chytrid fungal pathogen, Batrachochytrium dendrobatidis (Bd), is implicated in the declines and extinctions of amphibians across the globe (Scheele et al. 2019). Although the pathogen has been documented in many species across the Afrotropics, our understanding of how this pathogen impacts Afrotropical anuran diversity lags far behind that of other regions (Zimkus et al. 2020). Surveys of freshly sampled and historical specimens confirmed that the pathogen is present on both São Tomé and Príncipe (Hydeman et al. 2013, 2017). The earliest infections date from the oldest specimens screened to date (2001), indicating that the pathogen has been present on the islands for at least 20 years (Hydeman et al. 2017). All of the endemic species have tested positive for Bd (including the caecilians), and genomic sequencing of the positive samples indicates that the more virulent Bd-GPL strain occurs on the islands (Byrne et al. 2019). No symptomatic individuals have been reported; however, we recommend careful monitoring of this emergent pathogen.
Future Research
The biogeographic history of the amphibian fauna of the Gulf of Guinea oceanic islands is emerging as our understanding of evolutionary relationships within African genera continues to improve. Robust phylogenies of Leptopelis and Phrynobatrachus, and better resolution within the Ptychadena mascareniensis species complex, will fill important gaps in our understanding of how and when representatives of these genera reached the islands. The ecology and behavior of all the island species are incompletely understood, including basic aspects of reproductive biology (e.g., L. palmatus) and dependence on particular habitats (e.g., P. newtoni, H. thomensis), which are likely important considerations for effective conservation management. Like most vertebrates, São Tomé and Príncipe’s amphibians also serve as hosts for many parasites, including the fungal pathogen Bd and a recently described nematode (Meteterakis saotomensis; Junker et al. 2015), but the diversity of this microfauna and potential impacts on the amphibian hosts are poorly known. For instance, a recent study identified cryptic infection of P. leveleve (misidentified as P. dispar in the study) tadpoles by Perkinsea protists (Chambouvet et al. 2015) that have caused mass mortality events in the United States (Davis et al. 2007). Further studies investigating the prevalence of this parasite in the tadpoles of the other endemics and whether it is associated with tadpole mortality are sorely needed. Beyond these more practical avenues for future research, the amphibians of São Tomé and Príncipe also exhibit intriguing phenotypic diversity for addressing long-standing hypotheses in evolutionary biology, including body size evolution and gigantism on islands (e.g., H. thomensis, L. palmatus, P. newtoni), intraspecific variation and interspecific divergence in coloration, and reproductive and dietary niche partitioning.
References
Ahl E (1931) Anura III, Polypedatidae. Das Tierreich 55:1–477
Amiet J-L, Schiøtz A (1974) Voix d’amphibiens camerounais III. Hyperoliinae: genre Leptopelis. Annales de la Faculté des Sciences du Cameroun 17:131–163
Andersson LG (1937) Reptiles and batrachians collected in the Gambia by Gustav Svensson and Birger Rudebeck (Swedish Expedition 1931). Arkiv för Zoologi Stockholm 29(16):1–28
Bedriaga J (1892) Notes sur les amphibiens et reptiles recueillis par M. Adolphe F. Moller aus îles de la Guinée. O Instituto Coimbra Série 2 39:642–648
Bell RC (2016) A new species of Hyperolius (Amphibia: Hyperoliidae) from Príncipe Island, Democratic Republic of São Tomé and Príncipe. Herpetologica 72:353–351
Bell RC, Irian CG (2019) Phenotypic and genetic divergence in reed frogs across a mosaic hybrid zone on São Tomé Island. Biological Journal of the Linnean Society 128:672–680
Bell RC, Scheinberg LA (2016) Hyperolius molleri (Moller’s reed frog) and Phrynobatrachus dispar (Peters’ river frog). Heterospecific amplexus. Herpetological Review 47(2016):109
Bell RC, Drewes RC, Channing A et al (2015a) Overseas dispersal of Hyperolius reed frogs from Central Africa to the oceanic islands of São Tomé and Príncipe. Journal of Biogeography 42:65–75
Bell RC, Drewes RC, Zamudio KR (2015b) Reed frog diversification in the Gulf of Guinea: overseas dispersal, the progression rule, and in situ speciation. Evolution 69:904–915
Bell RC, Parra JL, Badjedjea G et al (2017) Idiosyncratic responses to climate-driven forest fragmentation and marine incursions in reed frogs from Central Africa and the Gulf of Guinea Islands. Molecular Ecology 26(19):5223–5244
Bergmann C (1847) Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien, Munich. 116 pp
Bocage JVB (1873) Mélanges erpétologiques. II. Sur quelques reptiles et batraciens nouveaux, rares ou peu connus d’Afrique occidentale. Jornal de Sciencias Mathematicas, Physicas e Naturaes 4:209–227
Bocage JVB (1886) Reptis e amphibios de S. Thomé. Jornal de Sciencias Mathematicas, Physicas e Naturaes 11:65–75
Boulenger GA (1882) Catalogue of the Batrachia Salientia s. Ecaudata in the collection of the British Museum. Taylor and Francis, London, 503 pp
Boulenger GA (1906) Report on the batrachians collected by the late L. Fea in West Africa. Annali del Museo Civico di Storia Naturale di Genova Series 3 2:157–172
Byrne AQ, Vredenburg VT, Martel A et al (2019) Cryptic diversity of a widespread global pathogen reveals expanded threats to amphibian conservation. Proceedings of the National Academy of Sciences 116(41):20382–20387
Ceríaco LMP, Marques MP, Bell RC, Bauer AM (2022) The terrestrial reptiles of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands: science and conservation. Springer, Cham, pp 505–534
Chambouvet A, Gower DJ, Jirků M et al (2015) Cryptic infection of a broad taxonomic and geographic diversity of tadpoles by Perkinsea protists. Proceedings of the National Academy of Sciences 112(34):E4743–E4751
Channing A, Rödel M-O (2019) Field guide to the frogs and other amphibians of Africa. Struik Nature, Cape Town
Darwin C (1859) On the origins of species by means of natural selection. Murray, London
Darwin C (1874) The descent of man, and selection in relation to sex, 2nd edn. Appleton, New York
Davis AK, Yabsley MJ, Keel MK, Maerz JC (2007) Discovery of a novel alveolate pathogen affecting Southern Leopard frogs in Georgia: description of the disease and host effects. EcoHealth 4:310–317
Delêtre M, Measey GJ (2004) Sexual selection vs ecological causation in a sexually dimorphic caecilian, Schistometopum thomense (Amphibia Gymnophiona Caeciliidae). Ethology Ecology and Evolution 16(3):243–253
Drewes RC (1984) A phylogenetic analysis of the Hyperoliidae (Anura): treefrogs of Africa, Madagascar and the Seychelles islands. Occasional Papers of the California Academy of Sciences 139:1–70
Drewes RC, Stoelting RE (2004) The California Academy of Sciences Gulf of Guinea Expedition (2001) II. Additions and corrections to our knowledge of the endemic amphibians of São Tomé and Príncipe. Proceedings of the California Academy of Sciences 55:573–587
Drewes RC, Wilkinson JA (2004) Gulf of Guinea Expedition (2001) I. The taxonomic status of the genus Nesionixalus Perret, 1976 (Anura: Hyperoliidae), treefrogs of São Tomé and Príncipe, with comments on the genus Hyperolius. Proceedings of the California Academy of Sciences 55:395–407
Ducey PK, Formanowicz DR, Boyet L et al (1993) Experimental examination of burrowing behavior in caecilians (Amphibia: Gynmophiona): effects of soil compaction on burrowing ability of four species. Herpetologica 49(4):450–457
Fahr J (1993) Ein Beitrag zur Biologie der Amphibien der Insel São Tomé (Golf von Guinea) (Amphibia). Faunistische Abhandlungen (Dresden) 19(1–16):75–84
Ferreira RB, Mônico AT, Zocca CZ et al (2019) Uncovering the natural history of the bromeligenous frog Crossodactylodes izecksohni (Leptodactylidae, Paratelmatobiinae). South American Journal of Herpetology 14:136–145
Gilbert CM, Bell RC (2018) Evolution of advertisement calls in an island radiation of African reed frogs. Biological Journal of the Linnean Society 123:1–11
Gorham SW (1962) Liste der rezenten Amphibien und Reptilien. Gymnophiona Das Tierreich 78:1–25
Gower DJ, Wilkinson M (2002) Phallus morphology in caecilians (Amphibia, Gymnophiona) and its systematic utility. Bulletin of the Natural History Museum, London (Zoology) 68(2):143–154
Gower DJ, Giri V, Dharne MS, Shouche YS (2008) Frequency of independent origins of viviparity among caecilians (Gymnophiona): evidence from the first “live-bearing” Asian amphibian. Journal of Evolutionary Biology 21:1220–1226
Greeff R (1884a) Ueber die Fauna der Guinea-Inseln S. Thomé und Rolas. Sitzungsberichte der Gesellschaft zur Beförderung der gesammten Naturwissenschaften zu Marburg 2:41–80
Greeff R (1884b) Über Siphonops thomensis Barboza du Bocage. Beitrag zur Kenntniss der Coecilien (Gymnophionen). Sitzungsberichte der Gesellschaft zur Beförderung der gesammten Naturwissenschaften zu Marburg 1:15–40
Guibé J, Lamotte M (1957) Révision systématique des Ptychadena (Batraciens, anoures ranidés) d’Afrique occidentale. Bulletin de l’Institut Française d’Afrique Noire. Série A, Sciences Naturelles 19:937–1003
Guimarães-Souza BA, Mendes GB, Bento L et al (2006) Limnological parameters in the water accumulated in tropical bromeliads. Acta Limnologica Brasiliensia 18:47–53
Haft J (1992) Bemerkungen zu den Blindwühlen der Gattung Schistometopum von São Tomé (Gymnophiona, Caeciliidae). Bonner Zoologische Beiträge 43:477–479
Haft J, Franzen M (1996) Freilandbeobachtungen, Verhalten und Nachzucht der São Tomé-Blindwühle Schistometopum thomense (Bocage, 1873). Herpetofauna 18(105):5–11
Herrel A, Measey GJ (2010) The kinematics of locomotion in caecilians: effects of substrate and body shape. Journal of Experimental Zoology 313:301–309
Herrel A, Measey GJ (2012) Feeding underground: kinematics of feeding in caecilians. Journal of Experimental Zoology 317:533–539
Himstedt W, Simon D (1995) Sensory basis of foraging behaviour in caecilians (Amphibia: Gymnophiona). Herpetological Journal 5:266–270
Hofer D (1998) Blindwühlen im Freiland und in Gefangenschaft. Beobachtungen aus 20-jähriger Amateurforschung (Amphibia: Gymnophiona). Herpetozoa 11:37–46
Hydeman ME, Bell RC, Drewes ZKR (2013) Amphibian chytrid fungus confirmed in endemic frogs and caecilians on the island of São Tomé, Africa. Herpetological Review 44:254–257
Hydeman ME, Longo AV, Velo-Antón G, Rodriguez D, Zamudio KR, Bell RC (2017) Prevalence and genetic diversity of Batrachochytrium dendrobatidis in central African island and continental amphibian communities. Ecology and Evolution 7:7729–7738
Idris ON (2004) Taxonomy, phylogeny, and biogeography of the African treefrog species of the genus Leptopelis (Hyperoliidae). PhD thesis. The University of Texas, Arlington
IUCN (2021) The IUCN Red List of Threatened Species: Version 2020-2. Available via International Union for Conservation of Nature. https://www.iucnredlist.org. Accessed 22 Oct 2021
Jaynes KE, Myers EA, Drewes RC, Bell RC (2021) New evidence for distinctiveness of the island-endemic Príncipe Giant Tree Frog, (Arthroleptidae: Leptopelis palmatus). Herpetological Journal 31(3):162–169
Jones DT, Loader SP, Gower DJ (2006) Trophic ecology of East African caecilians (Amphibia: Gymnophiona), and their impact on forest soil invertebrates. Journal of Zoology 269:117–126
Junker K, Mariaux J, Measy GJ, Mutafchiev Y (2015) Meteterakis saotomensis n. sp. (Nematoda: Heterakidae) from Schistometopum thomense (Bocage) (Gymnophiona: Dermophiidae) on São Tomé island. Systematic Parasitology 92:131–139
Kang C, Sherratt TN, Kim YE et al (2017) Differential predation drives the geographical divergence in multiple traits in aposematic frogs. Behavioral Ecology 28:1122–1130
Laurent RF (1941) Contribution à l’ostéologie et à la systematique des ranides africains. Deuxième note. Revue de Zoologie et de Botanique Africaines 34:192–234
Lavelle P, Bignell D, Lepage M et al (1997) Soil function in a changing world: the role of invertebrate ecosystem engineers. European Journal of Soil Biology 33:159–193
Lehtinen RM, Lannoo MJ, Wassersug RJ (2004) Phytotelm-breeding anurans: past, present and future research. In: Lehtinen RM (ed) Ecology and evolution of phytotelm-breeding anurans, vol 193. Miscellaneous publications. Museum of Zoology, University of Michigan, pp 1–9
Loader SP, Pisani D, Cotton JA, Gower DJ, Day JJ, Wilkinson M (2007) Relative time scales reveal multiple origins of parallel disjunct distributions of African caecilian amphibians. Biology Letters 3(5):505–508
Loumont C (1992) Les amphibiens de São Tomé et Príncipe: Révision systématique, cris nuptiaux et caryotypes. Alytes 10:37–62
Luiselli L, Bikikoro L, Odegbune E et al (2004) Feeding relationships between sympatric Afrotropical tree frogs (genus Hyperolius): the effects of predator body size and season. Animal Biology 54:293–302
Manaças S (1958) Anfíbios e répteis das ilhas de São Tomé e do Príncipe e do Ilhéo das Rolas. Conferência Internacional dos Africanistas Ocidentais, 6ª Sessão, S. Tomé, Comunicações 4:179–192
Manaças S (1973) Alguns dos anfíbios e répteis da província de S. Tomé e Príncipe. In: Livro de Homenagem ao Prof. Fernando Frade. Junta Investigação do Ultramar, Lisbon, pp 219–230
Measey GJ (2006) Surveying biodiversity of soil herpetofauna: towards a standard quantitative methodology. European Journal of Soil Biology 42:S103–S110
Measey GJ, Herrel A (2006) Rotational feeding in caecilians: putting a spin on the evolution of cranial design. Biology Letters 2:485–487
Measey GJ, Van Dongen S (2006) Bergmann’s rule and the terrestrial caecilian Schistometopum thomense (Amphibia: Gymnophiona: Caeciliidae). Evolutionary Ecology Research 8:1049–1059
Measey GJ, Vences M, Drewes RC, Chiari Y, Melo M, Bourles B (2007) Freshwater paths across the ocean: molecular phylogeny of the frog Ptychadena newtoni gives insights into amphibian colonization of oceanic islands. Journal of Biogeography 34:7–20
Melo M, Ceríaco LMP, Bell RC (2022) Biogeography and evolution in the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands: science and conservation. Springer, Cham, pp 141–170
Mertens R (1965) Die Amphibien von Fernando Poo. Bonner Zoologische Beiträge 16(1965):14–29
Mocquard F (1902) Sur des reptiles et batraciens de l’Afrique orientale anglaise, du Gabon et de la Guinée française (région de Kouroussa). Bulletin du Muséum d’Histoire Naturelle de Paris 8:404–417
Mohun SM, Davies WL, Bowmaker JK et al (2010) Identification and characterization of visual pigments in caecilians (Amphibia: Gymnophiona), an order of limbless vertebrates with rudimentary eyes. Journal of Experimental Biology 213(20):3586–3592
Nieden F (1910) Die Reptilien (ausser den Schlangen) und Amphibien. R. Friedländer & Sohn, Berlin, 74 pp
Nussbaum RA, Pfrender ME (1998) Revision of the African caecilian genus Schistometopum Parker (Amphibia: Gymnophiona: Caeciliidae), vol 187. Miscellaneous publications. Museum of Zoology, University of Michigan, pp 1–32
O’Connell KA, Prates I, Scheinberg LA, Mulder KP, Bell RC (2021) Speciation and secondary contact in a fossorial island endemic, the São Tomé caecilian. Molecular Ecology 30:2859–2871
Parker HW (1936) The amphibians of the Mamfe Division, Cameroons.—I. Zoogeography and systematics. Proceedings of the Zoological Society of London 106(1):135–163
Parker HW (1941) The caecilians of the Seychelles. The Annals and Magazine of Natural History 7(37):1–17
Parker HW (1956) Viviparous caecilians and amphibian phylogeny. Nature 178(4527):250–252
Parker HW, Dunn ER (1964) Dentitional metamorphosis in the Amphibia. Copeia 1964(1):75–86
Perret J-L (1962) Révision des types de Leptopelis et note sur quelques Hyperolius (Amphibia Salientia) de la région camerounaise, conservés au Museum de Berlin. Revue Zoologique Botanique Africaine 65:235–246
Perret J-L (1966) Les amphibiens du Cameroun. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 8:289–464
Perret J-L (1973) Leptopelis palmatus et Leptopelis rufus (Amphibia Salientia), deux espèces distinctes. Annales del al Faculté des Sciences du Yaoundé 15-16:81–90
Perret J-L (1976) Révision des amphibiens africains et principalement des types, conservés au Musée Bocage de Lisbonne. Arquivos do Museu Bocage Série 2(6):15–34
Perret J-L (1988) Sur quelques genres d’Hyperoliidae (Anura) restés en question. Bulletin de la Société Neuchâteloise des Sciences Naturelles 111:35–48
Peters WCH (1868) Über eine neue Nagergattung, Chiropodomys penicillatus, so wie über einige neue oder weniger bekannte Amphibien und Fische. Monatsberichte der Königlich Preussischen Akademie der Wissenschaften zu Berlin, Berlin, pp 448–460
Peters WCH (1870) Eine Mittheilung über neue Amphibien (Hemidactylus, Urosaura, Tropdolepisma, Geophis, Uriechis, Scaphiophis, Hoplocephalus, Rana, Entomoglossus, Cystignathus, Hylodes, Arthroleptis, Phyllobates, Cophomantis) des Königlich-Zoologischen Museums. Monatsberichte der Königlich Preussischen Akademie der Wissenschaften zu Berlin, Berlin, pp 641–652
Peters WCH (1874) Über neue Amphibien (Gymnopis, Siphonops, Polypedates, Rhacophorus, Hyla, Clyclodus, Euprepes, Clemmys). Monatsbericht der Königlich Preussischen Akademie der Wissenschaften zu Berlin, Berlin, pp 616–624
Peters WCH (1879) Über die Eintheilung der Caecilien und insbesondere über die Gattungen Rhinatrema und Gymnopis. Monatsbericht der Königlich Preussischen Akademie der Wissenschaften zu Berlin, Berlin, pp 924–943
Peters WCH (1880) Über neue oder weniger bekannte Amphibien des Berliner Zoologischen Museums (Leposoma dispar, Monopeltis (Phractogonus) jugularis, Typhlops depressus, Leptocalamus trilineatus, Xenodon punctatus, Elapomorphus erythronotus, Hylomantis fallax). Monatsbericht der Königlich Preussischen Akademie der Wissenschaften zu Berlin, Berlin, pp 217–224
Portik DM, Blackburn DC (2016) The evolution of reproductive diversity in Afrobatrachia: a phylogenetic comparative analysis of an extensive radiation of African frogs. Evolution 70(9):2017–2032
Portik DM, Scheinberg LA, Blackburn DC, Saporito RA (2015) Lack of defensive alkaloids in the integumentary tissue of four brilliantly colored African reed frog species (Hyperoliidae: Hyperolius). Herpetological Conservation and Biology 10(3):833–838
Portik DM, Bell RC, Blackburn DC et al (2019) Sexual dichromatism drives diversification within a major radiation of African amphibians. Systematic Biology 68(6):859–875
Portik DM, Blackburn DC, McGuire JA (2020) Macroevolutionary patterns of sexual size dimorphism among African tree frogs (Family: Hyperoliidae). Journal of Heredity 111(4):379–391
Rhymer JM, Simberloff D (1996) Extinction by hybridization and introgression. Annual Review of Ecology and Systematics 27:83–109
Sabaj M (2020) Codes for natural history collections in ichthyology and herpetology. Copeia 108:593–669
Sánchez-Vialas A, Calvo-Revuelta M, Castroviejo-Fisher S, De la Riva I (2020) Synopsis of the amphibians of Equatorial Guinea based upon the authors’ field work and Spanish natural history collections. Proceedings of the California Academy of Sciences 66(8):137–230
San Mauro D, Gower DJ, Müller H et al (2014) Life-history evolution and mitogenomic phylogeny of caecilian amphibians. Molecular Phylogenetics and Evolution 73:177–189
Schätti B, Loumont C (1992) Ein Beitrag zur Herpetofauna von São Tomé (Golf von Guinea) (Amphibia et Reptilia). Zoologische Abhandlungen. Staatliches Museum für Tierkunde Dresden 47:22–36
Scheele BC, Pasmans F, Skerratt LF et al (2019) Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363(6434):1459–1463
Schick S, Kielgast J, Roedder D et al (2010) New species of reed frog from the Congo basin with discussion of paraphyly in Cinnamon-belly reed frogs. Zootaxa 2501(1):23–36
Schiøtz A (1963) The amphibians of Nigeria. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjøbenhavn 125:1–92
Schiøtz A (1967) The treefrogs (Rhacophoridae) of West Africa. Spolia Zoologica Musei Hauniensis 25:1–346
Schiøtz A (1999) Treefrogs of Africa. Edition Chimaira, Franfurt
Sherratt E, Gower DJ, Klingenberg CP, Wilkinson M (2014) Evolution of cranial shape in caecilians (Amphibia: Gymnophiona). Evolutionary Biology 41:528–545
Smits AW, Flanagin JI (1994) Bimodal respiration in aquatic and terrestrial apodan amphibians. American Zoologist 34:247–263
Stoelting RE, Measey GJ, Drewes RC (2014) Population genetics of the São Tomé caecilian (Gymnophiona: Dermophiidae: Schistometopum thomense) reveals strong geographic structuring. PLoS One 9(8):e104628
Strauss L, Lima RF, Riesbeck F, Rödel M-O (2018) São Tomé Island endemic treefrogs (Hyperolius spp.) and land-use intensification: a tale of hope and caution. Tropical Conservation Science 11:1940082918776434
Taylor EH (1965) New Asiatic and African caecilians with redescriptions of certain other species. University of Kansas Science Bulletin 46:253–302
Taylor EH (1968) The caecilians of the world: a taxonomic review. University of Kansas Press, Lawrence
Teodecki EE, Brodie ED Jr, Formanowicz DR, Nussbaum RA (1998) Head dimorphism and burrowing speed in the African caecilian Schistometopum thomense. Herpetologica 54(2):154–160
Tolojanahary NL, Fatroandrianjafinonjasolomiovazo N, Rasoamampionona R, Vieites DR, Vences M (2011) Diet of the Mascarene grass frog, Ptychadena mascareniensis, in Madagascar. Malagasy Nature 5:68–74
Uyeda JC, Drewes RC, Zimkus BM (2007) The California Academy of Sciences Gulf of Guinea Expeditions (2001, 2006) VI. A new species of Phrynobatrachus from the Gulf of Guinea Islands and a reanalysis of Phrynobatrachus dispar and P. feae (Anura: Phrynobatrachidae). Proceedings of the California Academy of Sciences 58(18):367–385
Vitt LJ, Caldwell JP (2014) Biogeography and phylogeography. In: Vitt LJ, Caldwell JP (eds) Herpetology: an introductory biology of amphibians and reptiles. Academic, London, pp 381–406
Wake MH (1977) Fetal maintenance and its evolutionary significance in the Amphibia: Gymnophiona. Journal of Herpetology 11(4):379–386
Wilkinson M, Loader SP, Gower DJ, Sheps JA, Cohen BL (2003) Phylogenetic relationships of African caecilians (Amphibia: Gymnophiona): insights from mitochondrial rRNA gene sequences. African Journal of Herpetology 52:83–92
Witte GF (1941) Exploration du Parc National Albert, batraciens et reptiles. Institut des Parcs Nationaux du Congo Belge 33:1–261
Wollenberg KC, Measey GJ (2009) Why colour in subterranean vertebrates? Exploring the evolution of colour patterns in caecilian amphibians. Journal of Evolutionary Biology 22:1046–1056
Womack MC, Bell RC (2020) Two-hundred million years of anuran body-size evolution in relation to geography, ecology and life history. Journal of Evolutionary Biology 33(10):1417–1432
Zimkus BM, Rödel M-O, Hillers A (2010) Complex patterns of continental speciation: molecular phylogenetics and biogeography of sub-Saharan puddle frogs (Phrynobatrachus). Molecular Phylogenetics and Evolution 55:883–900
Zimkus BM, Lawson LP, Barej MF et al (2017) Leapfrogging into new territory: how Mascarene ridged frogs diversified across Africa and Madagascar to maintain their ecological niche. Molecular Phylogenetics and Evolution 106:254–269
Zimkus BM, Baláž V, Belasen AM et al (2020) Chytrid pathogen (Batrachochytrium dendrobatidis) in African amphibians: a continental analysis of occurrences and modeling of its potential distribution. Herpetologica 76(2):201–215
Acknowledgments
We thank Eng. Arlindo de Ceita Carvalho, Director General of the Ministry of Environment, Daniel Pontes, Director of the Príncipe Obo National Park, and the former President of Príncipe Autonomous Region, Dr. José Cassandra, for permission to collect and export specimens for study. We were assisted in the field by Pedro Ceríaco, Ostelino da Conceição Rocha, Ana Carolina Sousa, Pedro Dias, Lauren Esposito, Maria Jerónimo, Mariana Pimentel Marques, Brian Simison, Felipe Spina, and Andrew Stanbridge. Expeditions to São Tomé and Príncipe by RCD, LAS and RCB were supported by donations from many generous friends and colleagues to the California Academy of Sciences Gulf of Guinea Fund. We are grateful to the curators and collection managers Giuliano Doria, Andreas Schmitz, Frank Tillack, Jakob Hallermann, and Patrick Campbell of the Museo Civico di Storia Naturale “Giacomo Doria” (Genoa, Italy), the Musée d’Histoire Naturelle de la Ville de Genéve (Genéve, Switzerland), the Museum für Naturkunde (Berlin, Germany) Zoologisches Museum (Hamburg, Germany), and the Natural History Museum (London, UK), respectively, for allowing access and providing information about the collections in their care. We thank David Blackburn, John Boyette, Vaclav Gvozdík, Kevin Mulder, Kyle O’Connell, Ivan Prates, Rachel Quock, Ryan Schott and Mike Yuan for discussion and feedback to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Bell, R.C., Ceríaco, L.M.P., Scheinberg, L.A., Drewes, R.C. (2022). The Amphibians of the Gulf of Guinea Oceanic Islands. In: Ceríaco, L.M.P., de Lima, R.F., Melo, M., Bell, R.C. (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham. https://doi.org/10.1007/978-3-031-06153-0_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-06153-0_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06152-3
Online ISBN: 978-3-031-06153-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)