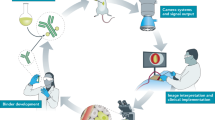
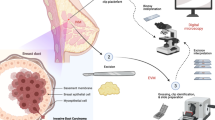
Abstract
Fluorescence Confocal Microscopy (FCM) is an imaging technique able to generate digital images from fresh tissue in real time, without the need for traditional processing. FCM’s capacity to discriminate prostate tissue on bioptic cores in real time was investigated in two prospective clinical trials. In this chapter we are presenting the updated data about the use of FCM applied to robotic prostatectomy in patients with prostate cancer.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bertoni L, Puliatti S, Reggiani Bonetti L, Maiorana A, Eissa A, Azzoni P, Bevilacqua L, Spandri V, Kaleci S, Zoeir A, Sighinolfi MC, Micali S, Bianchi G, Pellacani G, Rocco B, Montironi R. Ex vivo fluorescence confocal microscopy: prostatic and periprostatic tissues atlas and evaluation of the learning curve. Virchows Arch. 2020;476(4):511–20. https://doi.org/10.1007/s00428-019-02738-y.
Paddock SW, Eliceiri KW. Laser scanning confocal microscopy: history, applications, and related optical sectioning techniques. Methods Mol Biol. 2014;1075:9–47. https://doi.org/10.1007/978-1-60761-847-8_2.
Longo C, Pampena R, Bombonato C, et al. Diagnostic accuracy of ex vivo fluorescence confocal microscopy in Mohs surgery of basal cell carcinomas: a prospective study on 753 margins. Br J Dermatol. 2019;180:1473–80.
Bertoni L, Azzoni P, Reggiani C, et al. Ex vivo fluoresccence confocal microscopy for intra-operative real-time diagnosis of cutaneous inflammatory diseases: a preliminary study. Exp Dermatol. 2018;27:1152–9.
Reggiani C, Pellacani G, Reggiani Bonetti L, et al. An intraoperative study with ex vivo fluorescence confocal microscopy: diagnostic accuracy of the 3 visualization modalities. J Eur Acad Dermatol Venereaol. 2020;35(1):e92–4. https://doi.org/10.1111/jdv.16831.
Krishnamurthy S, Sabir S, Ban K, et al. Comparison of real-time fluorescence confocal microscopy with hematoxylin eosin stained sections of core-needle biopsy specimens. JAMA Net Open. 2020;3:e200476.
Mir MC, Bancalari B, Calatrava A, Casanova J, Dominguez Escrig JL, Ramirez-Backhaus M, Gomez-Ferrer A, Collado A, Wong A, Iborra I, Sanmarti O, Rubio-Briones J. Ex-vivo confocal fluorescence microscopy for rapid evaluation of renal core biopsy. Minerva Urol Nefrol. 2020;72(1):109–13. Epub 2019 Dec 12. PMID: 31833726. https://doi.org/10.23736/S0393-2249.19.03627-0.
Minsky M. Memoir on inventing the confocal scanning microscope. Scanning. 1988;10:128–38.
Pawley JB. Handbook of biological confocal microscopy. 3rd ed. NY: Plenum Press; 2006.
Amos WB, White JG. How the confocal laser scanning microscope entered biological research. Biol Cell. 2003;95:335–42.
MAVIG. Datasheet VivaScope® 2500M-G4. 2018. https://www.vivascope.de/wp-content/uploads/2019/06/DS_VS-2500M-G4_ 287_0219-ohne-Mohs.pdf. Accessed 4 Jan 2020.
Puliatti S, Bertoni L, Pirola GM, Azzoni P, Bevilacqua L, Eissa A, Elsherbiny A, Sighinolfi MC, Chester J, Kaleci S, Rocco B, Micali S, Bagni I, Bonetti LR, Maiorana A, Malvehy J, Longo C, Montironi R, Bianchi G, Pellacani G. Ex vivo fluorescence confocal microscopy: the first application for real-time pathological examination of prostatic tissue. BJU Int. 2019;124(3):469–76. https://doi.org/10.1111/bju.14754.
Rocco B, Sighinolfi MC, Cimadamore A, Reggiani Bonetti L, Bertoni L, Puliatti S, Eissa A, Spandri V, Azzoni P, Dinneen E, Shaw G, Nathan S, Micali S, Bianchi G, Maiorana A, Pellacani G, Montironi R. Digital frozen section of the prostate surface during radical prostatectomy: a novel approach to evaluate surgical margins. BJU Int. 2020;126(3):336–8. https://doi.org/10.1111/bju.15108.
Rocco B, Sighinolfi MC, Sandri M, et al. Digital biopsy with fluorescence confocal microscopy for effective real time diagnosis of prostate cancer: a prospective, comparative study. Eur Urol Oncol. 2021;4(5):784–91.
Rocco B, Sighinolfi MC, Bertoni L, et al. Real-time assessment of surgical margins during radical prostatectomy: a novel approach that uses fluorescence confocal microscopy for the evaluation of peri-prostatic soft tissue. GBJU Int. 2020;125(4):487–9.
Cicione A, De Nunzio C, Manno S, et al. An update on prostate biopsy in the era of magnetic resonance imaging. Minerva Urol Nephrol. 2018;70:264–74.
Mottet N, Cornford P, van der Bergh RCN et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer. 2019 edn.
Alshieban S, Al-Surimi K. Reducing turnaround time of surgical pathology reports in pathology and laboratory medicine departments. BMJ Qual Improv Rep. 2015;4(1):u209223.w3773.
Marenco J, Calatrava A, Casanova J, et al. Evaluation of fluorescent confocal microscopy for intraoperative analysis of prostate biopsy core. Eur Urol Focus. 2021;7(6):1254–9.
Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–7.
Mauermann J, Fradet V, Lacombe L, et al. The impact of solitary and multiple positive surgical margins on hard clinical end points in 1712 adjuvant treatment-naive pT2-4 N0 radical prostatectomy patients. Eur Urol. 2013;64:19–25.
Yossepowitch O, Briganti A, Eastham JA, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65:303–13.
Lopez A, Zlatev DV, Mach KE, et al. Intraoperative optical biopsy during robotic assisted radical prostatectomy using confocal endomicroscopy. J Urol. 2016;195:1110–7.
Boyette LB, Reardon MA, Mirelman AJ, et al. Fiberoptic imaging of cavernous nerves in vivo. J Urol. 2007;178:2694–700.
Jaudlim A, Aydin A, Hebrain F, et al. Imaging modalities aiding nerve sparing during radical prostatectomy. Turk J Urol. 2019;45:325–30.
Sighinolfi MC, Rocco B. Re: EAU guidelines: prostate cancer 2019. Eur Urol. 2019;76(6):871.
Dinneen EP, Van Der Slot M, Adasonla K, et al. Intraoperative frozen section for margin evaluation during radical prostatectomy: a systematic review. Eur Urol Focus. 2020;6(4):664–73.
Schlomm T, Tennstedt P, Huxhold C, et al. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients. Eur Urol. 2012;62(2):333–40.
Oxley J, Bray A, Rowe E. Could a Mohs technique make NeuroSAFE a viable option? BJU Int. 2018;122(3):358–9.
Sighinolfi MC, Rocco B. Reply to Alessia Cimadamore, Marina Scarpelli, Liang Cheng, et al.’s Letter to the Editor, re: Maria Chiara Sighinolfi, Bernardo Rocco’s words of wisdom, re: EAU guidelines: prostate cancer 2019. Mottet N, van den Bergh RCN, Briers E, et al. https://uroweb.org/guideline/prostate-Cancer/. Eur Urol. 2019;76:871. Eur Urol. 2020;77(5):e128–9.
Rocco B, Sarchi L, Assumma S, et al. Digital frozen sections with fluorescence confocal microscopy during robot-assisted radical prostatectomy: surgical technique. Eur Urol. 2021;80(6):724–9. https://doi.org/10.1016/j.eururo.2021.03.021.
Metter DM, Colgan TJ, Leung ST, et al. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2:e194337.
Bulten W, Pinckaers H, van Boven H, et al. Automated deep-learning system for Gleason grading of prostate cancer using biopsies: a diagnostic study. Lancet Oncol. 2020;21:233–41.
Ström P, Kartasalo K, Olsson H, et al. Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: a population-based, diagnostic study. Lancet Oncol. 2020;21:222–32.
Nir G, Karimi D, Goldenberg SL, et al. Comparison of artificial intelligence techniques to evaluate performance of a classifier for automatic grading of prostate cancer from digitized histopathologic images. JAMA Netw Open. 2019;2:e190442.
Chen PHC, Gadepalli K, MacDonald R, et al. An augmented reality microscope with real-time artificial intelligence integration for cancer diagnosis. Nat Med. 2019;25:1453–7.
Huang EY, Knight S, Guetter CR, et al. Telemedicine and telementoring in the surgical specialties: a narrative review. Am J Surg. 2019;218:760–6.
Montironi R, Cheng L, Cimadamore A, et al. Uropathologists during the COVID-19 pandemic: what can be learned in terms of social interaction, visibility, and social distance. Eur Urol. 2020;78(3):478–81. Epub 2020 May 11.
Cimadamore A, Lopez-Beltran A, Scarpelli M, et al. Digital pathology and COVID-19 and future crises: pathologists can safely diagnose cases from home using a consumer monitor and a mini PC. J Clin Pathol. 2020;73(11):695–6.
Comperat E. What does COVID-19 mean for the urology-pathology interaction? Eur Urol. 2020;78(1):e43–4.
Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–4.
Gerston KF, Blumberg L, Tshabalala VA, Murray J. Viability of mycobacteria in formalin-fixed lungs. Hum Pathol. 2004;35:571–5.
Badia JM, Rubio-Pérez I, Arias Díaz J, et al. Protocolo de actuación quirúrgica en casos confirmados o sospechosos de enfermedad por Ébola y otras enfermedades víricas altamente transmisibles. Cir Esp. 2016;94:11–5.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rocco, B. et al. (2022). Ex Vivo Fluorescence Confocal Microscopy. In: Ren, S., Nathan, S., Pavan, N., Gu, D., Sridhar, A., Autorino, R. (eds) Robot-Assisted Radical Prostatectomy. Springer, Cham. https://doi.org/10.1007/978-3-031-05855-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-05855-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05854-7
Online ISBN: 978-3-031-05855-4
eBook Packages: MedicineMedicine (R0)