Abstract
The field of pathology, which provides tissue diagnoses for clinical and research purposes, is at the heart of medical decision-making. The current move to digital pathology (DP) is a fundamental change in how primary diagnostic work, consultations, education, and multidisciplinary conferences are performed. DP is the prerequisite for computational pathology (CPATH), the big-data approach to pathology that extracts information from images, typically using artificial intelligence (AI) methods. While CPATH offers significant new capabilities and workflows, it also brings new challenges. There will be knock-on effects on other specialties and in teaching and research. The emerging next-generation pathology will be more quantitative, will have more diagnostic consistency, and will be more connected to its medical peers.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Transformations of the Past
Pathology, the study of the causes and effects of disease, has gone through several transformations in its history (Fig. 1). Nothing has changed about the basic goal of rendering a tissue diagnosis from the interpretation of macroscopic and especially microscopic morphology. The introduction of microscopy in the 1850s–1870s (van den Tweel & Taylor, 2010) brought about a modern, histology-based understanding of disease (i.e., histopathology) and revolutionized medicine. In the 1980s, immunohistochemistry, which uses antibodies to highlight specific antigens in tissue, entered clinical practice (Gatter et al., 1985; Taylor, 1986). Molecular pathology, which was widely adopted in the 2010s and is still a developing field, goes beyond the microscope with a broad array of methods for analyzing nucleic acids in particular.
Among the many challenges in the specialty are an aging workforce (Colgan & Geldenhuys, 2012; Metter et al., 2019; Robboy et al., 2013), an insufficient number of trainees to match future demands, and rising workloads (Metter et al., 2019; Märkl et al., 2021; Bonert et al., 2021). The complexity of case assessment is increasing in tandem with the demands of precision medicine, adding further to the workload (Warth et al., 2016). A trend toward subspecialization is related to the growing complexity of the specialty (Sarewitz, 2014; Ohori et al., 2016; Conant et al., 2017), as is the case in internal medicine, surgery, and radiology. However, the required subspecialty expertise is not always available. In addition, there are external pressures. As life expectancy continues to rise, so does the prevalence of chronic illnesses and neoplasms (Halaweish & Alam, 2015).
New technologies are now converging to reshape the specialty once again. DP makes possible novel use cases and innovative workflows. DP is also the foundation for CPATH (Louis et al., 2014; Abels et al., 2019), which is based on machine learning. Together, DP and CPATH will create many effective responses to the challenges pathology faces. CPATH will bring many new capabilities in classifying lesions, segmenting tissue elements, discovering biomarkers, and using image features to predict therapy and outcome. These developments will require new ways of training medical students and trainees in various specialties. DP will make the wealth of information in pathology much more accessible to colleagues in other specialties. An opportunity is emerging to rebrand a specialty that contributes much to medical decision-making yet is poorly understood by the public and colleagues in other specialties. The chapter concludes with an attempt to envision an age when the changes CPATH offers have become routine practices.
2 Digital Pathology
The current practice of pathology still centers on conventional microscopes and glass slides for the most part, but this is changing. The digital transformation has gone much more slowly than initially expected due to high up-front costs, an unclear business case, insufficient standardization, as well as regulatory issues. Comparisons have been made to radiology (Montalto, 2008; Cornish et al., 2012; Hipp et al., 2011; Patterson et al., 2011), which went digital decades ago, but there are fundamental differences. Microscopic images are at least 10×, sometimes 100× larger than black-and-white radiology images and require more resources. Workflow in radiology was simplified with the elimination of films, which cut costs. In contrast, pathologists still have to generate glass slides as before, but now they must also scan them and manage the data, which adds expenses. Radiology adopted an image standard long ago (i.e., DICOM, digital imaging and communications in medicine), while proprietary standards are still the rule in pathology. DICOM for pathology is under development (Herrmann et al., 2018; Clunie, 2021). It appears that digitization in pathology presents greater challenges than in other areas of medical imaging.
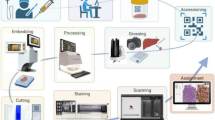
DP is the practice of pathology using digital imaging technology. It “includes the acquisition, management, sharing and interpretation of pathology information—including slides and data—in a digital environment” (Royal College of Pathologists, 2021). The definition of DP should not be limited to microscopy since pathology also involves macroscopic and molecular information. The laboratory information system (LIS) has interfaces to laboratory equipment, sample archiving, and more. DP has its roots in virtual microscopy (i.e., viewing microscopic images with a computer) and telepathology (i.e., pathology over a distance) (Weinstein, 1986). A significant catalyst for the field was whole slide imaging (WSI) (Farahani et al., 2015; Pantanowitz et al., 2018) in which a specialized robotic microscope scans an entire histologic slide rapidly and at high resolution. Most digital microscopy involves bright-field images, but fluorescent images (fluorescent immunohistochemistry, fluorescence in situ hybridization) and multispectral images (information across the spectrum of light) can also be scanned. After over two decades of technological innovation (high-throughput WSI scanners, LIS interfaces, storage, networks, etc.), DP appears sufficiently robust and affordable for deployment in clinical use. A vibrant DP commercial sector has emerged for hardware (scanners, servers, storage, communication) and software (LISs, WSI viewers, image analysis). Regulatory approval by the US Food and Drug Administration of WSI scanners for primary diagnosis (Evans et al., 2018) was an important milestone. Vendor neutral archives can manage images from different sources. An exemplary DP cockpit is shown in Fig. 2a. The workflows of conventional pathology, DP, and CPATH are shown in Fig. 2b–d.
(a) A digital pathology “cockpit”: The laboratory information system (LIS) (left screen) shows a list of patients and case overview while the WSI viewer (right screen) displays the corresponding histologic images in slide-by-slide mode. (b–d) Comparison of workflows: (b) In conventional (analog) pathology, glass slides are delivered from the lab to the pathologist, who reads them with a conventional light microscope and reports the findings in the LIS. (c) In DP, the slide is digitized with a WSI scanner and stored locally. The pathologist reviews the case on a monitor and reports it in the LIS, and WSIs are subsequently available for easy retrieval and sharing. (d) In CPATH, after digitization, the WSI undergoes preanalytical quality control and extensive precomputing. The pathologist reviews the case on a CPATH workstation and runs additional computational tools. The case is stored locally and in cloud-based services and may also be submitted to a repository for research
DP has many advantages over glass-and-microscope pathology. Workflow improvements include faster case delivery from the lab, leading to shorter turnaround times (Hanna et al., 2019b). For urgent cases, multiple colleagues can view a case simultaneously. The pathologist can annotate directly in the slide for diagnostic documentation, education, and research. Access to a patient’s prior cases is easier and faster. Information storage is more reliable with no degradation of tissue over time (i.e., fading) or breakage of glass. The retrieval of similar cases for comparison and research is also easier. Images can be transmitted quickly over vast distances for applications such as telepathology, teleconsultation, education, and clinical trials. The recent COVID-19 pandemic was an important catalyst for DP as it suddenly made remote viewing, reporting, and teaching necessary in many centers (Hanna et al., 2020; Stathonikos et al., 2020; Williams et al., 2020; Araújo et al., 2021; Browning et al., 2021). For multidisciplinary tumor boards, WSI has advantages over static images (because it is interactive) and multiheaded microscopes (because it accommodates remote participants). The possible disadvantages of DP include the initial cost, data storage requirements, insufficient LIS integration, lack of Z-stacking in most scanners, and the risk of workload increases (e.g., expansion of second opinions) (Farahani et al., 2015; Jahn et al., 2020).
A number of use cases for DP exist, including clinical and nonclinical applications (Dash et al., 2021). For primary diagnosis (Volynskaya et al., 2018; Asa & Evans, 2020; Retamero et al., 2020; Schüffler et al., 2021; Borowsky et al., 2020), many validation studies indicate high concordance between glass slides and DP (Snead et al., 2016; Hanna et al., 2019a, 2020; Mukhopadhyay et al., 2018; Azam et al., 2021). In case review (e.g., internal or external review, consultation, and second opinions, including telepathology and remote work) (Zhao et al., 2015; Chong et al., 2019), DP is faster, safer, and often cheaper than sending slides by mail, as was done previously. These review capabilities will make it easier to generate consensus diagnoses among pathologists, thereby helping to reduce diagnostic variability and diagnostic errors. A long-established use case is intraoperative consultation (e.g., frozen or “quick” sections) that can be performed by telepathology (Horbinski et al., 2007; Evans et al., 2009; Ribback et al., 2014; Dietz et al., 2020) and is therefore appealing for multisite hospitals or those without sufficient subspecialty expertise. Multidisciplinary tumor boards benefit from DP because it eliminates much preparation work and enables presentations from WSI, which greatly improves their quality (Krupinski et al., 2018). Digital microscopy has been used in teaching for many years (Mea et al., 2017; Rodrigues-Fernandes et al., 2020; Hassell et al., 2021; Evans et al., 2021) and has proven to be an effective learning tool (Ordi et al., 2015; Saco et al., 2016) that high proportions of trainees accept. DP also brings new opportunities in quality assurance and quality control (QA/QC) (Janowczyk et al., 2019; Wright et al., 2020; Chen et al., 2021).
The business case for DP is improving. As initially mentioned, DP adoption by mainstream pathologists has been slow. One reason was that the business case was still challenging. However, this is now changing, and many centers have reported productivity gains from DP adoption (Baidoshvili et al., 2018; Hanna et al., 2019b; Retamero et al., 2020). In addition, new long-term revenue opportunities are opening up in the areas of telepathology and clinical trials (Barisoni & Hodgin, 2017; Pell et al., 2019). Several studies have examined the business case to provide guidance (Lujan et al., 2021; Williams et al., 2019). CPATH enables new use cases in image analysis that are impossible with conventional microscopes, further improving the economic case for DP adoption. Indeed, comparisons are sometimes made between the iPhone, which created the basis for apps, and WSI scanners that make pathology AI applications possible.
DP will likely lead to profound changes in workflows in ways that are difficult to predict. The DP environment is not simply a new user interface (i.e., a modernized version of the conventional microscope) but a foundation for a very different way to practice that opens up entirely new capabilities, such as CPATH and biomarker analysis.
3 Computational Pathology
3.1 Background and Definitions
A discussion of the new capabilities and use cases enabled by CPATH should be prefaced by a reminder that definitions in this area are still evolving. Experts from the Digital Pathology Association define CPATH broadly as “a branch of pathology that involves computational analysis of a broad array of methods to analyze patient specimens for the study of disease” (Abels et al., 2019, p. 287). They point out that definitions of CPATH may be affected by the context in which they are presented, which may sometimes lead to confusion. A more comprehensive earlier description of CPATH (Louis et al., 2014, p. 1133) stated that it “incorporates multiple sources of raw data (e.g., clinical and laboratory data, imaging); extracts biologically and clinically relevant information; uses mathematical models at the levels of molecules, individuals, and populations for diagnostic inferences and predictions; and presents that clinically actionable knowledge to customers.” Important foundational technologies for CPATH are machine learning (a type of AI that learns from examples of measurements and can generalize them after a learning phase to predict a variable of interest) and especially deep learning (i.e., a type of machine learning that is based on artificial neural networks that contain many intermediate, hidden layers of increasing complexity). In supervised machine learning, a label (ground truth) is attached to the data. In contrast, unsupervised machine learning does not use such assigned labels and is instead based on clustering and principal component analysis. In end-to-end deep learning (i.e., the model learns to connect initial input data and the final output result), images are the input and a corresponding clinical variable or molecular data is used for training. The output may be a therapy prediction or a prognostic assessment directly from raw images. A term that is sometimes used interchangeably with CPATH is “pathomics” (Saltz et al., 2017), also called “histomics” or “tissue phenomics.” It is the “pieces and parts” approach to pathology data. A WSI is broken down into very small units for further integration with molecular data, omics data, or other biomedical imaging data. Features are then extracted from tissue with the help of computational algorithms and are subsequently used to create prediction models. Pathomics enables reproducible and quantitative data mining for histology. The parallel field in radiology is called radiomics (Mayerhoefer et al., 2020; Yip & Aerts, 2016). The AI revolution in pathology is connected to and influenced by other fields of AI research, including other medical imaging and developments in more distant fields, such as autonomous vehicles.
3.2 Applications of CPATH
When machine learning methods were first introduced to the field, the goal was often to recapitulate the pathologist’s approach (e.g., automate tumor grading in a way that a human would proceed). As machine learning and deep learning methods have evolved, they have become increasingly able to perform tasks that are beyond the capabilities of a pathologist. Histopathologic image analysis, whether based on machine learning or deep learning techniques or more conventional methods, falls into several general categories. Segmentation is the precise delineation of the borders of one particular tissue element from its surroundings. Examples of frequently segmented objects are epithelia, glands, stroma, cells, or nuclei. In order to obtain sufficient training and validation data for segmentation, large numbers of manual annotations by pathologists are often required—a time-consuming and expensive step. Classification is often based on various clustering methods, such as supervised clustering (when labeled or annotated data are available) or unsupervised clustering (label scarcity). Images are grouped into categories, such as tumor vs. non-tumor, tumor subtypes, or grades of tumors.
There is more information in histopathology images than meets the human eye. However, with machine learning and deep learning methods, these “subvisual” features may be used for tasks such as classification or predicting molecular findings from hematoxylin and eosin (H&E) images. Certain histomorphologic features correlate with molecular features, and given sufficient training data, a prediction of molecular signatures from WSIs may be possible (Coudray et al., 2018; Yamashita et al., 2021). Advanced deep learning-based approaches make it possible to infer molecular features and therapy responses from tissue biomarkers (Krause et al., 2021; Echle et al., 2021). In the workflow of the future, diagnostic images could be prescreened for molecular predictions to flag those with a high likelihood of a negative result. Subsequent molecular testing may then not be necessary on these cases. Some mutations are mutually exclusive, and the availability of such image-based preliminary testing could allow for better triage of molecular testing. Much actual testing would still have to be done, but this approach may help cut costs (Kacew et al., 2021). Similarly, information about prognosis may also be obtained in this way although it requires training with clinical outcome data (Bychkov et al., 2018; Kather et al., 2019).
Numerous specific CPATH use cases have emerged. Some of them are depicted in a hypothetical app store shown in Fig. 3. They include QA/QC tools, detection and segmentation tools, diagnostic assistance for common tasks such as finding mitotic figures, predictive tools, grading tools, and more advanced analysis tools. Also included is the ability to find matching cases in large databases with image search (see below). Some of the tools in Fig. 3 are tongue-in-cheek, hinting at the hype surrounding AI.
3.3 AI Applications Beyond Histopathologic Analysis
Interest in applying AI to anatomic pathology has largely been focused on histologic image analysis, mostly tasks related to visual recognition and understanding. Many other potential uses have not been studied as thoroughly. Among them is the extraction of a patient’s relevant clinical history from various sources and automated writing and standardization of pathology reports (e.g., automated generation of synoptic reports for cancer cases). For patient engagement, automated reports in a language patients can understand could be accessed through a patient portal (Krasowski et al., 2017). Another area is laboratory automation (Naugler & Church, 2019) and laboratory QA/QC (Janowczyk et al., 2019). The potential to generate virtual stains has been demonstrated, which may involve scanning unstained paraffin sections and computing staining patterns or transforming one stain into another (de Haan et al., 2021). Other potential applications involve assisting with managing workload, multi-omics (correlation, integration), and multimodal data fusion (e.g., radiology-pathology).
3.4 Challenges in Pathology AI
Despite the very impressive progress in pathology AI in recent years, there are many risks and challenges. An immediate problem is the lack of sufficiently large training datasets that represent the extensive variability that exists in clinical data across different institutions (e.g., among institutions, regions, ethnicities). Such datasets are the prerequisite for clinical-grade CPATH (as opposed to research-grade) and will benefit from the establishment of repositories (see below). Low availability of annotations by pathologists for training data has also been a problem. However, this may improve with the wider adoption of automated high-throughput methods that make the annotation process far more efficient (Miao et al., 2021). AI could lead to much larger volumes of data to be extracted from histologic images. This deluge of data could expose the workforce to the risk of information overload. Pathologists may get bogged down in too much data that does not have much benefit for clinical care. There are also concerns related to human/computer interaction, such as decision fatigue (i.e., frequent inappropriate alerts leading the human operator to ignore the signal) (Ancker et al., 2017; Baron et al., 2021). Another potential risk is automation bias; the human operator may become complacent and rely on a machine’s output without doing sufficient double-checking (Parasuraman & Riley, 1997). There are concerns that when deep learning algorithms take over some very specialized diagnostic functions, pathologists’ skills may fall into disuse and atrophy (Sarwar et al., 2019).
New areas of research and development will certainly emerge from these topics. The topic of AI explainability has received much attention as AI approaches in pathology have become increasingly successful. There are concerns that the models with the best performance are also the least transparent ones. Labels such as “opacity” or “black box” are sometimes used when discussing AI. The field of explainable AI (xAI) in medicine “deals with the implementation of transparency and traceability of statistical black-box machine learning methods, particularly deep learning” (Holzinger et al., 2019, p. 1). In other words, xAI intends to bring transparency to the process by revealing the reasons for the decisions a model makes. When applied to anatomic pathology, xAI mechanisms will likely be embedded in the analysis of WSIs (Tosun et al., 2020) to improve trust in the models and the safety of the CPATH workflow.
3.5 Pathologist vs. AI?
Will AI replace pathologists? There are some concerns in the specialty about the possible elimination of jobs (Sarwar et al., 2019). The threat of automation may discourage potential trainees from choosing the specialty, thus increasing the growing shortage of pathologists. However, it appears unlikely that machines or AI will replace pathologists as the latter are needed to do more integrative tasks, to examine the data products coming from CPATH, and to do new types of research on topics such as biomarkers and pathomics (Saltz et al., 2017). Human pathologists are also needed to detect rare pathologies that the algorithms have not been trained on. For the near future, it seems more likely that pathologists using AI will replace those not using it, creating a combination of human and machine intelligence (augmented intelligence). AI-based diagnostic support systems can help avoid decision fatigue and enable the pathologist to do more work. For the longer term, however, emerging technologies will bring significant changes in workflow. These changes will enable machines to perform diagnostic tasks in ways that are very different from how human pathologists operate.
3.6 Reference Databases, DP Repositories, and Large-Scale Initiatives in CPATH
With image search, a reference database containing tens of thousands of curated and annotated cases can be queried with a current case to identify similar cases. The image search can involve histologic and other features. As a new capability in CPATH, this opens up new opportunities for diagnostic support and may lead to significant changes in workflow. The technologies for reverse image search developed at Google were applied to histologic images; the resulting search tool is called “Similar Medical Images Like Yours,” or “SMILY” (Hegde et al., 2019). Another example is “Yottixel,” which uses barcoding technology to represent images (Kalra et al., 2020a, b). “Pathobot” implements AI-driven image search and uses social media data for similarity ranking (Schaumberg et al., 2020).
The advances in applying AI technologies to pathology over the last 6–7 years have also spurred investments in large-scale infrastructure projects, such as repositories and large CPATH initiatives. Some of the problems with the robustness of AI applications seem to be primarily problems of data availability. More data from different institutions that represent a larger number of laboratories, staining conditions, scanners, and so forth are likely to improve robustness. The “BIGPICTURE” project is a large public-private consortium led by Radboud University in the Netherlands that is funded by the EU Innovative Medicines Initiative. BIGPICTURE is building a central repository of millions of annotated DP slides to support the development of AI models (Moulin et al., 2021). The “EMPAIA” project (EcosysteM for Pathology Diagnostics with AI Assistance), funded by the German Federal Ministry for Economic Affairs and Climate Action (Homeyer et al., 2021), is building a platform for the development and validation of AI services that will include data repositories and a marketplace. Its goal is to support an entire ecosystem of stakeholders, including AI developers, clinical laboratories, research institutions in academia and the private sector, certification bodies, and other participants. Five collaborative committees are working to remove regulatory, legal, technical, and organizational obstacles to pathology AI. The initiative also produces advanced half-day training events called “EMPAIA Academy” for AI skills development. The Pathology Innovation Collaborative Community (PIcc, formerly the Alliance for Digital Pathology) (Marble et al., 2020) is a significant regulatory science initiative where public and private stakeholders work with the US Food and Drug Administration to accelerate advances in the precompetitive phase of DP. Additional large-scale initiatives exist in the UK with funding from UK Research and Innovation (UKRI), including iCAIRD (https://icaird.com), PathLAKE (https://www.pathlake.org), and NPIC (https://npic.ac.uk/). These public-private consortia are building large repositories of annotated and curated WSI data with corresponding clinical information.
4 Broader Effects on Other Specialties, Education, and Research
4.1 Integrative Pathology and Other Specialties
Pathologists have always been integrators of information from different sources, combining their morphologic and molecular findings (morpho-molecular pathology) (Jones et al., 2017) and putting them in the context of clinical practice and basic science. The term “integrative” even became part of the name of a professional society, the European Society of Integrative Digital Pathology (Eloy et al., 2021). As pathology becomes even more data-rich, the role of the integrator will become stronger and will involve combining histomorphology, molecular pathology, omics data, AI-derived data products, clinical information, and other medical imaging modalities, such as radiology.
Significant opportunities are emerging for cross-disciplinary practice. Analogous to the way that radiologic images are now viewed by physicians in many specialties but only formally reported by radiologists, changes will happen with pathology images in the future. Once histologic images are decoupled from the microscope, other specialties can use them in the clinical setting and in teaching and research. Use cases for DP and CPATH are emerging for various specialties. For example, during surgery, when tissue is sent to pathology for an intraoperative consultation (“frozen section”), the surgeon will not just receive a verbal diagnosis but can see the actual histology on screen during the procedure without leaving the operating room. A radiologist can review not just the pathology report but the actual histology images, receiving much more granular feedback on the earlier radiology diagnosis (Lundström et al., 2017; Mun et al., 2020). Diagnosis and therapy will be more closely coupled, following the example of oncologic pathology (“companion diagnostics,” “theranostics”). The impact will be significant in dermatology and dermatopathology, fields that are already adopting novel imaging technologies (Glines et al., 2020).
4.2 Education and Outreach
The DP/CPATH revolutions will significantly impact the training of medical students, pathology trainees, and trainees in other specialties. Curricular changes at many medical schools in North America have resulted in the integration of various topics into core curricula. In the process, pathology teaching has often been curtailed or entirely eliminated. As a result, many medical students no longer have adequate exposure to this field and increasingly lack a tissue-based understanding of disease. This is a problem for the future of pathology because it is vitally important to attract good trainees. In response, digital platforms such as PathElective (Lilley et al., 2021) have sprung up that offer high-quality content to those who are willing to invest the time and effort beyond their required curriculum. The COVID-19 pandemic presented additional opportunities to use DP techniques to teach pathology (Hassell et al., 2021; Hassell & Afzal, 2021; Patel et al., 2021).
Whenever new topics emerged in recent decades, the specialty has responded by creating new training programs for pathology postgraduates, such as fellowships in molecular diagnostics (Rosenbaum et al., 2021) and pathology informatics (Levy et al., 2012; Mandelker et al., 2014; Quinn et al., 2014). The introduction of CPATH will necessitate an overhaul of existing anatomic pathology training programs and will lead to the creation of new fellowship programs.
DP/CPATH topics can support outreach. As mentioned earlier, when histologic images are “freed” from the microscope with DP, new ways to use them become possible. One such application is patient education and engagement. In the past, most pathologists have had minimal contact with patients, but this may change as digital technologies allow for much easier dialog. Indeed, the concept of a patient-pathologist consultation program has been presented (Booth et al., 2019; Jug et al., 2021; Lapedis et al., 2020; Shachar et al., 2021). If this becomes popular, it could give much more visibility to pathologists. Also significant for outreach is the small but vibrant presence of pathology on social media (Deeken et al., 2020; El Hussein et al., 2020; Mukhopadhyay et al., 2021). On platforms such as Twitter, Facebook, and LinkedIn, pathologists share educational material, announce events, interact with applicants, and much more. In the future, pathologists will likely move to dedicated, more formal services based on WSIs and CPATH functionality.
The ongoing transformation provides excellent opportunities for rebranding the specialty (El Hussein et al., 2021). Some of the ingredients of rebranding are the capabilities of DP/CPATH, integrative pathology, and improved outreach. Rebranding is essential because the perception of pathology is suboptimal among the general public, where autopsy activities predominate, and among colleagues in other medical specialties, who often have little knowledge about what pathology does.
4.3 Nonclinical Settings: Biobanking, Experimental and Veterinary Pathology, Toxicology, and Pharmaceutical Development
As a translational discipline, pathology is a bridge between basic biomedical science and various clinical fields. The same technologies that underpin the digital and computational pathology revolution are entering all other areas that perform tissue analysis. Because the workflows in those areas can differ significantly from routine clinical workflow, technologies will need to be adapted. For example, slide viewers designed for clinical diagnostic work are often case-centric and may not be suitable for preclinical pharmaceutical development that is more batch-centric.
Biobanking and biospecimen science are closely tied to the clinical diagnostic space and have started to use WSI scans as part of specimen annotation (Hamilton et al., 2014; Wei & Simpson, 2014). There is great potential in incorporating pathologist annotations and CPATH-derived data products and further combining these with molecular data, such as proteomics and methylomics. There is also great potential for robotics in biobanking and basic tissue-based research. For example, combining laser capture microdissection with robotics and AI will make possible new applications that were previously too time-consuming if performed by a human operator. Veterinary medicine has started to integrate DP techniques for teaching and diagnostic use (Bertram & Klopfleisch, 2017; Brown et al., 2016; Jones-Hall et al., 2021). CPATH techniques will be applied to organoids, tumoroids, xenografts, avatars, and other microanatomic models.
In drug discovery and development, DP is already changing the way clinical trials are conducted. Pharmaceutical companies are digitizing central pathology review (Mroz et al., 2013; Barisoni & Hodgin, 2017; Pell et al., 2019), bringing improvements in efficiency and accuracy. Compared to clinical diagnostics, some aspects of applying CPATH to preclinical pharmaceutical development and toxicological pathology may be easier to implement as the analyses are often highly standardized. AI tools are especially well suited for highly repetitive tasks, which will make it possible to expand preclinical development and toxicological pathology activities (Turner et al., 2020).
5 Conclusions and Perspectives
Pathology is entering another transformative phase. The introduction of DP will be the foundation for CPATH. Parallel changes will come from a larger number of additional areas that are beyond the scope of this chapter. Among them are novel imaging and visualization technologies, such as augmented reality, in vivo microscopy, and 3D pathology, as well as multiplexing, multi-omics, and multimodal data fusion. Advances in robotics and laboratory automation will also influence this convergence.
As pathologists reinvent themselves, their task will remain the same: extracting, simplifying, and distilling information. The role of pattern recognition abilities may become somewhat less important as AI-based methods will take over some of these tasks. However, managing complexity and performing integrative tasks will likely become more important. Some of the broader changes that characterize current and future practice are contrasted in Table 1. With AI and multiplexing, pathology will become more quantitative (e.g., tumor markers Ki-67 and PD-L1). Variability of diagnosis and reporting can be reduced significantly. Large reference databases can support standardization. With CPATH-enabled multimodal data fusion from different imaging sources, diagnostic imaging will become more cross-disciplinary.
What will the practice of pathology look like when this transformation is complete, perhaps 10 years after publication of this book? Workflow will be different in many ways, including AI-based QC in the lab to improve and standardize quality; the routine, automatic preordering of special stains and immunohistochemistry by AI (Chatrian et al., 2021); and the precomputation of diagnostic parameters even before pathologist review. DP will be the norm for primary diagnosis, consultation, teaching, and other functions. Previously unavailable diagnostic tools such as heat maps will be commonplace. The pathologists of the future will be sorting through AI-generated data products. They will be spending more time doing QA/QC. Automated AI-based preliminary diagnoses will be available for most indications. Even sooner, AI-based “second-read” mechanisms could be introduced that work in the background, surfacing only when a discrepancy occurs. Large reference case databases will be available for diagnostic support and standardization. Large image repositories will enhance research. Based on new biomarkers (“pathomics”), a new golden age of tissue-based research is likely. Pathologists will be more connected to each other as telepathology will improve ways to work collaboratively. They will also be communicating more with their patients. These changes also have the potential to address many currently unmet diagnostic needs. There is considerable demand for diagnostic services in developing countries that do not have sufficient numbers of pathologists and very little subspecialization. With telepathology plus automation of some diagnostic tasks, many gaps in service can be closed, even with a stagnating professional workforce.
In summary, major changes are underway and pathology will likely get even more exciting. Machines will take over some functions from pathologists but will also enable them to extract more information. The changes will introduce new workflows and entirely new capabilities. Wide-ranging effects for other specialties and for education and research can be expected.
References
Abels, E., Pantanowitz, L., Aeffner, F., Zarella, M. D., van der Laak, J., Bui, M. M., Vemuri, V. N., Parwani, A. V., Gibbs, J., Agosto-Arroyo, E., Beck, A. H., & Kozlowski, C. (2019). Computational pathology definitions, best practices, and recommendations for regulatory guidance: A white paper from the Digital Pathology Association. The Journal of Pathology, 249(3), 286–294. https://doi.org/10.1002/path.5331
Ancker, J. S., Edwards, A., Nosal, S., Hauser, D., Mauer, E., Kaushal, R., & HITEC Investigators. (2017). Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Medical Informatics and Decision Making, 17(1), 36. https://doi.org/10.1186/s12911-017-0430-8
Araújo, A. L. D., do Amaral-Silva, G. K., Pérez-de-Oliveira, M. E., Gallagher, K. P. D., López de Cáceres, C. V. B., Roza, A. L. O. C., Leite, A. A., Mariz, B. A. L. A., Rodrigues-Fernandes, C. I., Fonseca, F. P., Lopes, M. A., Speight, P. M., Khurram, S. A., Júnior, J. J., Martins, M. D., de Almeida, O. P., Santos-Silva, A. R., & Vargas, P. A. (2021). Fully digital pathology laboratory routine and remote reporting of oral and maxillofacial diagnosis during the COVID-19 pandemic: A validation study. Virchows Archiv: An International Journal of Pathology, 479(3), 585–595. https://doi.org/10.1007/s00428-021-03075-9
Asa, S. L., & Evans, A. (2020). Issues to consider when implementing digital pathology for primary diagnosis. Archives of Pathology and Laboratory Medicine, 144(11), 1297. https://doi.org/10.5858/arpa.2020-0168-LE
Azam, A. S., Miligy, I. M., Kimani, P. K.-U., Maqbool, H., Hewitt, K., Rajpoot, N. M., & Snead, D. R. J. (2021). Diagnostic concordance and discordance in digital pathology: A systematic review and meta-analysis. Journal of Clinical Pathology, 74(7), 448–455. https://doi.org/10.1136/jclinpath-2020-206764
Baidoshvili, A., Bucur, A., van Leeuwen, J., van der Laak, J., Kluin, P., & van Diest, P. J. (2018). Evaluating the benefits of digital pathology implementation: Time savings in laboratory logistics. Histopathology, 73(5), 784–794. https://doi.org/10.1111/his.13691
Barisoni, L., & Hodgin, J. B. (2017). Digital pathology in nephrology clinical trials, research, and pathology practice. Current Opinion in Nephrology and Hypertension, 26(6), 450–459. https://doi.org/10.1097/MNH.0000000000000360
Baron, J. M., Huang, R., McEvoy, D., & Dighe, A. S. (2021). Use of machine learning to predict clinical decision support compliance, reduce alert burden, and evaluate duplicate laboratory test ordering alerts. JAMIA Open, 4(1), ooab 006. https://doi.org/10.1093/jamiaopen/ooab006
Bertram, C. A., & Klopfleisch, R. (2017). The pathologist 2.0: An update on digital pathology in veterinary medicine. Veterinary Pathology, 54(5), 756–766. https://doi.org/10.1177/0300985817709888
Bonert, M., Zafar, U., Maung, R., El-Shinnawy, I., Kak, I., Cutz, J.-C., Naqvi, A., Juergens, R. A., Finley, C., Salama, S., Major, P., & Kapoor, A. (2021). Evolution of anatomic pathology workload from 2011 to 2019 assessed in a regional hospital laboratory via 574,093 pathology reports. PLoS One, 16(6), e0253876. https://doi.org/10.1371/journal.pone.0253876
Booth, A. L., Katz, M. S., Misialek, M. J., Allen, T. C., & Joseph, L. (2019). “Please help me see the dragon I am slaying”: Implementation of a novel patient-pathologist consultation program and survey of patient experience. Archives of Pathology and Laboratory Medicine, 143(7), 852–858. https://doi.org/10.5858/arpa.2018-0379-OA
Borowsky, A., Glassy, E., Wallace, W., Kallichanda, N., Behling, C., Miller, D. V., Oswal, H. N., Feddersen, R., Bakhtar, O. R., Mendoza, A. E., Molden, D., Saffer, H. L., Wixom, C. R., Albro, J. E., Cessna, M. H., Hall, B. J., Lloyd, I. E., Bishop, J., Darrow, M. A., et al. (2020). Digital whole slide imaging compared with light microscopy for primary diagnosis in surgical pathology: A multicenter, double-blinded, randomized study of 2045 cases. Archives of Pathology and Laboratory Medicine, 144(10), 1245–1253. https://doi.org/10.5858/arpa.2019-0569-OA
Brown, P. J., Fews, D., & Bell, N. J. (2016). Teaching veterinary histopathology: A comparison of microscopy and digital slides. Journal of Veterinary Medical Education, 43(1), 13–20. https://doi.org/10.3138/jvme.0315-035R1
Browning, L., Fryer, E., Roskell, D., White, K., Colling, R., Rittscher, J., & Verrill, C. (2021). Role of digital pathology in diagnostic histopathology in the response to COVID-19: Results from a survey of experience in a UK tertiary referral hospital. Journal of Clinical Pathology, 74(2), 129–132. https://doi.org/10.1136/jclinpath-2020-206786
Bychkov, D., Linder, N., Turkki, R., Nordling, S., Kovanen, P. E., Verrill, C., Walliander, M., Lundin, M., Haglund, C., & Lundin, J. (2018). Deep learning based tissue analysis predicts outcome in colorectal cancer. Scientific Reports, 8(1), 3395. https://doi.org/10.1038/s41598-018-21758-3
Chatrian, A., Colling, R. T., Browning, L., Alham, N. K., Sirinukunwattana, K., Malacrino, S., Haghighat, M., Aberdeen, A., Monks, A., Moxley-Wyles, B., Rakha, E., Snead, D. R. J., Rittscher, J., & Verrill, C. (2021). Artificial intelligence for advance requesting of immunohistochemistry in diagnostically uncertain prostate biopsies. Modern Pathology, 34, 1780–1794. https://doi.org/10.1038/s41379-021-00826-6
Chen, Y., Zee, J., Smith, A., Jayapandian, C., Hodgin, J., Howell, D., Palmer, M., Thomas, D., Cassol, C., Farris, A. B., Perkinson, K., Madabhushi, A., Barisoni, L., & Janowczyk, A. (2021). Assessment of a computerized quantitative quality control tool for whole slide images of kidney biopsies. The Journal of Pathology, 253(3), 268–278. https://doi.org/10.1002/path.5590
Chong, T., Palma-Diaz, M. F., Fisher, C., Gui, D., Ostrzega, N. L., Sempa, G., Sisk, A. E., Valasek, M., Wang, B. Y., Zuckerman, J., Khacherian, C., Binder, S., & Wallace, W. D. (2019). The California telepathology service: UCLA’s experience in deploying a regional digital pathology subspecialty consultation network. Journal of Pathology Informatics, 10, 31. https://doi.org/10.4103/jpi.jpi_22_19
Clunie, D. A. (2021). DICOM format and protocol standardization: A core requirement for digital pathology success. Toxicologic Pathology, 49(4), 738–749. https://doi.org/10.1177/0192623320965893
Colgan, T. J., & Geldenhuys, L. (2012). The practice of pathology in Canada: Decreasing pathologist supply and uncertain outcomes. Archives of Pathology and Laboratory Medicine, 136(1), 90–94. https://doi.org/10.5858/arpa.2011-0188-OA
Conant, J. L., Gibson, P. C., Bunn, J., & Ambaye, A. B. (2017). Transition to subspecialty sign-out at an academic institution and its advantages. Academic Pathology, 4, 2374289517714767. https://doi.org/10.1177/2374289517714767
Cornish, T. C., Swapp, R. E., & Kaplan, K. J. (2012). Whole-slide imaging: Routine pathologic diagnosis. Advances in Anatomic Pathology, 19(3), 152–159. https://doi.org/10.1097/PAP.0b013e318253459e
Coudray, N., Ocampo, P. S., Sakellaropoulos, T., Narula, N., Snuderl, M., Fenyö, D., Moreira, A. L., Razavian, N., & Tsirigos, A. (2018). Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nature Medicine, 24(10), 1559–1567. https://doi.org/10.1038/s41591-018-0177-5
Dash, R. C., Jones, N., Merrick, R., Haroske, G., Harrison, J., Sayers, C., Haarselhorst, N., Wintell, M., Herrmann, M. D., & Macary, F. (2021). Integrating the health-care enterprise pathology and laboratory medicine guideline for digital pathology interoperability. Journal of Pathology Informatics, 12(1), 16. https://doi.org/10.4103/jpi.jpi_98_20
de Haan, K., Zhang, Y., Zuckerman, J. E., Liu, T., Sisk, A. E., Diaz, M. F. P., Jen, K.-Y., Nobori, A., Liou, S., Zhang, S., Riahi, R., Rivenson, Y., Wallace, W. D., & Ozcan, A. (2021). Deep learning-based transformation of H&E stained tissues into special stains. Nature Communications, 12(1), 4884. https://doi.org/10.1038/s41467-021-25221-2
Deeken, A. H., Mukhopadhyay, S., & Jiang, X. (2020). Social media in academics and research: 21st-century tools to turbocharge education, collaboration, and dissemination of research findings. Histopathology, 77(5), 688–699. https://doi.org/10.1111/his.14196
Dietz, R. L., Hartman, D. J., & Pantanowitz, L. (2020). Systematic review of the use of telepathology during intraoperative consultation. American Journal of Clinical Pathology, 153(2), 198–209. https://doi.org/10.1093/ajcp/aqz155
Echle, A., Rindtorff, N. T., Brinker, T. J., Luedde, T., Pearson, A. T., & Kather, J. N. (2021). Deep learning in cancer pathology: A new generation of clinical biomarkers. British Journal of Cancer, 124(4), 686–696. https://doi.org/10.1038/s41416-020-01122-x
El Hussein, S., Lyapichev, K. A., Crane, G. M., Mirza, K. M., Pemmaraju, N., Medeiros, L. J., Khoury, J. D., & Loghavi, S. (2020). Social media for hematopathologists: Medical practice reinvented-#Hemepath. Current Hematologic Malignancy Reports, 15(5), 383–390. https://doi.org/10.1007/s11899-020-00600-6
El Hussein, S., Khoury, J. D., Lyapichev, K. A., Tashakori, M., Khanlari, M., Miranda, R. N., Kanagal-Shamanna, R., Wang, S. A., Ahmed, A., Mirza, K. M., Crane, G. M., Medeiros, L. J., & Loghavi, S. (2021). Next-generation scholarship: Rebranding hematopathology using Twitter: The MD Anderson experience. Modern Pathology, 34(5), 854–861. https://doi.org/10.1038/s41379-020-00715-4
Eloy, C., Zerbe, N., & Fraggetta, F. (2021). Europe unites for the digital transformation of pathology: The role of the new ESDIP. Journal of Pathology Informatics, 12(1), 10. https://doi.org/10.4103/jpi.jpi_80_20
Evans, A. J., Chetty, R., Clarke, B. A., Croul, S., Ghazarian, D. M., Kiehl, T.-R., Perez Ordonez, B., Ilaalagan, S., & Asa, S. L. (2009). Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The University Health Network experience. Human Pathology, 40(8), 1070–1081. https://doi.org/10.1016/j.humpath.2009.04.012
Evans, A. J., Bauer, T. W., Bui, M. M., Cornish, T. C., Duncan, H., Glassy, E. F., Hipp, J., McGee, R. S., Murphy, D., Myers, C., O’Neill, D. G., Parwani, A. V., Rampy, B. A., Salama, M. E., & Pantanowitz, L. (2018). US Food and Drug Administration approval of whole slide imaging for primary diagnosis: A key milestone is reached and new questions are raised. Archives of Pathology and Laboratory Medicine, 142(11), 1383–1387. https://doi.org/10.5858/arpa.2017-0496-CP
Evans, A. J., Depeiza, N., Allen, S.-G., Fraser, K., Shirley, S., & Chetty, R. (2021). Use of whole slide imaging (WSI) for distance teaching. Journal of Clinical Pathology, 74(7), 425–428. https://doi.org/10.1136/jclinpath-2020-206763
Farahani, N., Parwani, A. V., & Pantanowitz, L. (2015). Whole slide imaging in pathology: Advantages, limitations, and emerging perspectives. Pathology and Laboratory Medicine International, 7, 23–33. https://doi.org/10.2147/PLMI.S59826
Gatter, K. C., Alcock, C., Heryet, A., & Mason, D. Y. (1985). Clinical importance of analysing malignant tumours of uncertain origin with immunohistological techniques. Lancet, 1(8441), 1302–1305. https://doi.org/10.1016/s0140-6736(85)92794-1
Glines, K. R., Haidari, W., Ramani, L., Akkurt, Z. M., & Feldman, S. R. (2020). Digital future of dermatology. Dermatology Online Journal, 26(10), 13030/qt75p7q57j.
Halaweish, I., & Alam, H. B. (2015). Changing demographics of the American population. The Surgical Clinics of North America, 95(1), 1–10. https://doi.org/10.1016/j.suc.2014.09.002
Hamilton, P. W., Bankhead, P., Wang, Y., Hutchinson, R., Kieran, D., McArt, D. G., James, J., & Salto-Tellez, M. (2014). Digital pathology and image analysis in tissue biomarker research. Methods (San Diego, Calif.), 70(1), 59–73. https://doi.org/10.1016/j.ymeth.2014.06.015
Hanna, M. G., Reuter, V. E., Hameed, M. R., Tan, L. K., Chiang, S., Sigel, C., Hollmann, T., Giri, D., Samboy, J., Moradel, C., Rosado, A., Otilano, J. R., England, C., Corsale, L., Stamelos, E., Yagi, Y., Schüffler, P. J., Fuchs, T., Klimstra, D. S., & Sirintrapun, S. J. (2019a). Whole slide imaging equivalency and efficiency study: Experience at a large academic center. Modern Pathology, 32(7), 916–928. https://doi.org/10.1038/s41379-019-0205-0
Hanna, M. G., Reuter, V. E., Samboy, J., England, C., Corsale, L., Fine, S. W., Agaram, N. P., Stamelos, E., Yagi, Y., Hameed, M., Klimstra, D. S., & Sirintrapun, S. J. (2019b). Implementation of digital pathology offers clinical and operational increase in efficiency and cost savings. Archives of Pathology and Laboratory Medicine, 143(12), 1545–1555. https://doi.org/10.5858/arpa.2018-0514-OA
Hanna, M. G., Reuter, V. E., Ardon, O., Kim, D., Sirintrapun, S. J., Schüffler, P. J., Busam, K. J., Sauter, J. L., Brogi, E., Tan, L. K., Xu, B., Bale, T., Agaram, N. P., Tang, L. H., Ellenson, L. H., Philip, J., Corsale, L., Stamelos, E., Friedlander, M. A., et al. (2020). Validation of a digital pathology system including remote review during the COVID-19 pandemic. Modern Pathology, 33(11), 2115–2127. https://doi.org/10.1038/s41379-020-0601-5
Hassell, L. A., & Afzal, A. (2021). Flattening the world of pathology education and training and shortening the curve of pathology learning. American Journal of Clinical Pathology, 156(2), 176–184. https://doi.org/10.1093/ajcp/aqab034
Hassell, L. A., Peterson, J., & Pantanowitz, L. (2021). Pushed across the digital divide: COVID-19 accelerated pathology training onto a new digital learning curve. Academic Pathology, 8, 2374289521994240. https://doi.org/10.1177/2374289521994240
Hegde, N., Hipp, J. D., Liu, Y., Emmert-Buck, M., Reif, E., Smilkov, D., Terry, M., Cai, C. J., Amin, M. B., Mermel, C. H., Nelson, P. Q., Peng, L. H., Corrado, G. S., & Stumpe, M. C. (2019). Similar image search for histopathology: SMILY. NPJ Digital Medicine, 2, 56. https://doi.org/10.1038/s41746-019-0131-z
Herrmann, M. D., Clunie, D. A., Fedorov, A., Doyle, S. W., Pieper, S., Klepeis, V., Le, L. P., Mutter, G. L., Milstone, D. S., Schultz, T. J., Kikinis, R., Kotecha, G. K., Hwang, D. H., Andriole, K. P., Iafrate, A. J., Brink, J. A., Boland, G. W., Dreyer, K. J., Michalski, M., et al. (2018). Implementing the DICOM standard for digital pathology. Journal of Pathology Informatics, 9, 37. https://doi.org/10.4103/jpi.jpi_42_18
Hipp, J. D., Fernandez, A., Compton, C. C., & Balis, U. J. (2011). Why a pathology image should not be considered as a radiology image. Journal of Pathology Informatics, 2, 26. https://doi.org/10.4103/2153-3539.82051
Holzinger, A., Langs, G., Denk, H., Zatloukal, K., & Müller, H. (2019). Causability and explainability of artificial intelligence in medicine. Wiley Interdisciplinary Reviews. Data Mining and Knowledge Discovery, 9(4), e1312. https://doi.org/10.1002/widm.1312
Homeyer, A., Lotz, J., Schwen, L. O., Weiss, N., Romberg, D., Höfener, H., Zerbe, N., & Hufnagl, P. (2021). Artificial intelligence in pathology: From prototype to product. Journal of Pathology Informatics, 12, 13. https://doi.org/10.4103/jpi.jpi_84_20
Horbinski, C., Fine, J. L., Medina-Flores, R., Yagi, Y., & Wiley, C. A. (2007). Telepathology for intraoperative neuropathologic consultations at an academic medical center: A 5-year report. Journal of Neuropathology and Experimental Neurology, 66(8), 750–759. https://doi.org/10.1097/nen.0b013e318126c179
Jahn, S. W., Plass, M., & Moinfar, F. (2020). Digital pathology: Advantages, limitations and emerging perspectives. Journal of Clinical Medicine, 9(11), E3697. https://doi.org/10.3390/jcm9113697
Janowczyk, A., Zuo, R., Gilmore, H., Feldman, M., & Madabhushi, A. (2019). HistoQC: An open-source quality control tool for digital pathology slides. JCO Clinical Cancer Informatics, 3, 1–7. https://doi.org/10.1200/CCI.18.00157
Jones, J. L., Oien, K. A., Lee, J. L., & Salto-Tellez, M. (2017). Morphomolecular pathology: Setting the framework for a new generation of pathologists. British Journal of Cancer, 117(11), 1581–1582. https://doi.org/10.1038/bjc.2017.340
Jones-Hall, Y. L., Skelton, J. M., & Adams, L. G. (2021). Implementing digital pathology into veterinary academics and research. Journal of Veterinary Medical Education, e20210068. doi:https://doi.org/10.3138/jvme-2021-0068
Jug, R., Booth, A. L., Buckley, A. F., Newell, J., Kesterson, J., Gardner, J. M., Ozcan, L., Liu, B., Green, C. L., Joseph, L., & Cummings, T. J. (2021). Multisite quality improvement study of a patient-pathologist consultation program. American Journal of Clinical Pathology, 155(6), 887–894. https://doi.org/10.1093/ajcp/aqaa202
Kacew, A. J., Strohbehn, G. W., Saulsberry, L., Laiteerapong, N., Cipriani, N. A., Kather, J. N., & Pearson, A. T. (2021). Artificial intelligence can cut costs while maintaining accuracy in colorectal cancer genotyping. Frontiers in Oncology, 11, 630953. https://doi.org/10.3389/fonc.2021.630953
Kalra, S., Tizhoosh, H. R., Choi, C., Shah, S., Diamandis, P., Campbell, C. J. V., & Pantanowitz, L. (2020a). Yottixel—An image search engine for large archives of histopathology whole slide images. Medical Image Analysis, 65, 101757. https://doi.org/10.1016/j.media.2020.101757
Kalra, S., Tizhoosh, H. R., Shah, S., Choi, C., Damaskinos, S., Safarpoor, A., Shafiei, S., Babaie, M., Diamandis, P., Campbell, C. J. V., & Pantanowitz, L. (2020b). Pan-cancer diagnostic consensus through searching archival histopathology images using artificial intelligence. NPJ Digital Medicine, 3, 31. https://doi.org/10.1038/s41746-020-0238-2
Kather, J. N., Krisam, J., Charoentong, P., Luedde, T., Herpel, E., Weis, C.-A., Gaiser, T., Marx, A., Valous, N. A., Ferber, D., Jansen, L., Reyes-Aldasoro, C. C., Zörnig, I., Jäger, D., Brenner, H., Chang-Claude, J., Hoffmeister, M., & Halama, N. (2019). Predicting survival from colorectal cancer histology slides using deep learning: A retrospective multicenter study. PLoS Medicine, 16(1), e1002730. https://doi.org/10.1371/journal.pmed.1002730
Krasowski, M. D., Grieme, C. V., Cassady, B., Dreyer, N. R., Wanat, K. A., Hightower, M., & Nepple, K. G. (2017). Variation in results release and patient portal access to diagnostic test results at an academic medical center. Journal of Pathology Informatics, 8, 45. https://doi.org/10.4103/jpi.jpi_53_17
Krause, J., Grabsch, H. I., Kloor, M., Jendrusch, M., Echle, A., Buelow, R. D., Boor, P., Luedde, T., Brinker, T. J., Trautwein, C., Pearson, A. T., Quirke, P., Jenniskens, J., Offermans, K., van den Brandt, P. A., & Kather, J. N. (2021). Deep learning detects genetic alterations in cancer histology generated by adversarial networks. The Journal of Pathology, 254(1), 70–79. https://doi.org/10.1002/path.5638
Krupinski, E. A., Comas, M., Gallego, L. G., & GISMAR Group. (2018). A new software platform to improve multidisciplinary tumor board workflows and user satisfaction: A pilot study. Journal of Pathology Informatics, 9(1), 26. https://doi.org/10.4103/jpi.jpi_16_18
Lapedis, C. J., Horowitz, J. K., Brown, L., Tolle, B. E., Smith, L. B., & Owens, S. R. (2020). The patient-pathologist consultation program: A mixed-methods study of interest and motivations in cancer patients. Archives of Pathology and Laboratory Medicine, 144(4), 490–496. https://doi.org/10.5858/arpa.2019-0105-OA
Levy, B. P., McClintock, D. S., Lee, R. E., Lane, W. J., Klepeis, V. E., Baron, J. M., Onozato, M. L., Kim, J., Brodsky, V., Beckwith, B., Kuo, F., & Gilbertson, J. R. (2012). Different tracks for pathology informatics fellowship training: Experiences of and input from trainees in a large multisite fellowship program. Journal of Pathology Informatics, 3, 30. https://doi.org/10.4103/2153-3539.100362
Lilley, C. M., Arnold, C. A., Arnold, M., Booth, A. L., Gardner, J. M., Jiang, X. S., Loghavi, S., & Mirza, K. M. (2021). The implementation and effectiveness of PathElective.com. Academic Pathology, 8, 23742895211006828. doi:https://doi.org/10.1177/23742895211006829
Louis, D. N., Gerber, G. K., Baron, J. M., Bry, L., Dighe, A. S., Getz, G., Higgins, J. M., Kuo, F. C., Lane, W. J., Michaelson, J. S., Le, L. P., Mermel, C. H., Gilbertson, J. R., & Golden, J. A. (2014). Computational pathology: An emerging definition. Archives of Pathology and Laboratory Medicine, 138(9), 1133–1138. https://doi.org/10.5858/arpa.2014-0034-ED
Lujan, G., Quigley, J. C., Hartman, D., Parwani, A., Roehmholdt, B., Meter, B. V., Ardon, O., Hanna, M. G., Kelly, D., Sowards, C., Montalto, M., Bui, M., Zarella, M. D., LaRosa, V., Slootweg, G., Retamero, J. A., Lloyd, M. C., Madory, J., & Bowman, D. (2021). Dissecting the business case for adoption and implementation of digital pathology: A white paper from the Digital Pathology Association. Journal of Pathology Informatics, 12, 17. https://doi.org/10.4103/jpi.jpi_67_20
Lundström, C. F., Gilmore, H. L., & Ros, P. R. (2017). Integrated diagnostics: The computational revolution catalyzing cross-disciplinary practices in radiology, pathology, and genomics. Radiology, 285(1), 12–15. https://doi.org/10.1148/radiol.2017170062
Mandelker, D., Lee, R. E., Platt, M. Y., Riedlinger, G., Quinn, A., Rao, L. K. F., Klepeis, V. E., Mahowald, M., Lane, W. J., Beckwith, B. A., Baron, J. M., McClintock, D. S., Kuo, F. C., Lebo, M. S., & Gilbertson, J. R. (2014). Pathology informatics fellowship training: Focus on molecular pathology. Journal of Pathology Informatics, 5(1), 11. https://doi.org/10.4103/2153-3539.129444
Marble, H. D., Huang, R., Dudgeon, S. N., Lowe, A., Herrmann, M. D., Blakely, S., Leavitt, M. O., Isaacs, M., Hanna, M. G., Sharma, A., Veetil, J., Goldberg, P., Schmid, J. H., Lasiter, L., Gallas, B. D., Abels, E., & Lennerz, J. K. (2020). A regulatory science initiative to harmonize and standardize digital pathology and machine learning processes to speed up clinical innovation to patients. Journal of Pathology Informatics, 11, 22. https://doi.org/10.4103/jpi.jpi_27_20
Märkl, B., Füzesi, L., Huss, R., Bauer, S., & Schaller, T. (2021). Number of pathologists in Germany: Comparison with European countries, USA, and Canada. Virchows Archiv: An International Journal of Pathology, 478(2), 335–341. https://doi.org/10.1007/s00428-020-02894-6
Mayerhoefer, M. E., Materka, A., Langs, G., Häggström, I., Szczypiński, P., Gibbs, P., & Cook, G. (2020). Introduction to radiomics. Journal of Nuclear Medicine, 61(4), 488–495. https://doi.org/10.2967/jnumed.118.222893
Mea, V. D., Carbone, A., Di Loreto, C., Bueno, G., De Paoli, P., García-Rojo, M., de Mena, D., Gloghini, A., Ilyas, M., Laurinavicius, A., Rasmusson, A., Milione, M., Dolcetti, R., Pagani, M., Stoppini, A., Sulfaro, S., Bartolo, M., Mazzon, E., Soyer, H. P., & Pantanowitz, L. (2017). Teaching digital pathology: The International School of Digital Pathology and proposed syllabus. Journal of Pathology Informatics, 8, 27. https://doi.org/10.4103/jpi.jpi_17_17
Metter, D. M., Colgan, T. J., Leung, S. T., Timmons, C. F., & Park, J. Y. (2019). Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Network Open, 2(5), e194337. https://doi.org/10.1001/jamanetworkopen.2019.4337
Miao, R., Toth, R., Zhou, Y., Madabhushi, A., & Janowczyk, A. (2021). Quick Annotator: An open-source digital pathology based rapid image annotation tool. ArXiv:2101.02183. http://arxiv.org/abs/2101.02183
Montalto, M. C. (2008). Pathology RE-imagined: The history of digital radiology and the future of anatomic pathology. Archives of Pathology and Laboratory Medicine, 132(5), 764–765. https://doi.org/10.5858/2008-132-764-PRTHOD
Moulin, P., Grünberg, K., Barale-Thomas, E., der Laak, J., & van. (2021). IMI-Bigpicture: A central repository for digital pathology. Toxicologic Pathology, 49(4), 711–713. https://doi.org/10.1177/0192623321989644
Mroz, P., Parwani, A. V., & Kulesza, P. (2013). Central pathology review for phase III clinical trials: The enabling effect of virtual microscopy. Archives of Pathology and Laboratory Medicine, 137(4), 492–495. https://doi.org/10.5858/arpa.2012-0093-RA
Mukhopadhyay, S., Feldman, M. D., Abels, E., Ashfaq, R., Beltaifa, S., Cacciabeve, N. G., Cathro, H. P., Cheng, L., Cooper, K., Dickey, G. E., Gill, R. M., Heaton, R. P., Kerstens, R., Lindberg, G. M., Malhotra, R. K., Mandell, J. W., Manlucu, E. D., Mills, A. M., Mills, S. E., et al. (2018). Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: A multicenter blinded randomized noninferiority study of 1992 cases (pivotal study). The American Journal of Surgical Pathology, 42(1), 39–52. https://doi.org/10.1097/PAS.0000000000000948
Mukhopadhyay, S., Kanakis, C., Golab, K., Hermelin, D., Crane, G. M., & Mirza, K. M. (2021). The network that never sleeps. Laboratory Medicine, 52(4), e83–e103. https://doi.org/10.1093/labmed/lmaa113
Mun, S. K., Wong, K. H., Lo, S.-C. B., Li, Y., & Bayarsaikhan, S. (2020). Artificial intelligence for the future radiology diagnostic service. Frontiers in Molecular Biosciences, 7, 614258. https://doi.org/10.3389/fmolb.2020.614258
Naugler, C., & Church, D. L. (2019). Automation and artificial intelligence in the clinical laboratory. Critical Reviews in Clinical Laboratory Sciences, 56(2), 98–110. https://doi.org/10.1080/10408363.2018.1561640
Ohori, N. P., Radkay, L. A., Macpherson, T. A., Yousem, S. A., & Schoedel, K. E. (2016). Changes in resident graduate characteristics in a large pathology training program, 1994 to 2013. Academic Pathology, 3, 2374289516643543. https://doi.org/10.1177/2374289516643543
Ordi, O., Bombí, J. A., Martínez, A., Ramírez, J., Alòs, L., Saco, A., Ribalta, T., Fernández, P. L., Campo, E., & Ordi, J. (2015). Virtual microscopy in the undergraduate teaching of pathology. Journal of Pathology Informatics, 6, 1. https://doi.org/10.4103/2153-3539.150246
Pantanowitz, L., Sharma, A., Carter, A. B., Kurc, T., Sussman, A., & Saltz, J. (2018). Twenty years of digital pathology: An overview of the road travelled, what is on the horizon, and the emergence of vendor-neutral archives. Journal of Pathology Informatics, 9, 40. https://doi.org/10.4103/jpi.jpi_69_18
Parasuraman, R., & Riley, V. (1997). Humans and automation: Use, misuse, disuse, abuse. Human Factors, 39(2), 230–253. https://doi.org/10.1518/001872097778543886
Patel, R., Hoppman, N. L., Gosse, C. M., Hagen-Moe, D. J., Dunemann, S. K., Kreuter, J. D., Preuss, S. A., Winters, J. L., Sturgis, C. D., Maleszewski, J. J., Solanki, M. H., Pritt, B. S., Rivera, M., Mairose, A. M., Nelsen, M. A., Hansing, K. L., Lehman, S. M., Gruhlke, R. C., & Boland, J. M. (2021). Laboratory medicine and pathology education during the COVID-19 Pandemic—Lessons learned. Academic Pathology, 8, 23742895211020490. https://doi.org/10.1177/23742895211020487
Patterson, E. S., Rayo, M., Gill, C., & Gurcan, M. N. (2011). Barriers and facilitators to adoption of soft copy interpretation from the user perspective: Lessons learned from filmless radiology for slideless pathology. Journal of Pathology Informatics, 2, 1. https://doi.org/10.4103/2153-3539.74940
Pell, R., Oien, K., Robinson, M., Pitman, H., Rajpoot, N., Rittscher, J., Snead, D., Verrill, C., & UK National Cancer Research Institute (NCRI) Cellular-Molecular Pathology (CM-Path) Quality Assurance Working Group. (2019). The use of digital pathology and image analysis in clinical trials. The Journal of Pathology. Clinical Research, 5(2), 81–90. https://doi.org/10.1002/cjp2.127
Quinn, A. M., Klepeis, V. E., Mandelker, D. L., Platt, M. Y., Rao, L. K. F., Riedlinger, G., Baron, J. M., Brodsky, V., Kim, J. Y., Lane, W., Lee, R. E., Levy, B. P., McClintock, D. S., Beckwith, B. A., Kuo, F. C., & Gilbertson, J. R. (2014). The ongoing evolution of the core curriculum of a clinical fellowship in pathology informatics. Journal of Pathology Informatics, 5(1), 22. https://doi.org/10.4103/2153-3539.137717
Retamero, J. A., Aneiros-Fernandez, J., & Del Moral, R. G. (2020). Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Archives of Pathology and Laboratory Medicine, 144(2), 221–228. https://doi.org/10.5858/arpa.2018-0541-OA
Ribback, S., Flessa, S., Gromoll-Bergmann, K., Evert, M., & Dombrowski, F. (2014). Virtual slide telepathology with scanner systems for intraoperative frozen-section consultation. Pathology, Research and Practice, 210(6), 377–382. https://doi.org/10.1016/j.prp.2014.02.007
Robboy, S. J., Weintraub, S., Horvath, A. E., Jensen, B. W., Alexander, C. B., Fody, E. P., Crawford, J. M., Clark, J. R., Cantor-Weinberg, J., Joshi, M. G., Cohen, M. B., Prystowsky, M. B., Bean, S. M., Gupta, S., Powell, S. Z., Speights, V. O., Gross, D. J., & Black-Schaffer, W. S. (2013). Pathologist workforce in the United States: I. Development of a predictive model to examine factors influencing supply. Archives of Pathology and Laboratory Medicine, 137(12), 1723–1732. https://doi.org/10.5858/arpa.2013-0200-OA
Rodrigues-Fernandes, C. I., Speight, P. M., Khurram, S. A., Araújo, A. L. D., da Perez, D. E. C., Fonseca, F. P., Lopes, M. A., de Almeida, O. P., Vargas, P. A., & Santos-Silva, A. R. (2020). The use of digital microscopy as a teaching method for human pathology: A systematic review. Virchows Archiv, 477(4), 475–486. https://doi.org/10.1007/s00428-020-02908-3
Rosenbaum, J. N., Berry, A. B., Church, A. J., Crooks, K., Gagan, J. R., López-Terrada, D., Pfeifer, J. D., Rennert, H., Schrijver, I., Snow, A. N., Wu, D., & Ewalt, M. D. (2021). A curriculum for genomic education of molecular genetic pathology fellows: A report of the Association for Molecular Pathology Training and Education Committee. The Journal of Molecular Diagnostics, 23(10), 1218–1240. https://doi.org/10.1016/j.jmoldx.2021.07.001
Royal College of Pathologists. (2021). Digital pathology. https://www.rcpath.org/profession/digital-pathology.html
Saco, A., Bombi, J. A., Garcia, A., Ramírez, J., & Ordi, J. (2016). Current status of whole-slide imaging in education. Pathobiology: Journal of Immunopathology, Molecular and Cellular Biology, 83(2–3), 79–88. https://doi.org/10.1159/000442391
Saltz, J., Almeida, J., Gao, Y., Sharma, A., Bremer, E., DiPrima, T., Saltz, M., Kalpathy-Cramer, J., & Kurc, T. (2017). Towards generation, management, and exploration of combined radiomics and pathomics datasets for cancer research. AMIA Joint Summits on Translational Science Proceedings. AMIA Joint Summits on Translational Science Proceedings, 2017, 85–94.
Sarewitz, S. J. (2014). Subspecialization in community pathology practice. Archives of Pathology and Laboratory Medicine, 138(7), 871–872. https://doi.org/10.5858/arpa.2014-0084-ED
Sarwar, S., Dent, A., Faust, K., Richer, M., Djuric, U., Van Ommeren, R., & Diamandis, P. (2019). Physician perspectives on integration of artificial intelligence into diagnostic pathology. NPJ Digital Medicine, 2, 28. https://doi.org/10.1038/s41746-019-0106-0
Schaumberg, A. J., Juarez-Nicanor, W. C., Choudhury, S. J., Pastrián, L. G., Pritt, B. S., Prieto Pozuelo, M., Sotillo Sánchez, R., Ho, K., Zahra, N., Sener, B. D., Yip, S., Xu, B., Annavarapu, S. R., Morini, A., Jones, K. A., Rosado-Orozco, K., Mukhopadhyay, S., Miguel, C., Yang, H., et al. (2020). Interpretable multimodal deep learning for real-time pan-tissue pan-disease pathology search on social media. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc, 33(11), 2169–2185. https://doi.org/10.1038/s41379-020-0540-1
Schüffler, P. J., Geneslaw, L., Yarlagadda, D. V. K., Hanna, M. G., Samboy, J., Stamelos, E., Vanderbilt, C., Philip, J., Jean, M.-H., Corsale, L., Manzo, A., Paramasivam, N. H. G., Ziegler, J. S., Gao, J., Perin, J. C., Kim, Y. S., Bhanot, U. K., Roehrl, M. H. A., Ardon, O., et al. (2021). Integrated digital pathology at scale: A solution for clinical diagnostics and cancer research at a large academic medical center. Journal of the American Medical Informatics Association, ocab085. doi:https://doi.org/10.1093/jamia/ocab085
Shachar, E., Hasson, S. P., Fayngor, R., Wolf, I., & Hershkovitz, D. (2021). Pathology consultation clinic for patients with cancer: Meeting the clinician behind the microscope. JCO Oncology Practice, 17(10), e1559–e1566. https://doi.org/10.1200/OP.20.00948
Snead, D. R. J., Tsang, Y.-W., Meskiri, A., Kimani, P. K., Crossman, R., Rajpoot, N. M., Blessing, E., Chen, K., Gopalakrishnan, K., Matthews, P., Momtahan, N., Read-Jones, S., Sah, S., Simmons, E., Sinha, B., Suortamo, S., Yeo, Y., El Daly, H., & Cree, I. A. (2016). Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology, 68(7), 1063–1072. https://doi.org/10.1111/his.12879
Stathonikos, N., van Varsseveld, N. C., Vink, A., van Dijk, M. R., Nguyen, T. Q., de Leng, W. W. J., Lacle, M. M., Goldschmeding, R., Vreuls, C. P. H., & van Diest, P. J. (2020). Digital pathology in the time of Corona. Journal of Clinical Pathology, 73(11), 706–712. https://doi.org/10.1136/jclinpath-2020-206845
Taylor, C. R. (1986). Immunomicroscopy: A diagnostic tool for the surgical pathologist. W. B. Saunders.
Tosun, A. B., Pullara, F., Becich, M. J., Taylor, D. L., Fine, J. L., & Chennubhotla, S. C. (2020). Explainable AI (xAI) for Anatomic Pathology. Advances in Anatomic Pathology, 27(4), 241–250. https://doi.org/10.1097/PAP.0000000000000264
Turner, O. C., Aeffner, F., Bangari, D. S., High, W., Knight, B., Forest, T., Cossic, B., Himmel, L. E., Rudmann, D. G., Bawa, B., Muthuswamy, A., Aina, O. H., Edmondson, E. F., Saravanan, C., Brown, D. L., Sing, T., & Sebastian, M. M. (2020). Society of Toxicologic Pathology Digital Pathology and Image Analysis Special Interest Group article: Opinion on the application of artificial intelligence and machine learning to digital toxicologic pathology. Toxicologic Pathology, 48(2), 277–294. https://doi.org/10.1177/0192623319881401
van den Tweel, J. G., & Taylor, C. R. (2010). A brief history of pathology: Preface to a forthcoming series that highlights milestones in the evolution of pathology as a discipline. Virchows Archiv: An International Journal of Pathology, 457(1), 3–10. https://doi.org/10.1007/s00428-010-0934-4
Volynskaya, Z., Chow, H., Evans, A., Wolff, A., Lagmay-Traya, C., & Asa, S. L. (2018). Integrated pathology informatics enables high-quality personalized and precision medicine: Digital pathology and beyond. Archives of Pathology and Laboratory Medicine, 142(3), 369–382. https://doi.org/10.5858/arpa.2017-0139-OA
Warth, A., Stenzinger, A., Andrulis, M., Schlake, W., Kempny, G., Schirmacher, P., & Weichert, W. (2016). Individualized medicine and demographic change as determining workload factors in pathology: Quo vadis? Virchows Archiv: An International Journal of Pathology, 468(1), 101–108. https://doi.org/10.1007/s00428-015-1869-6
Wei, B.-R., & Simpson, R. M. (2014). Digital pathology and image analysis augment biospecimen annotation and biobank quality assurance harmonization. Clinical Biochemistry, 47(4–5), 274–279. https://doi.org/10.1016/j.clinbiochem.2013.12.008
Weinstein, R. S. (1986). Prospects for telepathology. Human Pathology, 17(5), 433–434. https://doi.org/10.1016/s0046-8177(86)80028-4
Williams, B. J., Bottoms, D., Clark, D., & Treanor, D. (2019). Future-proofing pathology part 2: Building a business case for digital pathology. Journal of Clinical Pathology, 72(3), 198–205. https://doi.org/10.1136/jclinpath-2017-204926
Williams, B. J., Fraggetta, F., Hanna, M. G., Huang, R., Lennerz, J., Salgado, R., Sirintrapun, S. J., Pantanowitz, L., Parwani, A., Zarella, M., & Treanor, D. E. (2020). The future of pathology: What can we learn from the COVID-19 pandemic? Journal of Pathology Informatics, 11, 15. https://doi.org/10.4103/jpi.jpi_29_20
Wright, A. I., Clarke, E. L., Dunn, C. M., Williams, B. J., Treanor, D. E., & Brettle, D. S. (2020). A point-of-use quality assurance tool for digital pathology remote working. Journal of Pathology Informatics, 11, 17. https://doi.org/10.4103/jpi.jpi_25_20
Yamashita, R., Long, J., Longacre, T., Peng, L., Berry, G., Martin, B., Higgins, J., Rubin, D. L., & Shen, J. (2021). Deep learning model for the prediction of microsatellite instability in colorectal cancer: A diagnostic study. The Lancet. Oncology, 22(1), 132–141. https://doi.org/10.1016/S1470-2045(20)30535-0
Yip, S. S. F., & Aerts, H. J. W. L. (2016). Applications and limitations of radiomics. Physics in Medicine and Biology, 61(13), R150–R166. https://doi.org/10.1088/0031-9155/61/13/R150
Zhao, C., Wu, T., Ding, X., Parwani, A. V., Chen, H., McHugh, J., Piccoli, A., Xie, Q., Lauro, G. R., Feng, X., Hartman, D. J., Seethala, R. R., Wu, S., Yousem, S., Liang, Y., & Pantanowitz, L. (2015). International telepathology consultation: Three years of experience between the University of Pittsburgh Medical Center and KingMed Diagnostics in China. Journal of Pathology Informatics, 6, 63. https://doi.org/10.4103/2153-3539.170650
Acknowledgments
Support for the writing of this chapter was provided by the EMPAIA project, which is funded by the German Federal Ministry for Economic Affairs and Climate Action (BMWK) under FKZ 01MK20002A. The author declares no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Authors
About this chapter
Cite this chapter
Kiehl, TR. (2022). Digital and Computational Pathology: A Specialty Reimagined. In: Ehsani, S., Glauner, P., Plugmann, P., Thieringer, F.M. (eds) The Future Circle of Healthcare. Future of Business and Finance. Springer, Cham. https://doi.org/10.1007/978-3-030-99838-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-99838-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99837-0
Online ISBN: 978-3-030-99838-7
eBook Packages: Business and ManagementBusiness and Management (R0)