Abstract
This chapter very briefly outlines site and constellation specific direct and indirect impacts of a hydropower scheme primarily on fishes. It describes potential effects of single elements of a hydropower scheme, such as available migration routes up- and downstream, impoundment, hydraulic head, turbine type and mode of operation. It summarises the state of knowledge, points out knowledge gaps and indicates potential mitigation options.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
4.1 Introduction
The detrimental effects hydropower plants have on aquatic ecosystems and biodiversity are manifold and comprehensively reviewed (e.g., Gasparatos et al. 2017, Hecht et al. 2019, Jungwirth et al. 2003. Lees et al. 2016, Reid et al. 2019, Schmutz and Sendzimir 2018, Stendera et al. 2012, Ziv et al. 2012). In the following section, however, we review, categorize and outline hydropower-related impacts on freshwater fishes only. This is due to various reasons: For one, fishes are of great socio-economic interest. Their unquestionable cultural and societal value has caused managing efforts to support self-sustained, exploitable fish stocks for several thousand years, and today they are a priority target for many restoration and conservation programs. Furthermore, fish are most affected by the operation of hydropower (Larinier 2001) and the high level of hydromorphological degradation and resulting habitat loss associated with hydropower has been identified as one of the bottlenecks in reaching the Water Framework Directive targets (Freyhof et al. 2019).
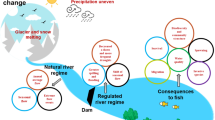
Therefore, this chapter draws a comprehensive conceptual model depicting what kinds of impacts on fish potentially happen beginning from habitat loss/modification upstream due to the impoundment, migration delays, indirect mortality due to increased predation, the hydropower plant (HPP) itself, with potential spillway, bypass, trash racks and also turbine effects (blade strike, shear forces, barotrauma) and down to tailwater effects, such as increased predation, residual flows, habitat and flow modifications (Fig. 4.1).
The resilience of fish species and populations as well as species most at risks will be addressed based on narratives derived as risk factors and the empirical evidence provided by the literature review.
4.2 Barrier Effects
The central, most prominent element of every hydropower scheme is undoubtedly a dam or a weir. Although these types of barriers are not exclusive to hydropower plants, they always have the same principal effects on fishes. Because barriers become impassable obstacles for fishes once they exceed certain dimensions, they segregate resident populations into isolated upstream and downstream components. Barriers disrupt the original river continuum (Allan and Castillo 2007; Mueller et al. 2011; Vannote et al. 1980) and the natural migration corridors for fishes (Jonsson et al. 1999). Dams and weirs act as migration barriers for migratory species that then face substantial migration delay (Buysse et al. 2015; Ovidio et al. 2017; Stich et al. 2015, Winter et al. 2006), and they render critical habitats inaccessible to fishes (Larinier 2001; Pelicice et al. 2015). However, unhindered upstream migration is particularly critical for diadromous migratory species like salmonids, lampreys, some clupeids or sturgeons that only spawn in the upper regions of rivers where hydraulic and geomorphic conditions support egg development and provide larval habitats (Katano et al. 2006; Lucas et al. 2009; Penczak et al. 1998). But also, migrations of potamodromous species are impaired by barriers (Britton and Pegg 2011; Lucas and Frear 1997). This can result in reduced natural recruitment (McCarthy et al. 2008), differences in population structure and species assemblages up- and downstream of the dam (Franssen and Tobler 2013; Morita and Yamamoto 2002; Mueller et al. 2011) and even result in the extinction of entire fish stocks (Larinier and Travade 1992), unless habitat heterogeneity and availability in the system remains high enough to support the native assemblage (Santos et al. 2006). Furthermore, because dams act as bi-directional nutrient traps that can cause a reduction of far-downstream fish biomass (Jackson and Marmulla 2001) and a lack of nutrients (i.e., due to a lower number of spawners remaining in the headwaters of streams), which directly affects the dietary composition of a range of different fish species (Piorkowski 1995). The mechanisms described in this paragraph primarily impact population endpoints that ultimately, cause a decline in recruitment, whereas individual mortality of affected fishes is only of secondary concern.
The negative ecological impacts of barriers can be partly mitigated by maintaining certain flow velocity through the impounded area that resembles the ecological functioning of the former stream. These flow patterns are cues for up- and downstream migrating species and ensure sediment transport and aeration.
4.3 Upstream Flow Alterations
Dams cause substantial alterations of the stream’s original discharge regime (Egré and Milewski 2002; Schiemer et al. 2001). Reservoirs and impoundments considerably slow down the stream’s flow velocity causing higher sedimentation rates of finer particles, stratification, increased temperature, and potential oxygen depletion in the hypolimnion due to an imbalance in aerobic production and consumption (Thornton et al. 1990). In principle, impoundments transform lotic habitats into ones with more lentic characteristics (Sá-Oliveira et al. 2015) that are unsuited for most riverine, lithophilic species that require well aerated, fast flowing coarse gravel beds as spawning habitats (Wood and Armitage 1997). These conditions result in habitat loss for a range of rheophilic species (Agostinho et al. 2002; Birnie-Gauvin et al. 2017; Larinier 2001; Tiffan et al. 2016), changes in water quality (Fantin-Cruz et al. 2016), shifts in biomass and ultimately, changes of species abundance and diversity relative to non-impounded reaches downstream (Sá-Oliveira et al. 2015). These conditions also affect species-specific length-frequency distributions, species richness (Gehrke et al. 2002) and species composition (Tundisi and Straškraba 1999). Manipulated abiotic conditions in impoundments were further associated with temperature-related changes of growth patterns (i.e., younger age of maturity and smaller individual sizes) (Reed et al. 1992). For example, another study by Yang et al. (2020) showed reduced energy transfer efficiency in impoundments, suggesting potential energetic bottlenecks of fish at higher trophic levels. In impoundments altered hydromorphological conditions have caused increased predation, most likely because of the novel environment, lack of navigation cues for diadromous species (Agostinho et al. 2002; Jepsen et al. 2000; Tiffan et al. 2016) and the resulting migration delay (Larinier 2001; Larinier and Travade 2002) and reinforce negative impacts of introduced predators (Pelicice and Agostinho 2009). This can lead to local extinction of native and proliferation of non-native species (Martinez et al. 1994).
4.4 Downstream Flow Alterations
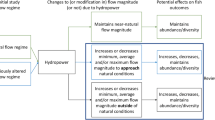
Different types of HPP have to be distinguished. There are run-of-river HPP of both instream or diversion-type schemes and storage HPP as well as pump-storage plants (Egré and Milewski 2002; Matt et al. 2019). Particularly storage, but to some extent also run-of-river hydropower plants dampen high natural discharge amplitudes by cutting flow peaks and increasing very low discharges. As such, they completely alienate the natural discharge regime of a stream, with flow fluctuations downstream being most problematic at all plants that do not release approximately as much water through the dam (i.e., through the turbines, spill gates or sluices) as would normally be discharged in the stream.
In diversion plants, the main purpose of the dam is to divert water away from the main stream towards the (potentially very remote) powerhouse where the water is turbinated and returned to the original river bed further downstream (Egré and Milewski 2002). The residual old river bed usually suffers from water scarcity, and methodological frameworks for defining sufficient environmental flow in the affected stretch are summarized by the CIS Guidance 31 “Ecological Flows in the Implementation of the Water Framework Directive” that can be consulted to mitigate the negative effects. At HPPs in which only a fraction of the original discharge remains in the residual river stretch severe consequences regarding water depths, flow velocities, and temperature extremes were observed. These do not support some fish populations anymore, cause species shifts and population declines (Anderson et al. 2006; Benejam et al. 2016; Habit et al. 2007) and sometimes even render whole river stretches uninhabitable. At some HPPs with state-of-the-art environmental flows of at least 10% mean annual stream flow (Huckstorf et al. 2008) these impacts are less pronounced. However, maintaining the comparably high environmental flow usually comes at the expense of hydroelectricity generation and loss of revenues.
Hydropeaking plants typically store larger amounts of water and release it for electricity generation in times of peak demand, mostly in the morning and evening (Moreira et al. 2019; Schmutz et al. 2015; Schmutz and Sendzimir 2018). Many species cannot cope with manipulated flow alterations induced by turbine operation which can lead to reduced food availability (De Jalon et al. 1994; Gandini et al. 2014; Young et al. 2011), erosion and habitat loss due to periodical dewatering (Almodóvar and Nicola 1999; Boavida et al. 2015, 2013; Choi et al. 2017; Person 2013; Shen and Diplas 2010; Young et al. 2011) and impaired egg development (Casas-Mulet et al. 2015a, b; Person 2013; Young et al. 2011), all of which commonly resulting either in reduced recruitment or increased direct mortality e.g., by stranding (Hedger et al. 2018, Schmutz et al. 2015, Tuthan et al. 2012, Young et al. 2011) in particular of smaller species or younger specimen with weaker swimming performance (Hayes et al. 2019; Person 2013).
If water shortage or pulse flows are not evident, manipulated flows can still exert major pressures on fishes e.g., because new habitat types immediately emerge beneath the dam that support accumulation of fishes (Jackson 1985) that attract unnaturally high abundances of predators able to deplete already impaired stocks (Larinier 2001; Stansell et al. 2010). In addition, hydropeaking can lead to altered sediment dynamics in rivers with severe consequences for lithophilic fish species (Casas-Mulet et al. 2015a, b).
4.5 Upstream Passage
Upstream migration needs of fishes have received much more attention relative to downstream migration needs, and respective efforts to increase passage rates date back longer, too (Katopodis and Williams 2012). The decline of the highly valued anadromous salmonids and the respective fisheries in response to damming became obvious very early on and had resulted in first legal acts that obliged e.g., mill owners to care about fish migration. In this context, attempts to facilitate upstream movement of fish that actively search for passage corridors have been more successful compared to attempts to guide fish following the main current in downstream direction (Geist 2021). Correspondingly, comprehensive guidelines exist to facilitate operational upstream migration facilities under varying environmental, technical and biotic conditions e.g., the DWA guidance M 509 (DWA 2014). However, upstream migration facilities show highly varying passage rates between 0 and 100% (Bunt et al. 2012; Gowans et al. 2003; Hershey 2021; Kemp et al. 2011), mostly due to the unique and highly complex interaction between the species’ internal state and motivation to migrate, their anatomy and swimming ability, ambient hydraulic conditions and type and design of the passage facility (Banks 1969, Castro-Santos et al., 2009, Crisp 2000, USFWS (U.S. Fish and Wildlife Service) 2019). In Europe the implementation of the WFD stipulated the re-establishment of the longitudinal connectivity (Schletterer et al. 2016) and various technical as well as natural fishways were developed or species-specifically improved (Clay 2017; Hershey 2021; Jungwirth et al. 1998; Katopodis 1992; Santos et al. 2014).
Factors determining passage success of an upstream fishpass include attraction efficiency mediated by position of entrance and attraction flow and passability mediated by slope, flow velocity in the migration corridor, height differences and physical dimensions (Banks 1969; Bunt et al. 2012; DWA 2014; Hershey 2021, USFWS 2019). Failing upstream passage success of fish result in excessive energy expenditure and migration delays (Noonan et al. 2012; Silva et al. 2019; Thorstad et al. 2008) and thus, delayed arrival at spawning events (Silva et al. 2019), and increased predation (Agostinho et al. 2012). When HPPs are aligned in cascades their cumulative barrier effects must be considered (Geist 2021) as it aggravates already significant delays, migration failures and mortalities threatening the persistence of fish populations (Caudill et al. 2007; Gowans et al. 2003; Muir et al. 2001; Roscoe et al. 2011; Williams et al. 2001).
4.6 Downstream Passage
Downstream passage attained attention only much more recently, but is of similar relevance especially for iteroparous species spawning more than once in a lifetime. Beside the target species (diadromous or potamodromous) and the biocoenotic region (upper vs. lower course and associated species guilds) also HPP constellation (size, turbine type, etc.) and operational mode need to be considered (Schmidt et al. 2018; Travade and Larinier 2002). Particularly, juveniles of anadromous and adults of catadromous guilds but also potamodromous species require unobstructed downstream migration corridors. Therefore, HPPs must be equipped with fish guiding structures that facilitate downstream fish migration. Generally, all routes downstream over barriers and through HPPs are inherently dangerous for fishes and may result in migration delay or elevated mortality.
Spillways, mostly used to release excess water in times of higher discharge, can serve as effective and comparably fish-friendly downstream paths through a hydropower plant with bypass efficiencies of >90% (Muir et al. 2001). However, water released through spillways, particularly from bigger heights, tends to supersaturate with nitrogen and oxygen and, together with shear forces, pressure changes and blunt trauma or abrasions, can cause substantial damages and high mortality rates: up to 2% at a height of <3 m, up to 40% at 10 m and up to 100% at 50 m (Algera et al. 2020; Heisey et al. 1996; Schilt 2007; Wolter et al. 2020), with larger fish being significantly more susceptible to drop-induced injuries than smaller ones (Ruggles and Murray 1983).
Sluice gates installed at hydropower plants are mostly opened to spill debris or discharge excess inflow and may constitute temporarily available pathways for downstream migrating fish, too. Because the hydraulic conditions around an open (esp. undershot) gate act as a strong cue for migrating species sluices have proven efficient in conveying e.g., European eels downstream (Egg et al. 2017). However, undershot pathways may expose passing fish to rapid pressure changes that by far exceed those at overshot routes (Pflugrath et al. 2019), causing up to 95% mortality rates, especially for juveniles, small species and those with pressure-sensitive swim bladders (Algera et al. 2020; Baumgartner et al. 2006; Marttin and De Graaf 2002), while passage efficiency varies between <20% (Kemp et al. 2011) and >90% (Gardner et al. 2016).
Bypasses are dedicated downstream migration routes for fishes and most often used in combination with deflection screens or behavioural guidance facilities (Ebel et al. 2015). Their set-up is usually relatively simple, comprising concrete or metal chutes, slides or pipes that flush entering fishes downstream. Operational and efficient bypasses must be easily accessible, sufficiently dimensioned and supplied with enough water (commonly measured as a proportion of the turbine flow rate), and the entering water should have a slightly higher flow velocity than the recommended approaching flow of deflection screens (Ebel et al. 2015; Larinier and Travade 2002). Studies quantifying bypass mortalities are comparably scarce (Algera et al. 2020), but documented bypass-related damages and mortalities are mainly caused by sheer forces, rapid pressure changes, collisions, disorientation and subsequent predation in the tailrace (Williams et al. 2001); however, mortalities remained generally lower compared to other downstream routes (Algera et al. 2020). Bypass passage rates of fish showed significant variation between 0 and 95% (Gosset et al. 2005; Nyqvist et al. 2018; Ovidio et al. 2017).
Trash racks are installed in front of turbine intakes to protect them from large debris like wood. Normally, they feature vertical bars that—depending on design requirements—may be slightly inclined. The bar spacing is usually very wide to minimize head loss and constitute a substantial risk for larger fish that may get impinged and damaged when the approaching flow velocity is too high, during trash cleaner operations or when debris accumulates in the forebay (Weibel 1991). Studies investigating mortality rates of fishes due to trash racks are methodologically very challenging and thus, scarce.
Deflection screens with much smaller bar spacing installed at HPP behind or instead of trash racks are mechanical and behavioural barriers that prevent fishes from entering the turbines. Fish deflection screens come in a wide variety of designs e.g., vertically inclined with vertical bars and horizontally angled screens with horizontal bars that mostly deflect fishes mechanically, or horizontally angled screens with vertical bars inducing an additional behavioural change that increases the deflection performance up to 95% (Albayrak et al. 2020; Amaral 2003; Beck 2019; Calles et al. 2013; Ebel 2013a; Ebel et al. 2015; Nyqvist et al. 2018). The purely mechanical deflection rate can be approximated using empirical length-width-regressions by (Ebel 2013b): for example, 18 mm bar spacing would deflect fusiform fish of approximately ≥16 cm and eel of approximately ≥55 cm length; 15 mm bar spacing would lower these values to 13.6 and 48 cm. In contrast, a common trash rack with 80–100 mm bar spacing is consequently passable for almost all native species. When the approaching flow exceeds the recommended value of approximately 0.5 m/s (Calles et al. 2013; DWA 2014; Ebel et al. 2015; Larinier and Travade 2002), fish may be impinged in the screen and get damaged (Calles et al. 2013; Larinier 2001). Typically, physical/behavioural deflection screens and downstream bypasses form a functional unit (Ebel et al. 2015; Gosset et al. 2005; Larinier and Travade 2002; Nyqvist et al. 2018; Økland et al. 2019) and are not considered operational in absence of each other.
Turbine passage is probably the best-studied, most dangerous downstream route for fishes (Algera et al. 2020, Eicher et al. 1987). Depending on type and size of the turbine, fishes can get damaged or killed usually by either one or a combination of i) abrupt pressure changes (barotrauma), ii) turbulent flow, iii) shear forces, and iv) turbine blade strikes (USFWS 2019). Generally, the consequences of direct and delayed mortality as well as external (Mueller et al. 2017) and internal (Mueller et al. 2020a, b, c, d, e, f, g, h, i) injuries following turbine passage must be distinguished. Reported mortalities were highly variable across and within turbine types e.g., 1–7.7% in “Very Low Head” (VLH) turbines (Hogan et al. 2014; Reuter and Kohout 2014), 2% in Alden turbines (Hogan et al., 2014), 2–2.4% for the “Minimum Gap Runner” (MGR) (Čada et al. 1997; Hogan et al. 2014), 0.1–2.5% in water wheels (Pulg and Schnell 2008; Quaranta and Wolter 2021; Reuter and Kohout 2014), 0–32.7% in Archimedes screws (Buysse et al. 2015; Hogan et al. 2014; Piper et al. 2018; Pulg and Schnell 2008; Reuter and Kohout 2014), 0.3–100% in Kaplan turbines (Anon et al. 1987, Čada et al. 1997, 2006; Čada 2001; Cramer and Oligher 1964; Reuter and Kohout 2014; Richmond et al. 2014), although the risk of lethal blade strike in large Kaplan turbines can be substantially reduced compared to that of smaller ones (Bell and Kynard 1985), 15 to >70% in Ossberger turbines (Gloss and Wahl 1983), 4–100% in Francis turbines (Anon et al. 1987, Cramer and Oligher 1964; Pulg and Schnell 2008; Reuter and Kohout 2014) and 100% in Pelton wheels (Reuter and Kohout 2014). Fish mortality increases with increasing rotational speed (Anon et al. 1987, Buysse et al. 2015; Cramer and Oligher 1964; Odeh 1999; Turnpenny et al. 2000) usually inversely correlates with turbine size and positively correlates with fish size (Čada 1990; Colotelo et al. 2012; Pracheil et al. 2016) and hydraulic head (Anon et al. 1987, Larinier 2001) i.e., with rapid decompression and lack of acclimation time (Brown et al. 2009, 2012; Colotelo et al. 2012; Cramer and Oligher 1964; Odeh 1999; Pracheil et al. 2016; Richmond et al. 2014; Stephenson et al. 2010; Turnpenny et al. 2000). Further, mortality decreases with increasing turbine load (Čada et al. 1997; Cramer and Oligher 1964) and depends on fish behaviour and species (Amaral et al. 2015; Calles et al. 2010; Coutant and Whitney 2000; Ebel 2013a; Havn et al. 2017). Even if direct mortality rates are not evident, fishes may die from their injuries later (Ferguson et al. 2006; Mueller et al. 2020c, 2020f, 2020a, 2020e, 2020d, 2020b, 2020g; Muir et al. 2006; Taylor and Kynard 1985). This delayed mortality can be substantial and not accounting for it might severely underestimate damage rates during field studies and therefore, must be considered in the experimental design.
Turbine entrainment can cause damages and mortalities, and thus, be a significant population impact factor not only for juveniles with weaker swimming abilities or migratory species (i.e., salmonid smolts) (Mathur et al. 2000; Thorne and Johnson 1993) but also for potamodromous (Harrison et al. 2019) and even resident adult fishes, mainly in fall and winter (Martins et al. 2013). However, survival for smaller (i.e., juvenile) fishes at turbine passage is often higher than for adults, and turbine entrainment may therefore contribute to the persistence of downstream populations, albeit at the expense of populations upstream (Amaral et al. 2018; Harrison et al. 2019). Entrainment and mortality of drifting fish larvae are severely understudied and have not been quantified so far.
4.7 Risk and Impact Assessment
Measuring, describing, and predicting the actual impact of a HPP or specific, hydropower-related stressors on fish populations is challenging and almost impossible, regardless of the knowledge about single, site- or constellation-specific factors. This is due to several reasons.
First, the lack of information on the reference state, that is the undisturbed condition of the system (Nijboer et al. 2004). The fundamental elements of many HPP (i.e., dams or weirs) are fairly old, and (at least in Europe) new, and particularly small hydropower plants are commonly built on top of existing infrastructure. This imposes serious constraints on typical means of impact investigations like BA (before-after) or BACI (before-after-control-impact) designs (Conner et al. 2015b; Eberhardt 1976; Green 1979; Smith 2014), unless the scientific objective is to assess the additional impact or mortality factor of the hydropower plant compared to that of the already existing dam. If construction work on the HPP or dam has not yet started studies applying BACI designs could be used to investigate hydropower-related impacts before and after completion (e.g., Almodóvar and Nicola 1999), but if a particular stressor is already in place meaningful conclusions about its impact are more difficult to obtain. Pressure-release studies, for example in the context of dam removals or restoration (Catalano et al. 2007; Conner et al. 2015a), could identify improvements from the prevalent condition without knowledge about the reference condition. However, such studies merely describe the “opportunistic” response of the ecosystem and not its resilience i.e., its proximity to the pre-disturbance state. Further, most river systems are facing multiple stressors (Mueller et al. 2020a, b, c, d, e, f, g, h, i) and the single impacts of HPPs are hard to disentangle.
Second, investigations of impacts from hydropower on fish populations are biased towards migratory (i.e., diadromous) species that express clearly distinguished, life stage-critical habitat shifts (Geist 2021). Species with a pronounced migration tendency like anadromous salmonids and lampreys will by default always attempt to pass the hydropower plant if their spawning or rearing grounds are located upstream of the plant. In contrast, it becomes much more difficult to detect impacts at the population level of resident, non-migratory or potamodromous species that do not express long-distance migratory behaviour, migrate within the river system or even stay in the impoundment.
Furthermore, the complexity of different hydropower-related stressors, their interactions, cumulative effects on river system scale (Geist 2021) and summed impact on resident or migratory fishes raise difficulties in predicting their impact in isolation, especially in relation to varying susceptibility of fish assemblages across sites. Conclusions drawn from observations at one site are not necessarily valid at another. While the constellation of a few hydropower components (e.g., turbine type and hydraulic head or turbine size, rotational speed and flow rate) will remain relatively constant across sites and applications, others are much more subject to either the operator’s intentions (e.g., operation modes), geo- and hydro-morphologically imposed structural design decisions (e.g., plant type, stream and discharge, mode of operation), spatial limitations (e.g., upstream migration facilities), composition and diversity of the ambient fish community, and fish protection facilities installed (e.g., dimensions of fish deflection screens and design or location of bypass systems). These elements can not only be combined in many different ways, they also interact uniquely with fish species and their life stages. Last but not least, site-specific environmental and conservation concerns do not only constrain the implementation details of a HPP, they also frame the environmental impact assessment. In conservation priority areas, even low impacts from hydropower might not be tolerable, while in heavily modified rivers HPPs of moderate impact might be acceptable.
References
Agostinho AA, Agostinho CS, Pelicice FM, Marques EE (2012) Fish ladders: safe fish passage or hotspot for predation? Neotrop Ichthyol 10(4):687–696. https://doi.org/10.1590/S1679-62252012000400001
Agostinho AA, Gomes LC, Fernandez R, Suzuki HI (2002) Efficiency of fish ladders for neotropical ichthyofauna. 306:299–306. https://doi.org/10.1002/rra.674
Albayrak I, Boes RM, Kriewitz-Byun CR, Peter A, Tullis BP (2020) Fish guidance structures: hydraulic performance and fish guidance efficiencies. J Ecohydraul 1–19. https://doi.org/10.1080/24705357.2019.1677181
Algera DA, Rytwinski T, Taylor JJ, Bennett JR, Smokorowski KE, Harrison PM, Clarke KD, Enders EC, Power M, Bevelhimer MS (2020) What are the relative risks of mortality and injury for fish during downstream passage at hydroelectric dams in temperate regions? Systemat Rev Environ Evid 9(1):3
Allan JD, Castillo MM (2007) Stream ecology: structure and function of running waters. Springer Science & Business Media
Almodóvar A, Nicola GG (1999) Effects of a small hydropower station upon brown trout Salmo trutta L. in the River Hoz Seca (Tagus basin, Spain) one year after regulation. Regulat Rivers Res Manage 15(5):477–484. https://doi.org/10.1002/(SICI)1099-1646(199909/10)15:5<477::AID-RRR560>3.0.CO;2-B
Amaral SV (2003) The use of angled bar racks and louvers for guiding fish at FERC-Licensed projects. In: FERC Fish Passage Workshop, November 13, 2003, 37
Amaral SV, Bevelhimer MS, Čada GF, Giza DJ, Jacobson PT, McMahon BJ, Pracheil BM (2015) Evaluation of behavior and survival of fish exposed to an axial-flow hydrokinetic turbine. North Am J Fish Manage 35(1):97–113. https://doi.org/10.1080/02755947.2014.982333
Amaral SV, Coleman BS, Rackovan JL, Withers K, Mater B (2018) Survival of fish passing downstream at a small hydropower facility. Mar Freshw Res 69(12):1870–1881
Amaral SV, Watson SM, Schneider AD, Rackovan J, Baumgartner A (2020) Improving survival: injury and mortality of fish struck by blades with slanted, blunt leading edges. J Ecohydraul 5(2):175–183. https://doi.org/10.1080/24705357.2020.1768166
Anderson EP, Freeman MC, Pringle CM (2006) Ecological consequences of hydropower development in Central America: impacts of small dams and water diversion on neotropical stream fish assemblages. River Res Appl 22(4):397–411. https://doi.org/10.1002/rra.899
Banks JW (1969) A review of the literature on the upstream migration of adult salmonids. J Fish Biol 1(2):85–136. https://doi.org/10.1111/j.1095-8649.1969.tb03847.x
Baumgartner LJ, Reynoldson N, Gilligan DM (2006) Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs. Mar Freshw Res 57(2):187–191
Beck C (2019) Hydraulic and fish-biological performance of fish guidance structures with curved bars. In: 38th International Association for Hydro-Environmental Engineering and Research World Congress (IAHR 2019)
Bell CE, Kynard B (1985) Mortality of adult American shad passing through a 17-megawatt Kaplan turbine at a low-head hydroelectric dam. North Am J Fish Manage 5(1):33–38. https://doi.org/10.1577/1548-8659(1985)5%3c33:moaasp%3e2.0.co;2
Benejam L, Saura-Mas S, Bardina M, Solà C, Munné A, García-Berthou E (2016) Ecological impacts of small hydropower plants on headwater stream fish: from individual to community effects. Ecol Freshw Fish 25(2):295–306. https://doi.org/10.1111/eff.12210
Birnie-Gauvin K, Aarestrup K, Riis TMO, Jepsen N, Koed A (2017) Shining a light on the loss of rheophilic fish habitat in lowland rivers as a forgotten consequence of barriers, and its implications for management. Aquat Conserv Mar Freshwat Ecosyst 27(6):1345–1349. https://doi.org/10.1002/aqc.2795
Boavida I, Santos JM, Ferreira MT, Pinheiro A, Zhaoyin W, Lee JHW, Jizhang G, Shuyou C (2013). Fish habitat-response to hydropeaking. In: Proceedings of the 35th Iahr World Congress, Vols I and Ii, August 2015, 1–8
Boavida I, Santos JM, Ferreira T, Pinheiro A (2015) Barbel habitat alterations due to hydropeaking. J Hydro Environ Res 9(2):237–247. https://doi.org/10.1016/j.jher.2014.07.009
Britton JR, Pegg J (2011) Ecology of European barbel Barbus barbus: implications for river, fishery, and conservation management. Rev Fish Sci 19(4):321–330. https://doi.org/10.1080/10641262.2011.599886
Brown RS, Carlson TJ, Gingerich AJ, Stephenson JR, Pflugrath BD, Welch AE, Langeslay MJ, Ahmann ML, Johnson RL, Skalski JR, Seaburg AG, Townsend RL (2012) Quantifying mortal injury of juvenile Chinook salmon exposed to simulated hydro-turbine passage. Trans Am Fish Soc 141(1):147–157. https://doi.org/10.1080/00028487.2011.650274
Brown RS, Carlson TJ, Welch AE, Stephenson JR, Abernethy CS, Ebberts BD, Langeslay MJ, Ahmann ML, Feil DH, Skalski JR (2009) Assessment of barotrauma from rapid decompression of depth-acclimated juvenile Chinook salmon bearing radiotelemetry transmitters. Trans Am Fish Soc 138(6):1285–1301
Bunt CM, Castro-Santos T, Haro A (2012) Performance of fish passage structures at upstream barriers to migration. River Res Appl 28(4):457–478. https://doi.org/10.1002/rra.1565
Buysse D, Mouton AM, Baeyens R, Coeck J (2015) Evaluation of downstream migration mitigation actions for eel at an Archimedes screw pump pumping station. Fish Manage Ecol 22(4):286–294. https://doi.org/10.1111/fme.12124
Čada GF (1990) A review of studies relating to the effects of propeller-type turbine passage on fish early life stages. North Am J Fish Manage 10(4):418–426. https://doi.org/10.1577/1548-8675(1990)010%3c0418:arosrt%3e2.3.co;2
Čada GF (2001) The development of advanced hydroelectric turbines to improve fish passage survival. Fisheries 26(9):14–23. https://doi.org/10.1577/1548-8446(2001)026%3c0014:tdoaht%3e2.0.co;2
Čada GF, Coutant CC, Whitney RR (1997) Development of biological criteria for the design of advanced hydropower turbines (Issue i). EERE Publication and Product Library, Washington, DC (United States)
Čada G, Loar J, Garrison L, Fisher R, Neitzel D (2006) Efforts to reduce mortality to hydroelectric turbine-passed fish: locating and quantifying damaging shear stresses. Environ Manage 37(6):898–906. https://doi.org/10.1007/s00267-005-0061-1
Calles O, Olsson IC, Comoglio C, Kemp PS, Blunden L, Schmitz M, Greenberg LA (2010) Size-dependent mortality of migratory silver eels at a hydropower plant, and implications for escapement to the sea. Freshw Biol 55(10):2167–2180. https://doi.org/10.1111/j.1365-2427.2010.02459.x
Calles O, Rivinoja P, Greenberg L (2013) A historical perspective on downstream passage at hydroelectric plants in Swedish rivers. Ecohydraulics: an integrated approach. John Wiley & Sons, Ltd, 309–321
Casas-Mulet R, Alfredsen K, Hamududu B, Timalsina NP (2015a) The effects of hydropeaking on hyporheic interactions based on field experiments. Hydrol Process 29(6). https://doi.org/10.1002/hyp.10264
Casas-Mulet R, Saltveit SJ, Alfredsen K (2015b) The survival of Atlantic salmon (Salmo salar) eggs during dewatering in a river subjected to hydropeaking. River Res Appl 31(4):433–446. https://doi.org/10.1002/rra.2827
Castro-Santos T, Cotel A (2015) Webb P (2009) fishway evaluations for better bioengineering : an integrative approach a framework for fishway. Am Fish Soc Symp 69:557–575
Catalano MJ, Bozek MA, Pellett TD (2007) Effects of dam removal on fish assemblage structure and spatial distributions in the Baraboo River, Wisconsin. North Am J Fish Manag 27(2):519–530
Caudill CC, Daigle WR, Keefer ML, Boggs CT, Jepson MA, Burke BJ, Zabel RW, Bjornn TC, Peery CA (2007) Slow dam passage in adult Columbia River salmonids associated with unsuccessful migration: delayed negative effects of passage obstacles or condition-dependent mortality? Can J Fish Aquat Sci 64(7):979–995. https://doi.org/10.1139/F07-065
Choi SU, Kim SK, Choi B, Kim Y (2017) Impact of hydropeaking on downstream fish habitat at the Goesan Dam in Korea. Ecohydrology 10(6). https://doi.org/10.1002/eco.1861
Clay CH (2017) Design of fishways and other fish facilities. CRC Press, In Design of Fishways and Other Fish Facilities. https://doi.org/10.1201/9781315141046
Colotelo AH, Pflugrath BD, Brown RS, Brauner CJ, Mueller R, Carlson TJ, Deng ZD, Ahmann ML, Trumbo BA (2012) The effect of rapid and sustained decompression on barotrauma in juvenile brook lamprey and Pacific lamprey: implications for passage at hydroelectric facilities. Fish Res 129–130:17–20. https://doi.org/10.1016/j.fishres.2012.06.001
Conner MM, Saunders WC, Bouwes N, Jordan C (2015a) Evaluating impacts using a BACI design, ratios, and a Bayesian approach with a focus on restoration. Environ Monit Assess 188(10). https://doi.org/10.1007/s10661-016-5526-6
Conner MM, Saunders WC, Bouwes N, Jordan C (2015b) Evaluating impacts using a BACI design, ratios, and a Bayesian approach with a focus on restoration. Environ Monit Assess 188(10). https://doi.org/10.1007/s10661-016-5526-6
Coutant CC, Whitney RR (2000) Fish behavior in relation to passage through hydropower turbines: a review. Trans Am Fish Soc 129(2):351–380. https://doi.org/10.1577/1548-8659(2000)129%3c0351:fbirtp%3e2.0.co;2
Cramer FK, Oligher RC (1964) Passing fish through hydraulic turbines. Trans Am Fish Soc 93(3):243–259. https://doi.org/10.1577/1548-8659(1964)93[243:pftht]2.0.co;2
Crisp DT (2000). Trout and Salmon: Ecology. Conservation and Rehabilitation, Fishing News Books, Blackwell, Oxford.
De Jalon DG, Sanchez P, Camargo JA (1994) Downstream effects of a new hydropower impoundment on macrophyte, macroinvertebrate and fish communities. Regul Rivers: Res Manage 9(4):253–261. https://doi.org/10.1002/rrr.3450090406
DWA (2014). Merkblatt DWA-M 509: Fischaufstiegsanlagen und fischpassierbare Bauwerke. Report: Merkblatt, 27
Ebel G (2013a) Fischschutz und Fischabstieg an Wasserkraftanlagen. Handbuch Rechen-Und Bypasssysteme. Bd, 4
Ebel G (2013b) Fish Protection and Downstream Passage at Hydro Power Stations Handbook of Bar Rack and Bypass Systems: Büro für Gewässerökologie und Fischereibiologie
Ebel G, Gluch A, Kehl M (2015) Einsatz des leitrechen-bypass-systems nach Ebel, Gluch & Kehl an wasserkraftanlagen—Grundlagen Erfahrungen Und Perspektiven. Wasserwirtschaft 105(7–8):49
Eberhardt LL (1976) Quantitative ecology and impact assessment. J Environ Manage (United Kingdom) 4(1)
Egg L, Mueller M, Pander J, Knott J, Geist J (2017) Improving European Silver Eel (Anguilla anguilla) downstream migration by undershot sluice gate management at a small-scale hydropower plant. Ecol Eng 106:349–357. https://doi.org/10.1016/j.ecoleng.2017.05.054
Egré D, Milewski JC (2002) The diversity of hydropower projects. Energy Policy 30(14):1225–1230. https://doi.org/10.1016/S0301-4215(02)00083-6
Eicher GJ, Bell MC, Campbell CJ, Craven RE, Wert MA (1987) Turbine-related fish mortality: review and evaluation of studies. Palo Alto, CA, Electric Power Research Institute, Report EPRI AP-5480
European Commission (2015) Ecological flows in the implementation of the Water Framework Directive. Guidance Document No. 31. European Commission Technical Report 2015-086. https://doi.org/10.2779/775712
Fantin-Cruz I, Pedrollo O, Girard P, Zeilhofer P, Hamilton SK (2016) Changes in river water quality caused by a diversion hydropower dam bordering the Pantanal floodplain. Hydrobiologia 768(1):223–238. https://doi.org/10.1007/s10750-015-2550-4
Ferguson JW, Absolon RF, Carlson TJ, Sandford BP (2006) Evidence of delayed mortality on juvenile pacific salmon passing through turbines at Columbia river dams. Trans Am Fish Soc 135(1):139–150. https://doi.org/10.1577/t05-080.1
Franssen NR, Tobler M (2013) Upstream effects of a reservoir on fish assemblages 45 years following impoundment. J Fish Biol 82(5):1659–1670
Freyhof J, Gessner M, Grossart HP, Hilt S, Jähnig S, Köhler J, Mehner T, Pusch M, Venohr M, Wolter C (2019) Strengths and weaknesses of the Water Framework Directive (WFD) IGB Policy Brief. https://doi.org/10.4126/FRL01-006416917
Gandini CV, Sampaio FAC, Pompeu PS (2014) Hydropeaking effects of on the diet of a Neotropical fish community. Neotrop Ichthyol 12(4):795–802. https://doi.org/10.1590/1982-0224-20130151
Gardner CJ, Rees-Jones J, Morris G, Bryant PG, Lucas MC (2016) The influence of sluice gate operation on the migratory behaviour of Atlantic salmon Salmo salar (L.) smolts. J Ecohydraul 1(1–2):90–101. https://doi.org/10.1080/24705357.2016.1252251
Gasparatos A, Doll CNH, Esteban M, Ahmed A (2017) Renewable energy and biodiversity: implications for transitioning to a green economy. Renew Sustain Energy Rev 70:161–184. https://doi.org/10.1016/j.rser.2016.08.030
Gehrke PC, Gilligan DM, Barwick M (2002) Changes in fish communities of the Shoalhaven River 20 years after construction of Tallowa Dam Australia. River Res Appl 18(3):265–286
Geist J (2021) Editorial: Green or red: challenges for fish and freshwater biodiversity conservation related to hydropower. Aquat Conserv Mar Freshwat Ecosyst 31(7):1551–1558. https://doi.org/10.1002/aqc.3597
Gloss SP, Wahl JR (1983) Mortality of Juvenile salmonids passing through Ossberger crossflow turbines at small-scale hydroelectric sites. Trans Am Fish Soc 112(2A):194–200. https://doi.org/10.1577/1548-8659(1983)112%3c194:mojspt%3e2.0.co;2
Gosset C, Travade F, Durif C, Rives J, Elie P (2005) Tests of two types of bypass for downstream migration of eels at a small hydroelectric power plant. River Res Appl 21(10):1095–1105. https://doi.org/10.1002/rra.871
Gowans ARD, Armstrong JD, Priede IG, Mckelvey S (2003) Movements of Atlantic salmon migrating upstream through a fish-pass complex in Scotland. Ecol Freshw Fish 12(3):177–189. https://doi.org/10.1034/j.1600-0633.2003.00018.x
Green RH (1979) Sampling design and statistical methods for environmental biologists. John Wiley & Sons
Habit E, Belk MC, Parra O (2007) Response of the riverine fish community to the construction and operation of a diversion hydropower plant in central Chile. Aquat Conserv Mar Freshwat Ecosyst 17(1):37–49. https://doi.org/10.1002/aqc.774
Harrison PM, Martins EG, Algera DA, Rytwinski T, Mossop B, Leake AJ, Power M, Cooke SJ (2019) Turbine entrainment and passage of potadromous fish through hydropower dams: developing conceptual frameworks and metrics for moving beyond turbine passage mortality. Fish Fish 20(3):403–418. https://doi.org/10.1111/faf.12349
Havn TB, Sæther SA, Thorstad EB, Teichert MAK, Heermann L, Diserud OH, Borcherding J, Tambets M, Økland F (2017) Downstream migration of Atlantic salmon smolts past a low head hydropower station equippped with Archimedes screw and Francis turbines. Ecol Eng 105:262–275. https://doi.org/10.1016/j.ecoleng.2017.04.043
Hayes DS, Moreira M, Boavida I, Haslauer M, Unfer G, Zeiringer B, Greimel F, Auer S, Ferreira T, Schmutz S (2019) Life stage-specific hydropeaking flow rules. Sustainability (switz#erland) 11(6):1547. https://doi.org/10.3390/su11061547
Hecht JS, Lacombe G, Arias ME, Dang TD, Piman T (2019) Hydropower dams of the Mekong River basin: a review of their hydrological impacts. J Hydrol 568:285–300. https://doi.org/10.1016/j.jhydrol.2018.10.045
Hedger RD, Sauterleute J, Sundt-Hansen LE, Forseth T, Ugedal O, Diserud OH, Bakken TH (2018) Modelling the effect of hydropeaking-induced stranding mortality on Atlantic salmon population abundance. Ecohydrology 11(5). https://doi.org/10.1002/eco.1960
Heisey PG, Mathur P, Euston ET (1996) Passing fish safely: a closer look at turbine vs. spillway survival. Hydro Rev 35(4)
Hershey H (2021) Updating the consensus on fishway efficiency: a meta-analysis. February, 1–14. https://doi.org/10.1111/faf.12547
Hogan TW, Čada GF, Amaral SV (2014) The status of environmentally enhanced hydropower turbines. Fisheries 39(4):164–172. https://doi.org/10.1080/03632415.2014.897195
Huckstorf V, Lewin WC, Wolter C (2008) Environmental flow methodologies to protect fisheries resources in human-modified large lowland rivers. River Res Appl 24(5):519–527. https://doi.org/10.1002/rra.1131
Jackson DC (1985) The influence of differing flow regimes on the Coosa River tailwater fishery below Jordan Dam. Diss Abst Int Pt B-Sci Eng 6(6):113
Jackson DC, Marmulla G (2001) The influence of dams on river fisheries. FAO Fish Tech Pap 419:1–44
Jepsen N, Pedersen S, Thorstad E (2000) Behavioural interactions between prey (trout smolts) and predators (pike and pikeperch) in an impounded river. Regul Rivers: Res Manage 16(2):189–198. https://doi.org/10.1002/(sici)1099-1646(200003/04)16:2%3c189::aid-rrr570%3e3.3.co;2-e
Jonsson B, Waples RS, Friedland KD (1999) Extinction considerations for diadromous fishes. ICES J Mar Sci 56(4):405–409. https://doi.org/10.1006/jmsc.1999.0483
Jungwirth M, Haidvogl G, Moog O, Muhar S, Schmutz S (2003) Angewandte Fischökologie an Fließgewässern. Facultas-Verlag, 547
Jungwirth M, Schmutz S, Weiss, S (1998) Fish migration and fish bypasses (Vol. 4). Fishing News Books Oxford
Katano O, Nakamura T, Abe S, Yamamoto S, Baba Y (2006) Comparison of fish communities between above-and below-dam sections of small streams; barrier effect to diadromous fishes. J Fish Biol 68(3):767–782
Katopodis C (1992) Introduction to fishway design. In Oceans (Issue January). Freshwater Institute, Central and Arctic Region, Department of Fisheries. http://www.wra.gov.tw/public/attachment/41110254871.pdf
Katopodis C, Williams JG (2012) The development of fish passage research in a historical context. Ecol Eng 48:8–18
Kemp PS, Russon IJ, Vowles AS, Lucas MC (2011) The influence of discharge and temperature on the ability of upstream migrant adult river lamprey (Lampetra fluviatilis) to pass experimental overshot and undershot weirs. River Res Appl 27(4):488–498
Larinier M (2001) Environmental issues, dams and fish migration. FAO Fish Tech Pap 419:45–90
Larinier M, Travade F (1992) La conception des dispositifs de franchissement pour les aloses. Bulletin Français De La Pêche Et De La Pisciculture 326–327:125–133. https://doi.org/10.1051/kmae:1992009
Larinier M, Travade F (2002) Downstream migration: problems and facilities. Bulletin Français De La Pêche Et De La Pisciculture 364:181–207
Lees AC, Peres CA, Fearnside PM, Schneider M, Zuanon JAS (2016) Hydropower and the future of Amazonian biodiversity. Biodivers Conserv 25(3):451–466. https://doi.org/10.1007/s10531-016-1072-3
Lucas MC, Bubb DH, Jang M-H, Ha K, Masters JEG (2009) Availability of and access to critical habitats in regulated rivers: effects of low-head barriers on threatened lampreys. Freshw Biol 54(3):621–634. https://doi.org/10.1111/j.1365-2427.2008.02136.x
Lucas MC, Frear PA (1997) Effects of a flow-gauging weir on the migratory behaviour of adult barbel, a riverine cyprinid. J Fish Biol 50(2):382–396. https://doi.org/10.1006/jfbi.1996.0302
Martinez PJ, Chart TE, Trammell MA, Wullschleger JG, Bergersen EP (1994) Fish species composition before and after construction of a main stem reservoir on the White River Colorado. Environ Biol Fishes 40(3):227–239
Martins EG, Gutowsky LFG, Harrison PM, Patterson DA, Power M, Zhu DZ, Leake A, Cooke SJ (2013) Forebay use and entrainment rates of resident adult fish in a large hydropower reservoir. Aquat Biol 19(3):253–263. https://doi.org/10.3354/ab00536
Marttin F, De Graaf GJ (2002) The effect of a sluice gate and its mode of operation on mortality of drifting fish larvae in Bangladesh. Fish Manage Ecol 9(2):123–125
Mathur D, Heisey PG, Skalski JR, Kenney DR (2000) Salmonid smolt survival relative to turbine efficiency and entrainment depth in hydroelectric power generation 1. JAWRA J Am Water Resourc Assoc 36(4):737–747
Matt P, Pirker O, Schletterer M (2019). Hydropower through time – The significance of Alpine rivers for the energy sector. In: Muhar S, Huhar A, Egger G, Siegrist D (eds) Rivers of the Alps—diversity in nature and culture, pp 248–259
McCarthy TK, Frankiewicz P, Cullen P, Blaszkowski M, O’Connor W, Doherty D (2008) Long-term effects of hydropower installations and associated river regulation on River Shannon eel populations: mitigation and management. Hydrobiologia 609(1):109–124. https://doi.org/10.1007/s10750-008-9395-z
Moreira M, Hayes DS, Boavida I, Schletterer M, Schmutz S, Pinheiro A (2019) Ecologically-based criteria for hydropeaking mitigation: a review. Sci Total Environ 657:1508–1522. https://doi.org/10.1016/j.scitotenv.2018.12.107
Morita K, Yamamoto S (2002) Effects of habitat fragmentation by damming on the persistence of stream-dwelling charr populations. Conserv Biol 16(5):1318–1323. https://doi.org/10.1046/j.1523-1739.2002.01476.x
Mueller M, Bierschenk AM, Bierschenk BM, Pander J, Geist J (2020a) Effects of multiple stressors on the distribution of fish communities in 203 headwater streams of Rhine, Elbe and Danube. Sci Total Environ 703:134523
Mueller M, Knott J, Pander J, Geist J (2020b) Fischökologisches Monitoring an innovativen Wasserkraftanlagen Abschlussbericht 2020b Band 3: Baiersdorf-Wellerstadt an der Regnitz. Lehrstuhl für Aquatische Systembiologie, Technische Universität München, Wissenschaftszentrum Weihenstephan
Mueller M, Knott J, Pander J, Geist J (2020c) Fischökologisches Monitoring an innovativen Wasserkraftanlagen Abschlussbericht 2020c Band 4: Lindesmühle an der Fränkischen Saale. Lehrstuhl für Aquatische Systembiologie, Technische Universität München, Wissenschaftszentrum Weihenstephan
Mueller M, Knott J, Pander J, Geist J (2020d) Fischökologisches Monitoring an innovativen Wasserkraftanlagen Abschlussbericht 2020d Band 5: Au an der Iller. Lehrstuhl für Aquatische Systembiologie, Technische Universität München, Wissenschaftszentrum Weihenstephan
Mueller M, Knott J, Pander J, Geist J (2020e) Fischökologisches Monitoring an innovativen Wasserkraftanlagen Abschlussbericht 2020e Band 6: Heckerwehr an der Roth. Lehrstuhl für Aquatische Systembiologie, Technische Universität München, Wissenschaftszentrum Weihenstephan
Mueller M, Knott J, Pander J, Geist J (2020f) Fischökologisches Monitoring an innovativen Wasserkraftanlagen Abschlussbericht 2020f Band 7: Eixendorf an der Schwarzach. Lehrstuhl für Aquatische Systembiologie, Technische Universität München, Wissenschaftszentrum Weihenstephan
Mueller M, Knott J, Pander J, Geist J (2020g) Fischökologisches Monitoring an innovativen Wasserkraftanlagen Abschlussbericht 2020g Band 8: Baierbrunn an der Isar. Lehrstuhl für Aquatische Systembiologie, Technische Universität München, Wissenschaftszentrum Weihenstephan
Mueller M, Knott J, Pander J, Geist J (2020h) Fischökologisches Monitoring an innovativen Wasserkraftanlagen Abschlussbericht 2020h Band 9: Höllthal an der Alz. Lehrstuhl für Aquatische Systembiologie, Technische Universität München, Wissenschaftszentrum Weihenstephan
Mueller M, Pander J, Geist J (2011) The effects of weirs on structural stream habitat and biological communities. J Appl Ecol 48(6):1450–1461. https://doi.org/10.1111/j.1365-2664.2011.02035.x
Mueller M, Pander J, Geist J (2017) Evaluation of external fish injury caused by hydropower plants based on a novel field-based protocol. Fish Manage Ecol 24(3):240–255. https://doi.org/10.1111/fme.12229
Mueller M, Sternecker K, Milz S, Geist J (2020i) Assessing turbine passage effects on internal fish injury and delayed mortality using X-ray imaging. PeerJ 8:e9977
Muir WD, Marsh DM, Sandford BP, Smith SG, Williams JG (2006) Post-hydropower system delayed mortality of transported snake river stream-type chinook salmon: unraveling the mystery. Trans Am Fish Soc 135(6):1523–1534. https://doi.org/10.1577/t06-049.1
Muir WD, Smith SG, Williams JG, Sandford BP (2001) Survival of Juvenile Salmonids passing through bypass systems, turbines, and spillways with and without flow deflectors at snake river dams. North Am J Fish Manage 21(1):135–146. https://doi.org/10.1577/1548-8675(2001)021%3c0135:sojspt%3e2.0.co;2
Nijboer RC, Johnson RK, Verdonschot PFM, Sommerhäuser M, Buffagni A (2004) Establishing reference conditions for European streams. Hydrobiologia 516(1–3):91–105. https://doi.org/10.1023/B:HYDR.0000025260.30930.f4
Noonan MJ, Grant JWA, Jackson CD (2012) A quantitative assessment of fish passage efficiency. Fish Fish 13(4):450–464. https://doi.org/10.1111/j.1467-2979.2011.00445.x
Nyqvist D, Elghagen J, Heiss M, Calles O (2018) An angled rack with a bypass and a nature-like fishway pass Atlantic salmon smolts downstream at a hydropower dam. Mar Freshw Res 69(12):1894–1904. https://doi.org/10.1071/MF18065
Odeh M (1999) A summary of environmentally friendly turbine design concepts. U.S. Department of Energy, Idaho Operations Office, Idaho Falls, ID, Leetown Science Center, Report 99-065/TF
Økland F, Havn TB, Thorstad EB, Heermann L, Sæther SA, Tambets M, Teichert MAK, Borcherding J (2019) Mortality of downstream migrating European eel at power stations can be low when turbine mortality is eliminated by protection measures and safe bypass routes are available. Int Rev Hydrobiol 104(3–4):68–79. https://doi.org/10.1002/iroh.201801975
Ovidio M, Dierckx A, Bunel S, Grandry L, Spronck C, Benitez JP (2017) Poor performance of a retrofitted downstream bypass revealed by the analysis of approaching behaviour in combination with a trapping system. River Res Appl 33(1):27–36. https://doi.org/10.1002/rra.3062
Pelicice FM, Agostinho AA (2009) Fish fauna destruction after the introduction of a non-native predator (Cichla kelberi) in a Neotropical reservoir. Biol Invasions 11(8):1789–1801
Pelicice FM, Pompeu PS, Agostinho AA (2015) Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish Fish 16(4):697–715. https://doi.org/10.1111/faf.12089
Penczak T, Głowacki Ł, Galicka W, Koszaliński H (1998) A long-term study (1985–1995) of fish populations in the impounded Warta River Poland. Hydrobiologia 368(1–3):157–173. https://doi.org/10.1023/A:1003246115666
Person É (2013) Impact of hydropeaking on fish and their habitat. In Communications du Laboratoire de Constructions Hydrauliques—55 (vol 5812, Issue 2013). EPFL-LCH. https://doi.org/10.5075/epfl-thesis-5812
Pflugrath BD, Boys CA, Cathers B (2019) Over or under? Autonomous sensor fish reveals why overshot weirs may be safer than undershot weirs for fish passage. Ecol Eng 132:41–48. https://doi.org/10.1016/j.ecoleng.2019.03.010
Piorkowski RJ (1995). Ecological effects of spawning salmon on several southcentral Alaskan streams
Piper AT, Rosewarne PJ, Wright RM, Kemp PS (2018) The impact of an Archimedes screw hydropower turbine on fish migration in a lowland river. Ecol Eng 118:31–42. https://doi.org/10.1016/j.ecoleng.2018.04.009
Pracheil BM, DeRolph CR, Schramm MP, Bevelhimer MS (2016) A fish-eye view of riverine hydropower systems: the current understanding of the biological response to turbine passage. Rev Fish Biol Fisheries 26(2):153–167. https://doi.org/10.1007/s11160-015-9416-8
Pulg U, Schnell J (2008) Untersuchungen zur Effektivität alternativer Triebwerkstechniken und Schutzkonzepte für abwandernde Fische beim Betrieb von Kleinwasserkraftanlagen. In Landesfischereiverband Bayern
Quaranta E, Wolter C (2021) Sustainability assessment of hydropower water wheels with downstream migrating fish and blade strike modelling. Sustain Energy Technol Assess 43:100943
Reed BC, Kelso WE, Rutherford DA (1992) Growth, fecundity, and mortality of Paddlefish in Louisiana. Trans Am Fish Soc 121(3):378–384. https://doi.org/10.1577/1548-8659(1992)121%3c0378:gfamop%3e2.3.co;2
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, Maccormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. 94:849–873. https://doi.org/10.1111/brv.12480
Reuter M, Kohout C (2014) Praxishandbuch für den umweltbewussten Einsatz von Turbinentechnologien im Bereich der Kleinstwasserkraft. Institut für Wasserwirtschaft, Siedlungswasserbau und Ökologie GmbH, Hydrolabor Schleusingen, Schleusingen
Richmond MC, Serkowski JA, Ebner LL, Sick M, Brown RS, Carlson TJ (2014) Quantifying barotrauma risk to juvenile fish during hydro-turbine passage. Fish Res 154:152–164. https://doi.org/10.1016/j.fishres.2014.01.007
Roscoe DW, Hinch SG, Cooke SJ, Patterson DA (2011) Fishway passage and post-passage mortality of up-river migrating sockeye salmon in the Seton River British Columbia. River Res Appl 27(6):693–705. https://doi.org/10.1002/rra.1384
Ruggles CP, Murray DG (1983) A Review of Fish Response to spillways. Can Tech Rep Fisher Aquat Sci 1172:29p
Santos JM, Branco P, Katopodis C, Ferreira T, Pinheiro A (2014) Retrofitting pool-and-weir fishways to improve passage performance of benthic fishes: effect of boulder density and fishway discharge. Ecol Eng 73:335–344. https://doi.org/10.1016/j.ecoleng.2014.09.065
Santos JM, Ferreira MT, Pinheiro AN, Bochechas JH (2006) Effects of small hydropower plants on fish assemblages in medium-sized streams in central and northern Portugal. Aquat Conserv Mar Freshwat Ecosyst 16(4):373–388. https://doi.org/10.1002/aqc.735
Sá-Oliveira JC, Hawe JE, Isaac-Nahum VJ, Peres CA (2015) Upstream and downstream responses of fish assemblages to an eastern Amazonian hydroelectric dam. Freshw Biol 60(10):2037–2050. https://doi.org/10.1111/fwb.12628
Schiemer F, Keckeis H, Winkler G, Flore L (2001) Large rivers: the relevance of ecotonal structure and hydrological properties for the fish fauna. River Syst 12(2–4):487–508. https://doi.org/10.1127/lr/12/2001/487
Schilt CR (2007) Developing fish passage and protection at hydropower dams. Appl Anim Behav Sci 104(3–4):295–325. https://doi.org/10.1016/j.applanim.2006.09.004
Schletterer M, Reindl R, Thonhauser T (2016) Options for re-establishing river continuity, with an emphasis on the special solution “fish lift”: examples from Austria. Revista Eletrônica de Gestão e Tecnologias Ambientais, 109–128
Schmidt MB, Tuhtan JA, Schletterer M (2018) Hydroacoustic and pressure turbulence analysis for the assessment of fish presence and behavior upstream of a vertical trash rack at a run-of-river hydropower plant. Appl Sci (switzerland) 8(10):1723. https://doi.org/10.3390/app8101723
Schmutz S, Bakken TH, Friedrich T, Greimel F, Harby A, Jungwirth M, Melcher A, Unfer G, Zeiringer B (2015) Response of fish communities to hydrological and morphological alterations in hydropeaking rivers of Austria. River Res Appl 31(8):919–930. https://doi.org/10.1002/rra.2795
Schmutz S, Sendzimir J (2018) Riverine ecosystem management: science for governing towards a sustainable future. Springer Nature
Shen Y, Diplas P (2010) Modeling unsteady flow characteristics of hydropeaking operations and their implications on fish habitat. J Hydraul Eng 136(12):1053–1066. https://doi.org/10.1061/(ASCE)HY.1943-7900.0000112
Silva S, Barca S, Vieira-Lanero R, Cobo F (2019) Upstream migration of the anadromous sea lamprey (Petromyzon marinus Linnaeus, 1758) in a highly impounded river: impact of low-head obstacles and fisheries. Aquat Conserv Mar Freshwat Ecosyst 29(3):389–396. https://doi.org/10.1002/aqc.3059
Smith EP (2014) BACI design. Wiley StatsRef: Statistics Reference Online
Stansell RJ, Gibbons KM, Nagy WT (2010) Evaluation of pinniped predation on adult salmonids and other fish in the Bonneville Dam tailrace, 2008–2010
Stendera S, Adrian R, Bonada N, Cañedo-Argüelles M, Hugueny B, Januschke K, Pletterbauer F, Hering D (2012) Drivers and stressors of freshwater biodiversity patterns across different ecosystems and scales: a review. Hydrobiologia 696(1):1–28. https://doi.org/10.1007/s10750-012-1183-0
Stephenson JR, Gingerich AJ, Brown RS, Pflugrath BD, Deng Z, Carlson TJ, Langeslay MJ, Ahmann ML, Johnson RL, Seaburg AG (2010) Assessing barotrauma in neutrally and negatively buoyant juvenile salmonids exposed to simulated hydro-turbine passage using a mobile aquatic barotrauma laboratory. Fish Res 106(3):271–278
Stich DS, Zydlewski GB, Kocik JF, Zydlewski JD (2015) Linking behavior, physiology, and survival of Atlantic salmon Smolts during estuary migration. Mar Coast Fisher 7(1):68–86. https://doi.org/10.1080/19425120.2015.1007185
Taylor RE, Kynard B (1985) Mortality of Juvenile American Shad and Blueback Herring passed through a low-head Kaplan hydroelectric turbine. Trans Am Fish Soc 114(3):430–435. https://doi.org/10.1577/1548-8659(1985)114%3c430:mojasa%3e2.0.co;2
Thorne RE, Johnson GE (1993) A review of hydroacoustic studies for estimation of salmonid downriver migration past hydroeiectric facilities on the Columbia and Snake Rivers in the 1980s. Rev Fish Sci 1(1):27–56
Thornton KW, Kimmel BL, Payne FE (1990) Reservoir limnology: ecological perspectives. John Wiley & Sons
Thorstad EB, Økland F, Aarestrup K, Heggberget TG (2008) Factors affecting the within-river spawning migration of Atlantic salmon, with emphasis on human impacts. Rev Fish Biol Fisher 18(4):345–371. https://doi.org/10.1007/s11160-007-9076-4
Tiffan KF, Hatten JR, Trachtenbarg DA (2016) Assessing juvenile salmon rearing habitat and associated predation risk in a lower snake river reservoir. 1038:1030–1038. https://doi.org/10.1002/rra
Travade F, Larinier M (2002) Fish locks and fish lifts. Bulletin Français de La Pêche et de La Pisciculture, 364 supplément, 102–118. https://doi.org/10.1051/kmae/2002096
Tuhtan JA, Noack M, Wieprecht S (2012) Estimating stranding risk due to hydropeaking for juvenile European grayling considering river morphology. KSCE J Civ Eng 16(2):197–206. https://doi.org/10.1007/s12205-012-0002-5
Tundisi JG, Straškraba M (1999) Theoretical reservoir ecology and its applications. International Institute of Ecology Ann Arbor
Turnpenny AWH, Clough S, Hanson KP, Ramsay R, McEwan D (2000) Risk assessment for fish passage through small, low-head turbines. Fawley Aquatic Research Laboratories, London, Report ETSU H/06/00054/REP
USFWS (U.S. Fish and Wildlife Service) (2019) Fish passage engineering design criteria. USFWS, Northeast Region R5, Hadley, Massachusetts, 5, 224
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37(1):130–137. https://doi.org/10.1139/f80-017
Weibel U (1991) Neue Ergebnisse zur Fischfauna des nördlichen Oberrheins ermittelt im Rechengut von Kraftwerken. Fischökologie 5:43–68
Williams JG, Smith SG, Muir WD (2001) Survival estimates for downstream migrant yearling Juvenile Salmonids through the Snake and Columbia rivers hydropower system, 1966–1980 and 1993–1999. North Am J Fish Manage 21(2):310–317. https://doi.org/10.1577/1548-8675(2001)021%3c0310:sefdmy%3e2.0.co;2
Winter HV, Jansen HM, Bruijs MCM (2006) Assessing the impact of hydropower and fisheries on downstream migrating silver eel, Anguilla anguilla, by telemetry in the River Meuse. Ecol Freshw Fish 15(2):221–228. https://doi.org/10.1111/j.1600-0633.2006.00154.x
Wolter C, Bernotat D, Gessner J, Brüning A, Lackemann J, Radinger J (2020) Fachplanerische Bewertung der Mortalität von Fischen an Wasserkraftanlagen. Bundesamt für Naturschutz
Wood PJ, Armitage PD (1997) Biological effects of fine sediment in the lotic environment. 21(2):203–217
Yang N, Li Y, Zhang W, Lin L, Qian B, Wang L, Niu L, Zhang H (2020). Cascade dam impoundments restrain the trophic transfer efficiencies in benthic microbial food web. Water Res 170:115351
Young PS, Cech JJ, Thompson LC (2011) Hydropower-related pulsed-flow impacts on stream fishes: a brief review, conceptual model, knowledge gaps, and research needs. Rev Fish Biol Fisher 21(4):713–731. https://doi.org/10.1007/s11160-011-9211-0
Ziv G, Baran E, Nam S, Rodríguez-Iturbe I, Levin SA (2012) Trading-off fish biodiversity, food security, and hydropower in the Mekong River Basin. Proc Natl Acad Sci USA 109(15):5609–5614. https://doi.org/10.1073/pnas.1201423109
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
van Treeck, R., Geist, J., Pander, J., Tuhtan, J., Wolter, C. (2022). Impacts and Risks of Hydropower. In: Rutschmann, P., et al. Novel Developments for Sustainable Hydropower. Springer, Cham. https://doi.org/10.1007/978-3-030-99138-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-99138-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99137-1
Online ISBN: 978-3-030-99138-8
eBook Packages: EngineeringEngineering (R0)