Abstract
Studies showed that the δ18OP is a useful tool to study P in the environment. Adequate extraction protocols for the targeted P pools of the study are a prerequisite for a successful study. Likewise, for most environmental samples, including water, soil, sediment and plant samples, it is crucial that the samples are processed as soon as possible after they have been taken to avoid any alterations of the original δ18OP signature. This is especially true when more bioavailable P pools, like soluble reactive P (SRP) in water samples, are extracted and analysed. Brucite precipitation of water samples should be directly done in the field, fresh soil and sediment samples have to be extracted within 7 days (if microbial P is targeted, on the day of sampling), and plant samples have to be extracted within a few hours of sampling or be frozen. The chapter briefly describes the P cycle in aquatic and terrestrial ecosystems and give an overview about extracting the most common P pools for δ18OP analysis: soluble reactive P in water samples, sequentially extracted P pools of soil, sediment, fertilizer and plant samples.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
2.1 An Overview of the Extraction Protocols
Studies showed that the δ18OP is a useful tool to study P in the environment (Helfenstein et al. 2018). Adequate extraction protocols for the targeted P pools of the study are a prerequisite for a successful study. Likewise, for most environmental samples, including water, soil, sediment and plant samples, it is crucial that the samples are processed as soon as possible after they have been taken in order to avoid any alterations of the original δ18OP signature. This is especially true when more bioavailable P pools, like soluble reactive P (SRP) in water samples, are extracted and analysed. Brucite precipitation of water samples should be directly done in the field (see Sect. 2.2), fresh soil (see Sect. 2.3) and sediment (see Sect. 2.4) samples have to be extracted within 7 days (if microbial P is targeted, on the day of sampling), and plant samples (see Sect. 2.5) have to be extracted within a few hours of sampling or be frozen.
The following chapters briefly describe the P cycle in aquatic and terrestrial ecosystems and give an overview about extracting the most common P pools for δ18OP analysis: soluble reactive P in water samples, sequentially extracted P pools of soil, sediment, fertilizer and plant samples. In general, P occurs in microbial, organic and inorganic forms in the environment (Condron and Newman 2011). These forms are interlinked with each other through inorganic and biological processes and are often clustered together according to their extractability with different chemicals. Which of these pools needs to be extracted and analysed for its δ18OP value depends on the research question.
2.2 Aquatic Systems
Phosphorus inputs into aquatic systems are mainly from non-point sources like runoff from roads and fields or point sources, for example, from wastewater treatment plants. Direct disposal of untreated wastewater, effluents and solid wastes to water ways from households and small-scale industries, and leachates from septic tanks also contributed to the enrichment of P in water ways. Atmospheric inputs of P into aquatic systems are often low but can still negatively affect water quality in some cases. Since bioavailability of P in aquatic systems is often low and hence aquatic biota like plants are efficient in taking up P (Reynolds and Davies 2001), those P inputs can have a drastic effect on aquatic ecosystems like eutrophication (Schindler et al. 2016). In marine ecosystems, P is often considered as the nutrient which is limiting primary production (Filippelli 2008).
Soluble reactive P (SRP) is considered most bioavailable and many studies and water quality guidelines focus on this pool (Fig. 2.1) (Directive 2000).
Particulate P in water is also important as it can contribute significantly to the bioavailable P in aquatic systems (Ellison and Brett 2006). Both particulate P and SRP contribute to sedimentary P, either by deposition or adsorption onto particles. Through resuspension and desorption processes their bioavailability for aquatic organisms increases again. Once taken up by the biota, inorganic P can be incorporated into organic P (Porg). The P bond in organic forms becomes available through the hydrolysis by enzymes. Organic P like organic matter, in general, can also be buried into the sediment.
2.2.1 Extraction of Soluble Reactive Phosphorus (SRP)
Concentrations of SRP can be very low in water bodies and thus it might be necessary to collect several litres of water for the determination of the δ18OP as 20–30 µmol P (= 0.65–0.97 µg P) which are necessary for the purification protocol. Granger et al. (2017b) sampled, for example, between 25 and 50 L of water. It is therefore recommended to measure the SRP concentration in the water before the actual sampling. The below-described sampling protocol is based on the method by Nisbeth et al. (2019) (Fig. 2.2).
As SRP concentrations are often low, it is recommended to thoroughly clean all equipment/consumables like polypropylene bottles with P-free detergent and rinse them with diluted HCl and ultrapure water before using.
2.2.2 Equipment and Consumables
-
Standard lab glassware and equipment.
-
Polypropylene bottles, different sizes.
-
Nylon mesh.
-
Vacutainers.
-
Plastic tubes.
-
GF/F filters.
2.2.3 Reagents
-
1.
3 M magnesium brine
Weigh out 1.6 kg of MgCl2 (hexahydrate; MW: 203.3 g/mol). Add 2.5 L of ultrapure water. After the salt has dissolved, filter the brine on a GF/F filter.
-
2.
1 M NaOH
Weigh out 40 g of NaOH pellets. Dissolve it in 1 L of ultrapure water.
-
3.
1 M HNO 3
In a volumetric flask add 66 mL concentrated HNO3 to 800 mL ultrapure water and make up to 1 L.
Step SRP_1—Water collection (field)
-
Choose two different sizes of polypropylene bottles: a smaller one (<5 L) to take the water samples and a larger one (<50 L) for collecting the water.
-
In shallow water ways and ponds, samples need to be collected carefully without disturbing the sediments deposited in bed of the water ways.
-
Attach a nylon mesh on top of the larger bottle to remove any coarse material like leaves and small branches from the water samples.
-
Fill up the larger bottle using the smaller one. Depending on the SRP concentration, more than one large bottle might be necessary.
-
In a small bottle, e.g. 125 mL, collect water to measure phosphate concentration. Fill up a vacutainer for the determination of the δ18O of water.
Step SRP_2—First Brucite precipitation (field)
-
Measure out 1 L of the Mg brine and pour it into the 50 L bottle. Shake or stir using a rod well. Measure out 250 mL of 1 M NaOH and add it. Shake again.
Step SRP_3—Discarding supernatant (field)
-
If it is cold, leave the 50 L bottle in the field and let the Mg(OH)2 (brucite) floc settle. After about 1 h, siphon out the supernatant by using the plastic tube. Thick pipes might be faster; however, thinner tubes can reduce losses and are therefore preferable. About 1/10 of the initial volume will remain. If it is warm, the bottle should be transferred to the lab, put into a fridge and shaken. After a couple of hours, the floc is settled and no brucite remains in suspension, the supernatant is discarded.
Step SRP_4—Centrifuging brucite flocs (laboratory)
-
The remaining brucite floc should be centrifuged at about 3000 rpm for 15 min. The supernatant is then eliminated.
Step SRP_5—Dissolution of brucite flocs (laboratory)
-
Brucite is removed from the centrifuging bottles by dissolving it with 1 M HNO3. Use the minimum amount of acid to dissolve the brucite from each bottle. Combine the solutions. pH should be around 1. If needed, the volume can be further reduced by performing additional MAGIC (steps SRP_2 to SRP_5) steps. This is done only by raising the pH to about 10–11 (e.g. by adding NaOH). Then, repeat step SRP_2–5. Generally, after three MAGIC steps, the volume is reduced from 50 L to about 250–300 mL.
Step SRP_6—Filtration
-
Once the desired volume is reached (generally around 100–150 mL), the solutions are filtered using GF/F filters.
2.3 Soils
Phosphorus occurs in inorganic and organic forms in soils (Fig. 2.3). The concentration of inorganic P in the soil solution (available P) is often relatively low, around 0.1 mg P L−1, and is controlled by several biotic and abiotic processes (Fig. 2.3).
Abiotic processes with no/little fractionation 2. Inorganic hydrolysis of condensed phosphates 3. Preferential uptake of P by, for example, microorganisms 4. Intracellular cycling of P leading to equilibrium between O in phosphate and water, mediated my inorganic pyrophosphatase 5. Hydrolysis of organic P by phosphoenzymes like acid and alkaline phosphatase
Immobilization of inorganic P by microorganisms, precipitation of P minerals, and sorption of P onto iron oxides or clay minerals reduce the concentration of inorganic P in the soil solution (Arai and Sparks 2007). Mineralization of organic P, dissolution of P minerals and desorption of Pi from soil particles increase the concentration of P in the soil solution (Shen et al. 2011). Organic P in soils typically makes up 30–65% of total P (Harrison 1987) and includes phosphomonoesters and diesters, such as DNA and RNA. These forms of organic P cannot directly be taken up by plants or microorganisms. Plants and microorganisms developed several strategies to make organic P and other immobilized P available again, such as exudation of organic acids and phosphoenzymes like acid phosphatase. Association with mycorrhizal fungi is also a common strategy among plants to increase P availability (Shen et al. 2011).
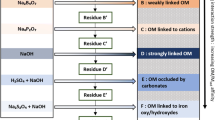
The extraction methods for different soil P pools vary greatly from country to country and soil to soil (Nawara et al. 2017). Sodium bicarbonate, anion exchange resin membranes and water are only some of the extractants used for available P. Not all existing methods for extraction different soil P pools are suitable for the analysis of the δ18OP. In some cases, P concentrations in the extracts would be too low so that a large amount of soil would be necessary, or the extraction would lead to an uncontrolled O exchange between phosphate and the extracting reagent. Other extracts might cause issues during the purification protocol. A new extraction method should therefore first be tested for its suitability for the δ18OP analysis. The most tested and used method is the extraction with 1 M HCl (Tamburini et al. 2010; Amelung et al. 2015; Granger et al. 2017a). It is also often the easiest when it comes to sample handling as the samples do not need to be fresh but can be dried. It might, however, not always be the most meaningful method, depending on the research hypothesis. When investigating the impact of biological processes on soil P cycling, the available (and microbial) P pool is better suited (see also Chap. 5). The following sequential extraction protocol is based on Tamburini et al. (2018) and consists of the extraction of resin and hexanol P, NaOH-EDTA inorganic and organic P and HCl P (Fig. 2.4). Tamburini et al. (2018) developed the method to purify inorganic and organic P in NaOH-EDTA extracts and follows the protocols by Weiner et al. (2011) and Kouno et al. (1995) for resin and hexanol P and Tamburini et al. (2010) for HCl P. The microbial P is calculated with a simple mass balance using the resin and hexanol P concentrations and δ18OP values (Tamburini et al. 2012).
Extraction scheme for sequential soil extraction for δ18OP analysis; modified after Tamburini et al. (2018). Pi = inorganic P; Porg = organic P; H2O = here: ultrapure water; AEMs = anion exchange resin membranes; RT = room temperature
Equipment and consumables
-
Polypropylene bottles, different sizes.
-
Vacutainers.
-
Standard lab glassware and equipment.
-
GF/F filters.
Reagents
-
1.
Anion exchange resin membranes
The resin membranes are stored in 1 M HNO3. Take out the number of membranes you need and wash with ultrapure water. Shake for 1 h in 0.5 M NaHCO3. Wash with ultrapure water. Shake for 1 h in 0.5 M NaHCO3. Wash three times with ultrapure water and store in ultrapure water before use.
-
2.
0.5 M NaHCO 3
Weigh out 42.3 g of sodium bicarbonate (NaHCO3; MW = 84.01 g/mol). Add 1 L of ultrapure water. Dissolve well by stirring on magnetic plate. Prepare fresh.
-
3.
Hexanol
-
4.
0.2 M HNO 3
Measure 986 mL ultrapure water and add 14 mL concentrated HNO3 (65% HNO3).
-
5.
1 M HNO 3
In a volumetric flask add 66 mL concentrated HNO3 to 800 mL ultrapure water and make up to 1 L.
-
6.
0.25 M NaOH–0.05 M EDTA solution
Weigh out 10 g NaOH and 18.612 g EDTA disodium salt and dissolve in 1 L ultrapure water.
-
7.
1 M HCl
In a volumetric flask add 82.7 mL concentrated HCl (37%) in 900 mL ultrapure water and make up to 1 L. Prepare two batches of 1 M HCl (18O-labelled and unlabelled HCl solutions). Take a subsample from each batch for the determination of the δ18Ow value of each batch.
Use a soil: solution ratio of 1:10 throughout the protocol.
Step S_1 Sample preparation
-
After sampling the soil, sieve it to 2 mm and store at 4 °C. Put a subsample into a vacutainer for the extraction of soil water and store it in the freezer. The soil sample should be processed as soon as possible.
-
Weigh fresh soil samples in duplicates into plastic bottles. Amounts weighed depend on P concentrations, since for each extract to be purified, 20 µmoles of P (0.65 µg of P) are needed:
-
Sample 1: Resin P.
-
Sample 2: Hexanol P.
-
Step S_2 Resin and hexanol P extraction
-
Add ultrapure water to sample 1 and 2.
-
Add hexanol to the sample 2, e.g. 20 mL hexanol to 600 mL ultrapure water.
-
Add previously conditioned anion exchange resin membranes to each sample and shake overnight at 4 °C.
Step S_3 Recovering resin strips
-
Recover the resin membranes from the samples, rinse them with ultrapure water to remove any adhering particles.
-
Put the resin membranes in 1 L plastic bottles. Add about 100 mL ultrapure water and shake the resin membranes for 1 h. Discard the water. Add 75 mL 0.2 M HNO3 to each sample. Place on a horizontal shaker and shake for 16 h at 160 rpm. Collect the 0.2 M HNO3 elution solution. Recover the resin membranes and store them in 1 M HNO3.
-
Use soil + solution from sample 2 for the remaining extractions, discard soil + solution from sample 1.
Step S_4 NaOH-EDTA extraction
-
Add NaOH and EDTA to the soil-solution mix of sample 2 to obtain a final concentration of 0.25 M NaOH and 0.05 M EDTA.
-
Put on a shaker at room temperature and shake overnight.
-
The following day remove the samples from the shaker. Centrifuge for 15 min at 5000 rpm. Filter the supernatant through a GF/F filter. Recover soil from each of the centrifuge bottles and dry at 40 °C.
-
The supernatant is stored in the freezer and freeze-dried ASAP after the extraction.
Step S_5 HCl extraction
-
Split the recovered soil from step S_4 into two equal parts.
-
Add 18O-labelled 1 M HCl to one part and unlabelled 1 M HCl to the second part. Put on the shaker at 160 rpm at room temperature for 16 h.
-
The following day remove the samples from the shaker. Centrifuge for 15 min at 5000 rpm. Filter the supernatant through a GF/F filter. Collect supernatant.
2.4 Extraction of P from Sediments
Extracting P from sediments for δ18OP analysis is similar to the extraction of P from soils (see Sect. 2.3; Fig. 2.4). Sediment samples can be extracted sequentially including extraction with a citrate-dithionite-bicarbonate (CDB) solution and a sodium acetate buffer prior to the extraction with 1 M HCl (Ruttenberg 1992; Ruttenberg et al. 2009). The CDB and acetate fractions can be discarded and the 1 M HCl fraction purified is described in Chap. 3.
2.5 Plants and Fertilizers
Plants and fertilizers are a source of P into aquatic and terrestrial ecosystems. They are therefore important endmembers in δ18OP studies. Both are relatively easy to extract since concentrations are usually higher than in other samples like soils and thus less material is necessary.
2.5.1 Fertilizers
Phosphorus fertilizers are divided into mineral (e.g. triple superphosphate (TSP)) and organic fertilizers (e.g. manure). In mineral P fertilizers most of the P is present as inorganic P and is readily available. Organic P fertilizers also contain less readily available P forms. It is thus useful to extract especially organic P fertilizers sequentially. 18O-labelled and unlabelled 1 M HCl have to be used for the extraction as 1 M HCl could hydrolyse some organic P and/or condensed phosphate, which can be present especially in organic fertilizers.
Equipment and consumables
-
Standard lab glassware and equipment.
-
Polypropylene bottles.
-
Pestle and mortar.
-
GF/F filters.
Reagents
-
1.
1 M HCl
In a volumetric flask add 82.7 mL concentrated HCl (37%) in 900 mL ultrapure water and make up to 1 L. Prepare two batches of 1 M HCl (18O-labelled and unlabelled HCl solutions). Take a subsample from each batch for the determination of the δ18Ow value of each batch.
Step Fert_1—Sample preparation
-
Homogenize fertilizer with a pestle and mortar. It might be useful to freeze dry organic fertilizer samples to obtain a homogeneous sample.
-
Weigh fertilizer sample into plastic bottle.
Step Fert_2—Water extraction
-
Add ultrapure water and shake overnight.
Step Fert_3—Filtration
-
The next day, filter extract using GF/F filters.
This will yield the readily available P of a fertilizer. For less available P, extract the fertilizer samples also with HCl. Ideally this is done sequentially; however, some fertilizers will almost completely dissolve during the water extraction. In that case, HCl extraction needs to be done on a separate subsample without prior water extraction.
Step Fert_4—HCl extraction
-
Either split the sample remaining on the filter in step Fert_3 into two equal parts or weigh fertilizer sample in duplicate.
-
Add 18O-labelled 1 M HCl to one part and unlabelled 1 M HCl to the second part. Put on the shaker at room temperature at 160 rpm for 16 h.
-
The following day remove the samples from the shaker. Centrifuge for 15 min at 5000 rpm. Filter the supernatant through a GF/F filter. Collect supernatant.
2.5.2 Plants
Plants contain inorganic and organic P. Inorganic P can be leached from plant material and contributes directly to the available P pool in soils. Organic P needs to be mineralized by enzymes first. Especially in low P soils with high biomass production like tropical rainforest, P from plants can contribute significantly to the P nutrition of the rainforest. Therefore, both inorganic and organic P from plants are important pools in δ18OP studies. The main challenge for extracting P from plant material is to stop enzymatic activity during the extraction (Bieleski 1964; Adu-Gyamfi et al. 1990). As enzymatic activity leads to O exchange between phosphate and water, it is essential to choose extraction conditions which minimize it. Trichloroacetic acid (TCA) is efficient in halting enzymatic activity and it has been successfully used to analyse the δ18OP in plant material (Pfahler et al. 2013). TCA could also extract some organic P and therefore 18O-labelled and unlabelled TCA solutions are used, similar to extracting samples with 1 M HCl. After the extraction with TCA, plant material can be extracted with NaOH-EDTA for the determination of the δ18OP of structural P (mainly organic P) (Helfenstein et al. 2018; Tamburini et al. 2018).
Equipment and consumables
-
Standard lab glassware and equipment.
-
Polypropylene bottles, 125 mL.
-
Vacutainers.
-
Scissors.
-
Liquid nitrogen.
-
Pestle and mortar.
-
GF/F filters.
-
Tweezers.
Reagents
-
1.
0.3 M Trichloroacetic acid
Weigh 49.02 g TCA (MW 163.39 g/mol) in a volumetric flask and fill it up to 1000 mL with ultrapure water. Prepare two batches of 0.3 M TCA (18O-labelled and unlabelled TCA solutions). Take a subsample from each batch for the determination of the δ18Ow value of each batch.
-
2.
0.25 M NaOH—0.05 M EDTA
Weigh out 10 g NaOH and 18.612 g EDTA disodium salt and dissolve in 1 L ultrapure water.
Step Pl_1—Sample preparation
-
After sampling, carefully wash the plant material with ultrapure water in case any soil/dust/sediment particles are attached to the plant material. Plot dry with paper towels.
-
Cut out middle vein from plant leaves with scissors, if they are relatively big compared to the whole leaf. This step is necessary only if the δ18OP is used to investigate P cycling within plants.
-
Cut plant material in small pieces using scissor. Put a subsample into a vacutainer for the extraction of plant water (only necessary if the δ18OP is used to investigate P cycling within plants). Freeze the plant material and the subsample as soon as possible at −20 °C and store in the freezer until used.
-
Prior to the extraction, the plant material is homogenized using liquid nitrogen and a pestle and mortar. Grasses could be cut in small pieces using scissors while still frozen.
Step Pl_2—Extraction
-
Weigh between 1 and 2 g of the plant material in duplicates into two 125 mL plastic bottles for the extraction with 18O-labelled and unlabelled 0.3 M TCA.
-
Between 20 and 40 mL 0.3 M TCA is added to each sample.
-
The TCA-plant mixture is then treated with a homogenizer for about 30 s. Wash the probe/generator of the homogenizer with ultrapure water after each sample to avoid cross contamination. The samples are then put on a shaker at 4 °C for 1 h.
Step Pl_3
-
The samples are filtered through GF/F filters and the supernatant is collected.
-
Take a subsample of the supernatant for the determination of the P concentration and purify the remaining extract as soon as possible.
If the δ18OP of the organic P in the plants needs to be analysed, proceed with steps Pl-4 and Pl_5.
Step Pl_4
-
Carefully remove the plant material remaining on the GF/F filter with tweezers and extract it with NaOH-EDTA (see step S_4 for more details).
Step Pl_5
-
Shake sample overnight at room temperature.
-
Filter through GF/F filter and collect supernatant.
-
Take a subsample of the supernatant for the determination of the P concentration and purify the remaining extract as soon as possible.
References
Adu-Gyamfi JJ, Fujita K, Ogata S (1990) Phosphorus fractions in relation to growth in pigeon pea (Cajanus cajan (L) Millsp.) at various levels of P supply. Soil Sci Plant Nutr 36:531–543
Amelung W, Antar P, Kleeberg I et al (2015) The delta O-18 signatures of HCl-extractable soil phosphates: methodological challenges and evidence of the cycling of biological P in arable soil. Eur J Soil Sci 66:965–972. https://doi.org/10.1111/ejss.12288
Arai Y, Sparks DL (2007) Phosphate reaction dynamics in soils and soil components: a multiscale approach. In: Advances. Academic Press, pp 135–179
Bieleski RL (1964) The problem of halting enzyme action when extracting plant tissues. Anal Biochem 9:431–442. https://doi.org/10.1016/0003-2697(64)90204-0
Condron LM, Newman S (2011) Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J Soils Sediments 830–840
Directive WF (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off J Eur Commun 22:2000
Ellison ME, Brett MT (2006) Particulate phosphorus bioavailability as a function of stream flow and land cover. Water Res 40:1258–1268. https://doi.org/10.1016/j.watres.2006.01.016
Filippelli GM (2008) The global phosphorus cycle: past, present, and future. Elements 4:89–95
Granger SJ, Harris P, Peukert S et al (2017) Phosphate stable oxygen isotope variability within a temperate agricultural soil. Geoderma 285:64–75. https://doi.org/10.1016/j.geoderma.2016.09.020
Granger SJ, Heaton THE, Pfahler V et al (2017) The oxygen isotopic composition of phosphate in river water and its potential sources in the Upper River Taw catchment, UK. Sci Total Environ 574:680–690. https://doi.org/10.1016/j.scitotenv.2016.09.007
Harrison AF (1987) Soil organic phosphorus: a review of world literature. Cab International Wallingford
Helfenstein J, Tamburini F, von Sperber C et al (2018) Combining spectroscopic and isotopic techniques gives a dynamic view of phosphorus cycling in soil. Nat Commun 9:3226. https://doi.org/10.1038/s41467-018-05731-2
Kouno K, Tuchiya Y, Ando T (1995) Measurement of soil microbial biomass phosphorus by an anion exchange membrane method. Soil Biol Biochem 27:1353–1357. https://doi.org/10.1016/0038-0717(95)00057-L
Nawara S, Van Dael T, Merckx R, et al (2017) A comparison of soil tests for available phosphorus in long-term field experiments in Europe. Eur J Soil Sci. https://doi.org/10.1111/ejss.12486
Nisbeth CS, Tamburini F, Kidmose J et al (2019) Analysis of oxygen isotopes of inorganic phosphate (δ18Op) in freshwater: a detailed method description. Hydrol Earth Syst Sci Discuss 2019:1–18. https://doi.org/10.5194/hess-2019-469
Pfahler V, Dürr-Auster T, Tamburini F et al (2013) 18O enrichment in phosphorus pools extracted from soybean leaves. New Phytol 197:186–193. https://doi.org/10.1111/j.1469-8137.2012.04379.x
Reynolds CS, Davies PS (2001) Sources and bioavailability of phosphorus fractions in freshwaters: a British perspective. Biol Rev 76:27–64. https://doi.org/10.1111/j.1469-185X.2000.tb00058.x
Ruttenberg KC (1992) Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol Oceanogr 37:1460–1482
Ruttenberg KC, Ogawa NO, Tamburini F et al (2009) Improved, high-throughput approach for phosphorus speciation in natural sediments via the SEDEX sequential extraction method. Limnol Oceanogr Methods. https://doi.org/10.4319/lom.2009.7.319
Schindler DW, Carpenter SR, Chapra SC et al (2016) Reducing phosphorus to Curb Lake Eutrophication is a success. Environ Sci Technol. https://doi.org/10.1021/acs.est.6b02204
Shen J, Yuan L, Zhang J et al (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156:997–1005. https://doi.org/10.1104/pp.111.175232
Tamburini F, Bernasconi SM, Angert A et al (2010) A method for the analysis of the δ18OP of inorganic phosphate extracted from soils with HCl. Eur J Soil Sci 61:1025–1032. https://doi.org/10.1111/j.1365-2389.2010.01290.x
Tamburini F, Pfahler V, Bünemann EK et al (2012) Oxygen isotopes unravel the role of microorganisms in phosphate cycling in soils. Environ Sci Technol 46:5956–5962. https://doi.org/10.1021/es300311h
Tamburini F, Pistocchi C, Helfenstein J, Frossard E (2018) A method to analyse the isotopic composition of oxygen associated with organic phosphorus in soil and plant material. Eur J Soil Sci 69:816–826. https://doi.org/10.1111/ejss.12693
Weiner T, Mazeh S, Tamburini F et al (2011) A method for analyzing the δ18OP of resin-extractable soil inorganic phosphate. Rapid Commun Mass Spectrom 25:624–628. https://doi.org/10.1002/rcm.4899
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Glossary
- 18O-labelled/unlabelled solutions
-
A set of 18O-labelled and unlabelled solutions is used whenever P is extracted with acids. A small amount of 18O-enriched water is added to the 18O-labelled solution (prepared with ultrapure water), whereas only ultrapure water is used for the unlabelled solution.
- δ 18 O
-
The oxygen isotope ratio is conventionally given in the delta notation: δ18O = (Rsample/Rstandard) − 1, where Rsample is the 18O/16O ratio of a sample and Rstandard is the 18O/16O ratio of the Vienna Standard Mean Ocean Water (V-SMOW). δ18OP is the δ18O of oxygen bound to P. δ18OW is the δ18O of water.
Rights and permissions
The opinions expressed in this chapter are those of the author(s) and do not necessarily reflect the views of the [NameOfOrganization], its Board of Directors, or the countries they represent
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 3.0 IGO license (http://creativecommons.org/licenses/by/3.0/igo/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the [NameOfOrganization], provide a link to the Creative Commons license and indicate if changes were made.
Any dispute related to the use of the works of the [NameOfOrganization] that cannot be settled amicably shall be submitted to arbitration pursuant to the UNCITRAL rules. The use of the [NameOfOrganization]'s name for any purpose other than for attribution, and the use of the [NameOfOrganization]'s logo, shall be subject to a separate written license agreement between the [NameOfOrganization] and the user and is not authorized as part of this CC-IGO license. Note that the link provided above includes additional terms and conditions of the license.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Pfahler, V., Adu-Gyamfi, J., O’Connell, D., Tamburini, F. (2022). Extraction Protocol. In: Adu-Gyamfi, J., Pfahler, V. (eds) Oxygen Isotopes of Inorganic Phosphate in Environmental Samples. Springer, Cham. https://doi.org/10.1007/978-3-030-97497-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-97497-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-97496-1
Online ISBN: 978-3-030-97497-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)