Abstract
In this chapter we introduce the climate signal in stable isotope tree-ring records, with the emphasis on temperate forests. The development of the subdiscipline is recapped followed by an exploration of isotope dendroclimatic records by geography and, broadly, by isotopic species. Whilst there are still questions to be answered around signal strength and age-related effects in different environments and in different species, the proxy is now contributing to palaeoclimatology in a far greater way than in the days of the first hints of ‘isotope tree thermometers’. We include two summary tables. Table 19.1 exemplifies the range of climate information available from stable carbon isotope time series and Table 19.2 explores oxygen isotope proxy signals. Due to the greater complexity seen in stable carbon isotope interpretations we explore response groupings with example references given for each category of proxy response. Finally, we summarize the state of the art in isotope dendroclimatology and discuss possible future directions.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Our understanding of the climate variability of the last two thousand years has been significantly aided by large, network tree-ring reconstructions, the dendroclimatic contribution to which is based almost exclusively on ring width and density data (e.g. PAGES 2k Consortium 2013, 2017; Neukom et al. 2019; Esper et al. 2014; Luterbacher et al. 2016). However, the increase in proxy data availability, for example of temperature reconstructions (Wilson et al. 2016; Mann et al. 1999; Helama et al. 2002; McCarroll et al. 2013; Linderholm et al. 2014; Dorado-Liñán et al. 2012, 2015; Klippel et al. 2019) has been accompanied by an understanding of the vital need for more information, at greater spatial resolutions, for more regions and including more variables than warm season temperatures (Smerdon and Pollack 2016; Gouirand et al. 2008; Frank et al. 2010). There is a particular need for additional tree-ring data from areas spatially underrepresented in climate proxy databases, in order to capture the regional signature of past climate variability (Smerdon and Pollack 2016). A key drive is an emerging picture of significant differences in the magnitude and dynamics of forced and internal climate variability which requires stronger regional detail through high-resolution regional reconstructions of multiple climate variables (e.g. Gagen et al. 2016; Smerdon and pollack 2016; Young et al. 2019).
Tree-ring width and density-based dendroclimatology has, in the last ten years, coalesced around four key efforts. To: (a) improve reconstructions of large scale changes to explore the hemispheric temperature signature of climate change, (b) to expand the geographic reach of regional temperature reconstructions to explore the dynamics of forced and internal variability, (c) to increase the breadth and depth of our understanding of the temporal variations in proxy records and the statistical fingerprints of different reconstruction methods and, (d) to expand the non-temperature (e.g. hydroclimate) potential of the tree-ring archive. Stable isotope measurements from tree rings, both annual and non-annual, contain valuable climatic, physiological and environmental information that can contribute to these research drives (McCarroll and Loader 2004). Here we review the research frontier in stable isotope dendroclimatology and explore the subject’s contribution to palaeoclimate science.
1.1 The Development of Stable Isotope Measurements from Tree Rings
A cluster of studies carried out in the 1970s (Libby and Pandolfi 1974; Epstein and Yapp 1976; Libby et al. 1976; Yapp and Epstein 1977), began the process of exploring what environmental information could be accessed by measuring isotopic variations in tree rings (see also Chap. 1). These early studies explored a range of isotope species and, whilst the methods were evolving, the earliest and most critical studies were of relevance to the exploration of both the water and carbon isotopes. Early tree-ring isotope time series were based largely on samples from a single tree, which required a huge time and monetary cost to produce and used a variety of measurement methodologies, analyzing pools of years together and often not extracting samples to cellulose before measurement. However, without the quantitative knowledge of how plant functions fractionate isotopes as they moved through the metabolic pathways of photosynthesis, and physically around the plant, and how sensitive these fractionations are to temperature changes, the tantalizing evidence for a new climate proxy was effectively stalled until the underlying fractionation theoretical frameworks were in place. When this happened in the 1980s and 1990s (e.g. Farquhar et al. 1982, 1989b; Francey and Farquhar 1982; Marshall and Monserud 1996) we were able to state unequivocally that stable isotope ratios measured from tree rings provided a usable environmental archive for the first time (see Chaps. 9, 10and 13).
Early isotopic investigations on blocks of whole wood showed relatively little similarity in common isotopic signal (see Craig 1954; Farmer and Baxter 1974; Pearman et al. 1976; Libby and Pandolfi 1974). Whole wood contains varying amounts of resins and waxes, as well as lignin and cellulose (see Chap. 5). Without extracting and separating such components prior to analysis, it is difficult to obtain a common environmental signal not masked by noise from the isotopically heterogeneous resins, waxes and lignin. This problem was worked through by Epstein and Yapp (1977) and Wilson and Grinsted (1977). From this point onwards, it gradually became the working norm to extract tree-ring samples from wood to cellulose (or cellulose nitrate for δD in order to ensure that only non-exchangeable hydrogen is analyzed (Epstein and Krishnamurthy 1990) and Chap. 11), prior to the analysis of stable isotope ratios (Bradley 1985). Wilson and Grinsted (1977) established the methodology for cellulose extraction, based on the works of Green (1963), the so called ‘Jayme-Wise’ method. It is this method, or the alternative Brendel method (Brendel et al. 2000) that are generally used to extract whole wood tree-ring samples to cellulose still, often in fast batch processing systems to reduce lab time (Loader et al. 1997). Andreu-Hayles et al. (2019) summarizes the state of the art in isotope dendroclimatology preparation options (see also Chap. 5).
There is evidence that, with non-resinous species in particular, using whole wood can increase the efficiency of sample processing and still retain common signal coherence (Loader et al. 2003). The use of whole wood in isotopic tree-ring studies was encouraged by the revelation that there is a high degree of coherence between δ13C derived from cellulose and that from (resin-extracted) whole wood, with an offset between 1.5 and 3‰ (e.g. Loader et al. 2003) with variability between tree taxa. As the majority of climate reconstructions derived from stable isotope time series do not use absolute isotopic values, such an offset does not have consequences for reconstruction accuracy, and analyzing resin-extracted whole wood can heavily reduce lab time. A suite of studies has now developed climate reconstructions from stable carbon isotope series based on whole wood measurements (e.g. Verheyden et al. 2005; Harlow et al. 2006).
Multiyear and multi-tree pooling methodologies have once again become popular, following increased knowledge about the between-tree levels of isotopic variability for commonly samples tree species (see Chaps. 4 and 6). These methods considerably increase time efficiency and reduce costs while providing comparable results to single tree studies, if certain conditions are met (Dorado-Liñán et al. 2011; Woodley et al. 2012).
For several decades after the palaeoclimatic potential of the stable isotope tree-ring proxies was first explored following the establishment of the models describing isotopic fractionation in C3 plants (e.g. Burk and Stuiver 1981; Farquhar et al. 1982), the literature remained dominated by single site studies with results from a few tree cores, with relatively low sample replication (Ramesh et al. 1986; Buhay and Edwards 1995; Anderson et al. 1998; Gagen et al. 2004, 2006; McCarroll and Pawellek 1998, 2001). Such studies offered exciting evidence of a strong, new climate proxy (McCarroll and Loader 2004) but its exploitation was limited by the time and costs associated with mass spectrometric analysis. The 2000s were thus marked by methodology developments that could be explored with low sample numbers, rather than large replication climate reconstructions from stable isotope dendroclimatology. There were hints of relatively simple age-related trends in carbon isotope time series (Gagen et al. 2007, 2008) and, eventually, in oxygen (Duffy et al. 2017, 2019; Büntgen et al. 2020) as compared to ring width proxies. Aspects remain to be explored, however, because there are mixed results from different species and sites (Treydte et al. 2006; Esper et al. 2010; Brienen et al. 2017). Non-climatic trends in multi-tree stable carbon isotope time series also revealed information on changing plant carbon water relationships (Gagen et al. 2011a; Andreu-Hayles et al. 2011) useful to future climate projections where an accurate estimate of physiological forcing from the biosphere is needed. Methods for pre-reconstruction corrections for the effects of the changing stable isotopic composition of atmospheric CO2 (McCarroll and Loader 2004) and for the bulk rise in atmospheric CO2 amount (McCarroll et al. 2009) were developed (see Chaps. 17 and 25).
Advances in analysis increased the sample replication that could be achieved in a reasonably equipped stable isotope dendroclimatology lab, by an order of magnitude (see Chap. 7) (Loader et al. 1997, 2017; Brendel et al. 2000; Gagen et al. 2012). By the 2010s isotope dendroclimatic reconstructions often had sample replications equivalent to studies using ring width or density data (Gagen et al. 2011b; Young et al. 2012a, b, c; Loader et al. 2013). However, the time and cost of producing these climate reconstructions, using stable isotope mass spectrometry, was astronomical in comparison to equivalent growth proxy times series based on ring width measurements. Moreover, the general dependence on a laboratory process that involves physically cutting each ring to be analyzed has limited the exploitation of the stable isotope tree-ring proxies at a time when they have much to contribute.
Whilst the first tree-ring network analyses using stable isotope data from multiple sites are emerging (e.g. Szejner et al. 2016; Young et al. 2019), exploitation of the full potential of the proxy is still held back by the maximum speed of mass spectrometry, as the primary measurement method. At present a high-end stable isotope dendroclimatology lab uses some version of the following standard methodologies (see McCarroll and Loader 2004) to prepare samples for, and measure, stable isotopes of carbon, oxygen and hydrogen.
-
1.
Dating via standard methods generally (see Loader et al. 2019 for an exception).
-
2.
Physically isolating each ring, or a portion of each ring (e.g. latewood), by cutting.
-
3.
Performing a variety of chemical extractions on individual samples, ranging from simple resin removal to full extraction to alpha-cellulose.
-
4.
Drain, freeze dry, weigh out and individually ‘wrap’ samples.
-
5.
Perform mass spectrometric analysis.
There is a pressing need to increase the speed, and reduce the cost, at which we can produce stable isotope tree-ring time series in order to reach the sample replication necessary for statistically high-quality networks of climate reconstructions.
The analysis method noted above typically achieves measurement precisions around 0.15‰ for δ13C and 0.3‰ for δ18O (σn−1 n = 10) (Loader et al. 2016). In terms of increasing sampling speeds, at the very best step 1 is carried out by an ablating laser (Loader et al. 2017) and step 2 skipped in the analysis of non-resinous tree species. However, a more typical analysis time, from start to finish, is in the region of one week per 100 samples. Whilst the laborious nature of measuring stable isotopes by mass spectrometry is a limiting factor, there are other limitations to the reliance on this technique. Mass spectrometry typically requires a sample of the ring equivalent to approximately 2–300 µg dry weight of alpha cellulose (Rinne et al. 2005). This can require a whole wood cut of several cubic mm of wood from each ring.
Stable isotope measurements are routinely reported using the delta notation (‰). This notation gives the ratio of the heavier to the lighter stable isotope relative to an isotope standard, in parts per mille. V-SMOW (Vienna Standard Mean Oceanic Water) is used as a standard for stable hydrogen and stable oxygen isotope measurements in tree-rings (δ2H and δ18O) and V-PDB (Vienna Pee Dee Belemnite) as the standard for δ13C analyses (see Chap. 8 for a full discussion of the relevant isotopic notations and standards).
2 Dendroclimatic Information from Stable Carbon isotope Time Series
The use of δ13C as a tree-ring proxy is based on a mechanistic framework for the leaf-level fractionations of 13C/12C, which allows us to understand the relationships between climate and variations in the stable carbon isotope ratio of photosynthate. In Table 19.1 we give an overview of the range of climate information, which can be derived from stable carbon isotope time series. In plants, discrimination against the heavier carbon isotope at the point that ambient CO2 enters the leaf, and again upon assimilation by the plant, depends on the internal leaf concentration of CO2 (ci) and on atmospheric CO2 concentration (ca) expressed as the ratio ci/ca (Farquhar et al. 1982, 1989b). Chapter 9 gives a full discussion of these fractionation and discrimination processes. In trees growing in climates which are not water limited, ci is regulated primarily by photosynthetic rate, controlled by variations in temperature and sunlight. In climates that are water limited, ci is more often regulated by stomatal conductance to CO2, controlled by antecedent precipitation and relative humidity (Gebrekirstos et al. 2009; Brienen et al. 2016). Thus, at sites where the fundamental laws of dendroclimatology apply (Fritts 1976) stable carbon isotope measurements in tree rings, have successfully been used to reconstruct summer sunshine (e.g. Young et al. 2019) temperature (e.g. Payomrat et al. 2018) as well as hydroclimate proxies (e.g. Liu et al.2004; Roden and Ehleringer 2007).
Dendroclimatic reconstructions based on stable carbon isotopes still represent a small fraction compared to those based on more readily measured variables such as tree-ring width and maximum latewood density. Although they have a higher production cost, stable carbon isotope measurements from tree rings also offer a record of changes in plant carbon–water relationships via Intrinsic Water Use Efficiency (iWUE) as it responds to changes in atmospheric CO2 (i.e., Leavitt and Long 1986, 1988; Granda et al. 2017). We can summarize the proxy potential of stable isotope dendroclimatology:
-
Stable carbon isotope tree-ring time series record the interaction between photosynthetic rate and stomatal conductance. As such they are not a growth proxy, their variability is sensitive to parameters including warm season temperature and sunlight as well as hydroclimate e.g. summer cloud cover at moist, high latitude sites.
-
Age related trends in stable carbon isotope tree-ring time series are less complicated than in growth proxy series, and their impacts on signal strength are easier to mitigate via simple mathematical standardizations and juvenile cut-off points.
-
The capture of additional information in stable carbon isotope series related to changes in plant carbon–water relationships through time (e.g. Intrinsic Water Use Efficiency, discussed below and in Chap. 17), which add to the range of paleoenvironmental information available from tree-ring records.
Carbon isotopes in tree rings have some advantages of interpretation, in comparison to growth proxies, because the fractionation mechanisms controlling carbon isotope variability are known (Farquhar et al. 1982, 1989), the signal-to-noise ratio between trees is high (McCarroll and Pawellek 1998), they can preserve high-to-low frequency common climate signals and the number of samples required to build a statistically robust chronology is often lower than for other tree-ring proxies (Leavitt and Long 1986; McCarroll and Loader 2004). The proxy is an integration of (1) the stable isotope composition of atmospheric carbon dioxide, (2) the relevant regulation rates within trees (photosynthetic and stomatal conductance rates), and (3) the environmental variables, which influence those rates, including temperature, sunlight, relative humidity and precipitation (McCarroll and Loader 2004). All climate reconstructions based on carbon isotope measurements from tree rings are built upon the theory of how carbon dioxide is fractionated at the entrance to the stomatal pores and in the C3 photosynthetic pathway (Farquhar et al. 1982, 1989b). A suite of environmental variables influences these fractionation set points by exerting control over two rates – stomatal conductance to CO2 (generally noted as gs) and photosynthetic assimilation rate (generally noted as AN). Sites at which stomatal conductance to CO2 dominates the stable carbon isotope signal are usually restricted to dry, or moisture stressed, forests. In non-moisture-limited settings carbon isotope discrimination is generally controlled by photosynthetic rate, driven by temperature and/or sunlight (Farquhar et al. 1989a; McCarroll and Loader 2004). Unlike ring-width and density tree-ring proxies, carbon isotope series contain simpler age-related trends and, at some sites, do not require detrending, allowing low-frequency variability to be fully preserved in the final reconstruction (McCarroll and Loader 2004; Gagen et al. 2007). Comparisons between paleoclimate reconstructions based on ring width and carbon isotopes in some cases reveal similar (Esper et al. 2015; Bale et al. 2011) or even larger (e.g., Lavergne et al. 2018) variability in the carbon isotope series and increased signal capture at variable frequencies. However, this is not the case for all species and all sites (Esper et al. 2010, 2015; Helama et al. 2015; Brienen et al. 2017).
At sites where significant age-related trends do exist in stable carbon isotopes, tree-ring density series, or their digital equivalent (McCarroll et al. 2002; Campbell et al. 2011; Wilson et al. 2014) are more suited to the reconstruction of past temperature variations because of their lower analysis costs. However, stable carbon isotopes offer additional advantages due to their increased frequency capture and as they appear be less prone to the ‘divergence problem’ (i.e., the loss of climate sensitivity in growth proxies, during recent decades, Jacoby and D’Arrigo 1995; Savard and Daux 2020).
2.1 Dendroclimatic Information from Stable Carbon Isotope Time Series in Temperate Regions
In temperate regions and high latitude forests, where adequate moisture is usually available throughout the growing season, stable carbon isotopes from tree rings have been successfully used to reconstruct past changes in summer sunshine and solar radiation (Hafner et al. 2014; Dorado-Liñan et al. 2016) and summer temperatures, further south into Europe (Lipp et al. 1991; Esper et al. 2015), Asia (Liu et al. 2014; Treydte et al. 2009) and South America (Lavergne et al. 2018). Temperature reconstructions at high latitude sites have traditionally been assessed based on tree-ring width and maximum density (Wilson et al. 2016, St. George and Esper 2019 and references therein). Heinrich et al. (2013) expanded the focus of European carbon isotope-based reconstructions of temperature to Turkey with an 850-year reconstructions of cool season (winter-to-spring) temperature, consistent with the atmospheric circulation patterns governing the cool season climatology of the region.
At higher latitude, moist, sites, stable carbon isotopes have proven a strong and reliable proxy for cloudiness and parameters related to summer sunshine hours (e.g., Gagen et al. 2011b; Loader et al. 2013; Young et al. 2010). However, the extension of successful reconstructions of sunshine from stable carbon isotopes into drier regions, below the high latitudes, also reflects the strength of the relationship between the variable and sunshine. Reconstructions include sunshine hours in the Alps (Hafner et al. 2014) and surface solar radiation in mountainous area of Spain (Dorado-Liñán et al. 2016). The strength of the carbon isotope ‘palaeocloud’ proxy is unsurprising given that the action of rubisco is far more strongly controlled by photon flux (sunlight) than temperature (Farquhar et al. 1982). At more mesic sites related climatic variables share influence over the carbon isotope signal and temperature can be reconstructed via its co variation with summer sunshine, solar radiation or vapor pressure deficit (McCarroll and Loader 2004; Treydte et al.2009; Dorado-Liñán et al. 2016). Care must be taken not to extend this assumption to areas where summer sunshine and temperatures do not co vary, particularly at lower frequencies (e.g. Young et al. 2019). Emerging reconstructions of past cloudiness and summer sunshine are of crucial importance in current climate research since it may allow for an integrated assessment of the cloud-climate feedback, an important cause of the large spread in climate sensitivity between climate models (Dessler 2010; Flato et al. 2013).
At sites where summer moisture stress restricts stomatal conductance to CO2, or where moisture stress is involved in the covariation of multiple climate variables (precipitation, temperature, cloudiness etc.) carbon isotope variability can be used to reconstruct summer precipitation and drought, in the USA (Leavitt and Long 1989a, b; Bale et al. 2011), and in central Europe (Kress et al. 2010) as well as spring relative humidity in Asia (Liu et al. 2018). Andreu-Hayles et al. (2017) used carbon isotopes to explore and corroborate extreme precipitation events in spring and summer in the Iberian Peninsula. This study also explored non stationarity in carbon isotopes, in this case shifts through time in the dominant climatic limiting factor (from control of carbon variability by spring precipitation to summer precipitation). Beyond the reconstruction of specific climate parameters, carbon isotope variability in trees has also been used to reconstruct global modes of atmospheric circulation such as ENSO (Bale et al. 2011; Liu et al. 2014; Lavergne et al. 2018).
The carbon isotope proxy, in the temperate latitudes where tree rings excel in climate reconstructions, has clear potential to develop the continental scale network reconstructions that now dominate efforts with growth proxy time series (D’Arrigo et al. 2006a; Schneider et al. 2015; Stoffel et al. 2015; Wilson et al. 2016). Leavitt and Long (1989b) represent one such attempt, to reconstruct regional July drought variability across the north American southwest using a stable carbon isotope tree-ring network. To date, however, the regional networks of carbon isotopes chronologies, spanning several centuries, that do exist across North America and Europe, have predominantly been used to track changes in plant physiological responses such as Intrinsic Water Use Efficiency (i.e., Saurer et al. 2014; Frank et al. 2015; Shestakova et al. 2019).
As the palaeoclimate potential of well-replicated carbon isotope series was fully explored for the first time in the high latitudes (e.g. McCarroll and Pawellek 1998, 2001) the discipline has tended to focus on the conifer species, which dominate the northern forest zones. Whilst there are many species used to explore stable carbon isotope proxies in temperate forest, there are some taxa that have contributed most significantly to palaeoclimate products. These are Fagus, Picea, Quercus, Abies, Larix and Pinus (e.g. Saurer et al. 1997; Skomarkova et al. 2006; McCarroll and Loader 2004; Gagen et al. 2007; Churakova et al. 2019; Esper et al. 2015; Ferrio and Voltas 2005; Hafner et al. 2014; Helama et al. 2015; Holzkämper et al. 2012; Kirdyanov et al. 2008).
Stable carbon isotopes in tree rings from temperate regions offer an incomparable source of information to reconstruct climate variables that are unlikely to be accessible using other proxy records, such as summer cloudiness and non-age detrended temperature reconstructions, as well as having the potential to enhance our picture of climate variability in the southern hemisphere. The dominance of conifers in our understanding of carbon isotope variability should be useful as the discipline starts to focus on the underrepresented southern hemisphere, where preliminary results in conifers have shown the potential of carbon isotopes (Gebrekirstos et al. 2009).
2.2 Dendroclimatic Information from Stable Carbon Isotope Time Series in Northern and Boreal Regions
The limitation of photosynthetic assimilation rate by temperature and/or sunlight is common across northern latitudinal and boreal forests (see also Chap. 20). In those regions, moisture limitation is not as common as in lower latitudes and carbon isotope variability in trees is found to be driven primarily by the limitation of photosynthetic rate by photon flux. The capacity of stable carbon isotopes in Fennoscandian conifer tree rings to track historical changes in sunlight and, where it covaries with cloudiness, summer temperatures too, has been consistently demonstrated (e.g. Gagen et al. 2011a; Loader et al. 2013; Young et al. 2010, 2019). Those reconstructions have not only described past changes in sunlight, but also revealed changes in atmospheric circulation patterns (Young et al. 2012b, 2019; Loader et al. 2013) providing the first attempt to better understand the largest source of uncertainty in modelling future changes in climate: the cloud-climate feedback (Dessler 2010; Flato et al. 2013; Boucher et al. 2013). Whilst the relationship between summer cloud and temperate was revealed, across the Fennoscandian boreal zone, as negative at interannual time scales, analysis of millennial length temperature and summer cloud cover reconstructions revealed the relationship to be positive at decadal time scales (Young et al. 2012b, 2019). Thus, warmer periods in the past were associated with increases in cloud cover at decadal time-scales, linked to the position of the polar vortex and the variability of the Arctic Oscillation (Young et al. 2012b, 2019; Loader et al. 2013). At these sites, cloudiness is likely modulating local summertime temperature at inter-annual time scales, but at longer timescales, cloud cover may respond to large-scale multidecadal temperature changes (Dessler 2010). Thus, assessments of the relationship between cloud cover and temperature at both hemispherical and millennial time-scales are needed to provide a more complete picture, spatially and temporally, of the cloud-climate feedback. In this context, carbon isotope records derived from subfossil wood (e.g. Helama et al. 2018) offer a promising opportunity for extending the Fennoscandia summer cloud reconstructions back in time to tease apart the high latitude cloud-temperature feedback further. This endeavor becomes more pressing the more we learn about how significantly clouds are involved in moderating or enhancing greenhouse gas induced warming in the past (Zhu et al. 2019).
Stable carbon isotopes in combination with other tree-ring variables have also been used to estimate July–August mean temperature in the Russian Altai for the last 200 years (Sidorova et al. 2013) and Canadian Boreal forests for the last millennium (Gennaretti et al. 2017). Bégin et al. (2015) reconstructed 200 years of June–August maximum temperature in northeast Canada by combining stable carbon and oxygen time series. Edwards et al. (2008) reconstructed growing season relative humidity for the last thousand years based on carbon isotopes from living and subfossil conifer species in boreal western Canada. In the highest Arctic environments methods have also been pioneered to explore carbon isotope variability in the annual stem growth increments of Arctic Bell Heather Cassiope tetragona (Rayback and Henry 2006) and Salix species (Schifman et al. 2012), with correlations to growing season precipitation in these strongly climate limited shrub species.
In addition to complexity of decoding the prime driver of isotopic fractionation in different climatic settings, carbon isotope records also experience common tree-ring proxy pitfalls such as non-linearities (Schleser et al. 1999) and changes in climate sensitivity through time (Daux et al. 2011; Andreu-Hayles et al. 2017). Non-linear responses to climate, and shifts or loss, of climate sensitivity are well-known issues in tree-ring growth, particularly at low latitude and high-altitude sites (Esper and Frank 2009; St. George and Esper 2019). Isotopic fractionation is an active mechanism that involves active physiological adjustments by the tree to changing environmental conditions. Non-linear responses due to shifts in the acclimation strategy to new conditions have been described in old boreal trees (Giguère-Croteaua et al. 2019) as water use efficiency changes in response to rising CO2 (see Chap. 17).
The regularity with which carbon isotope series from moist high latitude sites are interpreted as temperature proxies may be partly related to the scarcity of reliable instrumental records of sunlight and cloud cover. These parameters are sometimes not even considered as potential drivers of isotopic fractionation (e.g., Kirdyanov et al. 2008; Naulier et al. 2014). Where cloud data is available, the often-limited length of those records can prevent testing of the co variance of summer cloudiness with temperature on all time scales or the stability of the climate signal through time leading to assumptions about the linearity of the climate-carbon isotope relationship through time (e.g., Helama et al. 2018; Churakova et al. 2019). As a result, carbon isotopes have been interpreted as palaeotemperature records where temperature is in fact not the primary driver of carbon fractionation at the site (e.g. Gagen et al.2007). Where temperature and cloud cover co vary in the summer, which should be explored in the instrumental record wherever possible before regression-based reconstruction of either parameter from carbon isotope records is attempted (see Young et al. 2019 for an example), the reconstruction of one variable from the other is not necessarily problematic. Where cloud cover and temperature do not co vary in a region a divergence between the temperature reconstruction from carbon and independent temperature reconstructions will appear back in time (e.g. Gagen et al.2007). The identification of periods in which temperature and sunshine or cloud cover may have diverged might be the key to unequivocally identify the primary driver of carbon fractionation at such sites, or its non-stationarity through time. In this context, the atmospheric alteration caused by large volcanic eruptions may cause a large-scale cooling, which is detectable in temperature sensitive tree-ring records (D’Arrigo et al. 2013; Stoffel et al. 2015) but may not affect the photosynthetic capacity of the tree, which translates in non-significant changes in carbon isotopes (Gu et al. 2003; Battipaglia et al. 2007; Dorado-Liñán et al. 2016).
Tree-ring stable carbon isotopes in boreal forests offer perhaps the best opportunity to accurately constrain one of the main sources of uncertainty in the simulation of climate models: the cloud-climate feedback. By focusing on unequivocally identifying the main limiting factor of carbon fractionation and ensuring the stability of the climate signal, it should be possible to assess hemispheric relationships and feedbacks between cloud cover and temperature and vapor pressure deficit (VPD) providing critical information for the climate modelling community.
2.3 Dendroclimatic Information from Stable Carbon Isotope Time Series in the Tropics
Tropical forests hold about half of the planet’s total terrestrial carbon biomass (Pan et al. 2011; Hunter et al. 2013). They home ecosystems spanning from humid to sub humid to dry (Fig. 19.1). They are considered one of the major environments under threat from habitat loss and climate change (Holm et al. 2017) and the drive to find ways to develop tree-based climate proxies for the tropical forest regions of the world has a long history (Worbes 2002; Stahle 1999). Carbon isotope studies in tropical trees have much to contribute to tropical environmental science because of debates around the future behavior of the tropical forest carbon sink (Körner 2009; Lloyd and Farquhar 2008), and because proxy climate records for the tropics are still profoundly limited (see also Chap. 22). The scarcity of long instrumental climate records for the tropics, and the importance of the tropics in global climate dynamics (Chiang 2009), drives the motivation to expand the field of tropical isotope dendroclimatology. Settling questions around carbon dynamics in tropical trees requires a more detailed understanding of tropical plant carbon water dynamics (e.g. Loader et al. 2011) as well as detailed analyses of physiological and environmental drivers of tropical wood formation at daily, seasonal and multi-annual timescales (Steppe et al. 2015, Chap. 22). Whilst studies on tropical wood remain scarce, compared to temperate and high-latitude regions, there is a common agreement about the need to integrate methodologies to explore tropical isotope dendroclimatology (Sass-Klaassen et al. 2016; Van der Sleen et al. 2017).
Ecosystems included in the tropical area and pictures of wood with A) poorly visible rings , B) suppressed rings , C) wedging rings : D) false (empty triangle) and real rings. Examples are given for lowland, mid (forested) and upper (tree line) elevations.
Tropical dendrochronology is a relatively new discipline in and of itself, because of the difficulties of cross dating tropical wood, caused by the lack of simple warm-cool seasonality in the tropics. Cross dating tree rings visually is, at best, challenging and in ‘ever wet’ areas of the humid tropics impossible due to the lack of the climate seasonality needed to form an annual growth cycle in trees. Early studies focused on seasonally dry regions where the climate was capable of forcing a ring boundary. Coster (1927) and Berlage (1931) described annual growth rings in teak trees from tropical Indonesia, because the dry season provoked the cambial dormancy necessary for annual ring formation. This has been found to be the case across the seasonally dry tropics, including Australia (e.g. Buckley et al. 2010; D'Arrigo et al. 2006b; Heinrich et al. 2008; Pumijumnong and Eckstein 2011), tropical Africa (e.g. Therrell et al. 2006; Trouet et al. 2010) and the American tropics (e.g. Biondi 2001; Worbes 1999). Elsewhere ring boundaries have also been found to be caused by cambial activity cessation in flood periods within seasonally flooded Amazonian forests (e.g. Schöngart et al. 2002, 2004) or during salinity fluctuations in mangrove forests (e.g. Robert et al. 2011; Verheyden et al. 2004b). Whilst a number of tropical trees do grow annual rings, which can be cross dated (see Worbes 2002), there are many other problems facing the tropical dendrochronologists once they have found a species that at least grows annual rings. Indistinct ring boundaries are common, and many of the above studies mention low correlations with climate when calibration is attempted (generally with the short instrumental climate data typical for much of the global south). Tropical isotope dendroclimatology (Evans and Schrag 2004) emerged as a drive to resolve seasonality in tropical trees that lack visible tree-ring-boundaries by using seasonal stable isotope variability (e.g. Poussart and Schrag 2005).
An early endeavor was to utilize stable isotope variability to define the ring boundary in tropical trees (Leavitt and Long 1991). In Thailand Poussart and Schrag (2005) and later Ohashi et al. (2009) applied a version of this method seeking to use seasonal peaks and troughs in dendrochemical variables to identify non-visible tree ring boundaries isotopically. However, Poussart and Schrag (2005) seminal study achieving dendro isotope dating in the tropics has not been widely replicated. Tropical oxygen isotope dendroclimatology, or the combination of both isotopes (carbon and oxygen), has also been used to facilitate boundary identification in Costa Rica (Evans and Schrag 2004; Anchukaitis et al. 2008), Kenya (Verheyden et al. 2004a), Central Guyana (Pons and Helle 2011) and Brazil (Ohashi et al. 2015). These methods take advantage of the fact that in tropical areas where temperature and photoperiod are more or less constant through the year (Fromm 2013) a driver for periodic wood formation can be identified through changes in precipitation seasonality (Worbes and Junk 1999) or seasonal shifts in isotopic source water signal (Anchukaitis et al. 2008).
Several studies also reveal strong correlations between stable carbon isotopes and climate in seasonal tropical trees. In tropical regions with a pronounced dry season, the limitation of stomatal conductance by moisture stress gives rise to robust climate variability in tree-rings that is effectively a seasonal drought signal (Gebrekirstos et al. 2009; Brienen et al. 2016), providing the growth rings can be dated by traditional means. The signal is generally thought to be linked to water availability and precipitation amount provoking stomatal limitation of the internal partial pressure of carbon dioxide (Leavitt and Long 1991; Schubert and Timmermann 2015; Li et al. 2011). However, in moist environments the relationship between stable carbon isotope variability and parameters related to sunlight availability seems to hold true in the tropics as it does elsewhere (Brienen et al. 2016; van der Sleen et al. 2014).
In a recent review by van der Sleen et al. (2017) the theory, methods and results of tropical isotope dendroclimatology are reviewed and summarized (see also Chap. 22). Their review of 50 studies revealed a dominance of controls over stable carbon isotope variability by water availability, light and nutrient supply in tropical studies. Whilst oxygen isotope variability was dominated by source water signals with the potential for additional variables influencing fractionation, such as rooting depth and evaporative enrichment at the leaf (van der Sleen et al. 2017). The review also mentions the emerging importance of nitrogen isotope investigations, which can be used to explore the nitrogen cycle in complex tropical ecosystems.
2.4 Carbon Isotope Climate Sensitivity in Response to Increasing CO2
Intrinsic Water Use Efficiency (iWUE) is a useful variable for studying spatial and temporal changes in plant carbon–water relations and provides information on climate–vegetation feedbacks at broad geographical scales (Dekker et al. 2016; Lavergne et al. 2019). In the context of stable isotope tree-ring science, iWUE can be derived from δ13C. A detailed description and explanation of iWUE is given in Chap. 17.
Assessing changes in iWUE inferred from the stable carbon isotopes across tree-ring networks reports an average global increase in tree iWUE of 0.1–0.3% per year over the last century (Peñuelas et al. 2011; Battipaglia et al. 2013; Saurer et al. 2014; Frank et al. 2015). Increases in iWUE are detected regardless of biome (Andreu-Hayles et al. 2011; Silva and Anand 2013), tree species (Martínez‐Sancho et al. 2018; Lévesque et al. 2014) or tree status (Hereş et al. 2014; Voltas et al. 2013). Rises in iWUE seem generally to be more rapid during the second half of the twentieth century (up to >0.5% per year) (see Gagen et al. 2011b) at some sites. However, the rate of change is strongly site and species specific (e.g. Saurer et al. 2004). iWUE time series have been observed where increases are halted by active stomatal control in response to rising atmospheric CO2 (Waterhouse et al. 2004; Gagen et al. 2011b; Bert et al. 1997; Saurer et al. 2004) and time series where ‘spikes’ of very large changes in iWUE occur against a background of a broadly passive response to rising CO2 (Belmecheri et al. 2014). Increases in iWUE are still observed when solely accounting for the effect of increasing atmospheric CO2 concentrations after removing the climatic signal from stable carbon isotope series (Frank et al. 2015). This suggests that trees may have adapted allowing the CO2 partial pressure within the stomatal cavities to rise in balance with atmospheric CO2 concentration.
The species’ and geographical variability in iWUE increases is unsurprising for two reasons, first, because of the range of carbon water economics that have evolved in trees, such that for some species it is beneficial to increase iWUE and for others less so (Lin et al. 2015). Second, CO2–induced increases in iWUE can be a response to more than one change within the tree (Franks et al. 2013). Elevated CO2 does stimulate photosynthesis (Lloyd and Farquhar 2008), reduce stomatal conductance to a minor degree (Ehleringer et al. 1993; Farqhar et al. 1989) and allow trees to absorb the same amount of carbon with less water loss. However, FACE (Free Air CO2 Enrichment) experiments have found little stomatal response to increasing CO2 in mature trees (see Chap. 21 and 24). Reductions in stomatal conductance can also, however, be linked to increases in the costs of respiration (Clark et al. 2013; Wright et al. 2009), heat stress (Corlett 2011) and drought stress due to increasing transpiration as our climate dries out (Wright et al. 2009). Climatic drying is considered to be causal at several sites where an increase in iWUE does not translate in enhanced tree growth (Peñuelas et al. 2011).
Spatial variability in iWUE also assists with interpreting temporal changes in δ13C derived iWUE time series. Large changes in iWUE are found across regional aridity gradients, in particular in isohydric species (gymnosperms), which respond to water stress by strongly regulating transpiration to maintain near constant leaf water potentials when soil moisture drops (Brodribb et al. 2014). In such species, at arid sites, negative correlations are found between iWUE and metrics of water availability such as annual precipitation (Pinus halepensis, Ferrio and Voltas 2005), groundwater access (Pinus sylvestris, Song et al. 2016), or rooting depth (Pinus sylvestris, Santini et al. 2018). Differences in iWUE (from pooled analyses) may exceed 30% between aridity extremes (Santini et al. 2018). Strong latitudinal gradients, linked to hydroclimatic conditions, have been reported for iWUE across Europe, with increasing values southwards (Saurer et al. 2014). However, factors such as nutrient supply and interactions with climate influence the regional variability in iWUE, which may confound water availability effects across spatial scales (Silva et al. 2015).
A better understanding of how plant carbon water relationships change across environmental gradients is needed to improve predictions of how water and carbon balance within forest ecosystems are changing with climate (Saurer et al. 2014; Frank et al. 2015). The extent to which variation in iWUE translates into carbon–water changes at the ecosystem scale remains unclear. The complexities of changes in leaf area index and canopy structure under climate change, along with atmospheric boundary‐layer feedbacks, challenge upscaling leaf-level information from tree rings to the whole ecosystem scale (Medlyn et al. 2017; Lavergne et al. 2019).
Outside the temperate regions, Rahman et al. (2019) summarize evidence for trends in tree growth and iWUE triggered by elevated CO2 against the background of climate change. The authors report that almost all studies show a consistent decrease of carbon discrimination over time, resulting in an increase in iWUE. Van der Sleen et al. (2014) estimate an increase of iWUE of 30–35% across the tropics in response to elevated CO2 but expect no similar increase in radial growth due to the impact of other factors in the nutrient-limited tropics. In a study of iWUE changes in non-annual ring forming trees in Borneo, Loader et al. (2011) found postindustrial increase in iWUE of similar magnitude as described by Van der Sleen et al. (2014). Rahman et al. (2019), reviewing iWUE histories in 81 species at 115 tropical sites, found increases in iWUE but no stimulated radial growth, as the negative effects of climate change overrode the fertilization potential of rising CO2.
Disentangling the iWUE/fertilization response in the tropics will take multi proxy ecophysiological studies of tropical wood formation, combining stable isotope, radiocarbon, wood anatomy and X-ray densitometry (De Mil et al. 2017). The climatic responses in these multiple proxies could then be combined to provide more robust long-term estimates of the response of tropical trees to past climate and improve model simulations of the impact of future climate change on tropical trees (De Micco et al. 2019).
3 Dendroclimatic Information from Stable Oxygen Isotope Time Series
3.1 Early Literature and Progress in Interpretations of δ18O Values in Tree-Rings
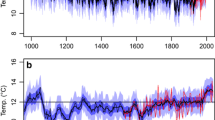
In the mid-1970s, trees were postulated to contain an ‘isotopic thermometer’ in the δ18O of tree-ring wood or extracted cellulose (Libby 1972; Libby and Pandolfi 1974). These early investigations were conducted on oak, cedar or spruce from temperate areas (Libby 1972; Libby and Pandolfi 1974; Libby et al. 1976; Gray and Thompson 1977). Following technical improvements in mass spectrometry, stable oxygen isotope tree-ring chronologies from sub-tropical to sub-arctic sites also began to be explored, revealing correlations with hydroclimate variables (including air humidity) and the isotopic signature of source water, whilst progress was also made in the mechanistic modeling of isotopes in soil and plant cellulose (Burk and Stuiver 1981; Edwards and Fritz 1986; Ramesh et al. 1986). In 2006 Treydte et al. published the first millennial length, annually resolved, well replicated stable oxygen isotope chronology, with results from seven Juniperus turcestanica from Northern Pakistan. This study also led the way in applying the standard statistical practices of traditional dendroclimatology - calibration/verification techniques—to a long, well replicated oxygen isotope tree-ring time series (Masson-Delmotte et al. 2005; Treydte et al. 2006).
Table 19.2 summarizes the range of climatic and environmental information, which can be derived from stable oxygen isotopes in tree rings. The climate signal in tree-ring stable oxygen isotopes arises from the influence of (a) meteorological processes before the water is incorporated into the tree (Chap. 18) and (b) the interaction between the atmosphere and the leaf (Chap. 10, see also Sects. 14.4.1 and 16.3). At the meteorological scale, origin of the water vapor, temperature, evaporation and condensation processes are the dominant controlling factors. During condensation of water vapor the ambient air temperature determines the 18O/16O ratio in meteoric water (Dansgaard 1964), resulting in a seasonal variation of the δ18O in precipitation water (Rozanski et al. 1993). During rain events some water evaporates at the plant and soil surfaces, leaving the remaining water enriched in H2 and 18O relative to the precipitation water. Water, infiltrating into the soil mixes with the residual soil water (Chap. 18). This mixing between infiltrated rain and residual soil water of different temporal origins (Allen et al. 2019) is reflected in tree rings mainly in temperate regions (Szejner et al. 2016), whereas in the tropics the δ18O values of tree rings corresponds to the wet season variables (Brienen and Helle 2012). During plant water uptake no measurable fractionation occurs. Differences in δ18O between xylem (source) water and soil water are due to a mixture of water absorbed from different soil depths with varying δ18O values (Sprenger et al., 2016). In Chap. 18 this topic is discussed in depth.
A detailed discussion about the variation in δ18O at leaf level is given in Chap. 10. Here, we present a summarizing overview. The 18O fractionation in the leaf is tightly linked to transpiration (E) and stomatal conductance (gs), both mainly controlled by air humidity with its δ18O value and leaf temperature. During transpiration the O isotope fractionation occurs as a result of the phase transition from liquid water to the gaseous phase. As the lighter H216O molecules evaporate more readily than the heavier ones the remaining leaf water pool becomes enriched in H218O relative to the source water (Dongmann et al. 1974; Craig and Gordon 1965). The 18O enrichment is increased, with decreasing air humidity. To maintain the leaf water balance the transpired water is replenished with unenriched source water, leading to a reduction in the leaf water 18O enrichment, which is inverse proportional to the transpiration rate and gs. This invers proportional relationship between E and gs is described as the Peclet effect (Farquhar and Lloyd 1993; Barbour 2007).
During photosynthetic assimilate production the δ18O signal of the leaf water is transferred to the assimilates. During the assimilate transport in the phloem, the δ18O of the assimilates is modified by a proportional oxygen exchange with the xylem water and at the sites of organic matter synthesis (Barbour 2007; Sternberg 2009; Gessler et al. (2014).
3.2 Dendroclimatic Information from Stable Oxygen Isotope Time Series in Temperate Regions
Traditional tree-ring parameters, such as tree-ring width or maximum latewood density have proved most useful where species ranges are strongly limited by a single climatic variable, such as summer rainfall or temperature, and it is these locations which dominate growth proxy climate reconstruction networks. In the past, stable-isotope studies have followed the same sampling strategy, starting at latitudinal or altitudinal limits to maximize the changes of accessing a strong common climate signal (Breshears et al. 2009; Loader et al. 2013). There is, however, increasing evidence, that stable oxygen isotope ratios in tree rings from temperate regions, where trees grow in favorable and moist climates, also carry strong climatic signals (Loader et al. 2019).
The strong source water signal in moist parts of the globe has steered the development of oxygen isotope dendroclimatology. Young et al. (2015) discuss such an example in UK oak that went on to reveal such strong climate signals in stable oxygen isotope variations that it led to the establishment of oxygen isotope dating of archaeological timbers, in sites where tree-ring growth was too complacent to allow for dendro dating via ring width variations (Loader et al. 2019). Similarly, δ18O measurements from subfossil wood (e.g. late glacial pines) is being explored as a method by which to reduce dating uncertainties in tree-ring width time series and radiocarbon dating (Pauly et al. 2018, 2020).
In the UK anticyclonic summers are dry and what precipitation does fall is enriched by short-lived, locally derived convective rain bearing clouds. This enriched source water signal is then further enhanced, in warm anticyclonic summers, by evaporative enrichment at the leaf, and thus high tree-ring stable oxygen isotope values in the wood. The opposite occurs in wet, cool cyclonic British summers where longer-lived Atlantic air masses, where many rainout events have depleted the source water, arrive on UK shores and ultimately lead to the synthesis of photosynthate with low tree-ring oxygen isotope values (Young et al. 2015; Loader et al. 2019).
In the early decades of stable isotope dendroclimatology many studies focused on exploring the different signal strengths in the isotope and growth proxies (e.g. McCarroll and Pawellek 1998). Now multi parameter studies, bolstered by improved understanding of the mechanistic models of the isotope-climate system, allow for the reconstruction of more climate variables. Hartl-Meier et al. (2015) systematically assessed the climate response of tree-ring width, δ13C and δ18O from a temperate mountain forest in the Austrian pre-Alps. Variations in stem growth and isotopic composition of Norway spruce, common beech and European larch from dry, medium and moist sites were compared with records of sunshine, temperature, hydroclimate, and cloud cover. Results indicated uniform year-to-year variations in δ13C and δ18O across sites and species, but distinct differences in ring width according to habitat and species. Whilst the climate sensitivity of ring widths was generally weak, the δ13C and δ18O chronologies contained strong common signals. This pattern is often found in areas where stand dynamics impact growth proxies but isotopic variables are more simply related to climate. It is in such ‘mesic’ growth environments where the isotopes proxies may have most to contribute. As discussed, stable oxygen isotopes have revealed particularly high common signal strength and climate reconstruction potential throughout the geographical ranges of temperate tree species, rather than just on the periphery of their distributions (Cernusak and English 2015). These findings are also in line with studies from temperate sites across Switzerland (Saurer et al. 2008), in China (Liu et al. 2012; Xu et al. 2020), in the Tibetan Plateu (Qin et al. 2015; Bräuning 2006; Wernicke et al. 2015; Holmes et al. 2007; Grießinger et al. 2011), and in India (Ramesh et al. 1986).
In Europe a tree-ring isotope network study, ISONET, explored δ18O variability across 35 sites from Northern Fennoscandia to the Mediterranean. The ISONET time series revealed that the strongest twentieth century climatic signals were contained in tree-ring δ18O from sites across the Northwest (UK, France) and Central (Germany, Austria, Switzerland) European sites (Treydte et al. 2007). The close association of tree-ring δ18O with precipitation and temperature variables was postulated to be due to isotopic fractionation being a function of air mass sourcing (temperature of condensation) and air mass trajectory (Treydte et al. 2007; Rozanski et al. 1993).
Studies on individual sites within the ISONET network made use of the strong dependency of δ18O variation on moisture conditions and created robust multi-century precipitation and drought reconstructions for both the UK and France. Similarly, Rinne et al. (2013) developed a 400-year May–August precipitation reconstruction for Southern England from tree-ring δ18O of pedunculate oak. The analysis demonstrated a statistically robust signal back to 1697, and was robust over a larger area of the UK, later confirmed to cover the entire UK (Loader et al. 2019; Young et al. 2015), such is the strength of the source water signal in the UK’s temperate oak. Critically UK oak stable oxygen isotope ratios retain both low- and high frequency precipitation variability (Rinne et al. 2013; Young et al. 2015). In northwestern Iberia, extremes in a centennial stable oxygen isotopic record tracked distinct seasonal hydroclimate conditions: wetter June-July periods were associated with extremely low δ18O values, while extreme high δ18O values were associated with warm and dry conditions in the spring (Andreu-Hayles et al. 2017).
Lowland sites in France have also revealed strong hydroclimate reconstruction potential. Labuhn et al. (2016) developed a long, continuous cellulose δ18O chronology for France, using living oak trees and timber from historic buildings. They provide a regional reconstruction of summer drought covering more than six centuries, coherent with other proxies of summer climate from the region. Again, the trees show very strong inter-annual variability, and highly significant correlations with temperature, precipitation and drought during the twentieth century. The changes in coherence between two sites within their chronology, during earlier centuries, indicated that the response of the proxy to climate might be non-linear, or that the spatial patterns of climate in France have changed (Labuhn et al. 2016). Such findings are supported by other studies revealing the sensitivity of tree-ring δ18O to the combined effects of temperature and humidity, and therefore of drought (Raffalli-Delerce et al. 2004; Masson-Delmotte et al. 2005; Etien et al. 2008b; Labuhn et al. 2014, see also further discussions in Chap. 14). Temperate stable oxygen isotope time series from tree rings, particularly in oak species, are now strongly evidenced to retain a strong hydroclimate signal (Levesque et al. 2019; Roden and Ehleringer 2007; Szejner et al. 2016; Roden et al. 2011).
In Argentina, the δ18O tree-ring of Nothofagus pumilio and Fitzroya cupressoides, at relatively humid sites, was shown to be comparable and related to temperature (December through May) (Lavergne et al. 2016). The temperature signal encompassed a large area in southern South America geographically under the influence of the Southern Annular Mode (SAM). In light of the significant correlation between the oxygen and carbon isotope records at the site the correlation between δ18O and temperature was ascribed to the effect of temperature on isotopic enrichment of leaf water, more than to its effect on precipitation δ18O (Lavergne et al. 2017a). A modeling experiment later confirmed this interpretation (Lavergne et al. 2017b). Further South, at Perito Moreno glacier (Santa Cruz Province, Argentina), also a moist site, the δ18O of Nothofagus pumilio was found to be sensitive both to temperature and hydroclimate (Grießinger et al. 2018; Meier et al. 2020). Though changes in moisture source origin may have been involved via the SAM.
In a pioneering Antipodean study, Wilson and Grinsted (1978) were early to hypothesize that δ18O in wood could be used to reconstruct past temperature and the δ18O of source water. Whilst regional dendrochronology challenges, due to the variations in ring width coherence, may have slowed progress in creating long-term isotope series, a larger number of prominent New Zealand and Australia ecophysiological investigations explore δ18O (e.g. Barbour et al. 2002; Cernusak et al. 2005; Ellsworth et al. 2013). Brookman (2014) demonstrated that tree-ring δ18O in New Zealand conifers was a promising tool for regional hydroclimate reconstruction via a comparison of the δ18O records from Agathis australis and Libocedrus bidwillii from sites on South Island. Again, a hydroclimate signal dominated (air humidity, soil moisture deficit and rainfall amount) from previous autumn through the concurrent growth season. Isotope climate correlations were strong in several different species. In the North Island, Lorrey et al. (2016) also explored the palaeoclimate potential of long-lived Agathis australis via δ18O in earlywood and evidenced statistically significant correlations with hydroclimate (October–December vapor pressure and May–December air humidity). The relationships were shown to be consistent with mechanistic δ18O simulations. These results suggested that Agathis australis δ18O has the potential to provide quantitative climate information to explore past ENSO activity in the region with sub-fossil chronologies having the potential to provide insights back into the late Quaternary.
3.3 Dendroclimatic Information from Stable Oxygen Isotope Time Series at High Latitudes and in the Boreal Forest
At high latitudes in the Northern Hemisphere annually resolved δ18O chronologies have been developed across the Boreal zone. Buhay and Edwards (1995) carried out an early exploration of hydroclimate in Canada based on tree-ring water isotopes. They used an isotope model to account for the impact of fractionation on source water signal in different moisture environments allowing for a reconstruction resolved by hydroclimatic environment, over 275 years prior to the instrumental period. Birks and Edwards (2009) described changes in atmospheric circulation patterns for the North Pacific from water isotopes, showing the strongest relationship with temperature variations and a precipitation amount effect. Temperature variability for the last millennia has also been reconstructed using δ18O tree-ring records in Canada from Boreal-lake subfossil trees, revealing the speed of recent climatic warming at that latitude as well as a connection between the coldest periods of the nineteenth century and the volcanic eruptions and low solar activity associated with the late Little Ice Age (Naulier et al. 2015). Gennaretti et al. (2017) combined δ18O, δ13C and ring-width chronologies to develop an improved multi-proxy temperature reconstruction (using a Bayesian framework) for the same region. Annual temperature and δ18O meteoric water records have also been estimated using δ18O tree-ring reconstructions from Pleistocene subfossil wood in Nunavut (Csank et al. 2013). Further studies in the Northwestern Territories showed positive correlations between a tree-ring δ18O record and summer temperatures and negative relationships with summer humidity, also involving an oxygen isotope evaporative enrichment signal in warmer summers (Porter et al. 2009). Using longer δ18O records for the same site, a ~200-year spring–summer temperature reconstruction was generated (Porter et al. 2013). Further Canadian examples show the strength of Boreal δ18O for reconstructing source water dominated precipitation and temperature histories, where the two climate parameters co-vary (Holzkämper et al. 2012; Bégin et al. 2015). Even further north, in High Arctic environments, a significant correlation between the δ18O of birch trees growing in Southwestern Greenland and NAO was evidenced (Xu et al. 2021). Similarly, tree-ring cellulose of the shrub Cassiope tetragona in Ellesmere Island in Canada at 79 °N recorded Arctic and NAO variability (Welker et al. 2005). Rayback and Henry (2006) reconstructed 100 years of summer temperatures using cellulose material from tree rings of the shrub Cassiope tetragona in Ellesmere Island.
Moving to consider the European context, δ18O records of Pinus sylvestris in Finland and Sweden also reveal hydroclimate sensitivity (Seftigen et al. 2011; Esper et al. 2018). In the Russian Altai, at the limit of the Boreal forest, summer temperature and precipitation signals were both preserved in δ18O chronologies of Larix decidua (Sidorova et al. 2013). High latitude Boreal forest has also formed the backdrop for ring width and densitometry-based dendroclimatology to develop networks reconstructions (e.g. Anchukaitis et al. 2017; Wilson et al. 2016) and so were a natural starting place for isotope dendroclimatology (e.g. McCarroll and Pawellek 1998, Robertson et al. 1997).
Trees are long lived in the Boreal and high-latitude forests and abundant subfossil material is available and well-preserved due to the cold environment. With the underlying isotope fractionation models described above and in Chaps. 10 and 18 suggesting that water isotopes in tree rings should sensitively record a source water signal in these moist, cool forests it is not surprising that tree-ring δ18O has proven to be a reliable hydroclimate proxy far into the northern forests. See also Chap. 20 for a discussion of relevant reconstructions.
3.4 Dendroclimatic Information from Stable Oxygen Isotope Time Series in the Tropics
A major research gap exists in the network of high-resolution proxy reconstructions for palaeoclimatology, in the terrestrial tropics (Evans and Schrag 2004). As discussed, this is in part due to the challenges of using traditional dendroclimatology in these regions (Worbes 2002). However, over the last two decades several authors have tried to address this research gap by exploring the source water signal and evaporative enrichment signal in cellulose δ18O from tropical trees to resolve both dating and climate signal (see Brienen et al. 2016; Rozendaal and Zuidema 2011; van der Sleen et al. 2017 for a review). In Chap. 22 we summarize major findings regarding the climatic information recorded in tree-ring δ18O from trees growing in tropical regions.
Away from the ‘ever wet’ regions, most parts of the tropics do experience precipitation seasonality, even with annually stable temperatures. Wet seasons are typified by δ18O depleted source water (Araguás-Araguás et al. 2000) with dryer periods seeing enriched source water. Moreover, even in regions with very little rainfall amount variability through the year, there is geographical source water variability, as the monsoon seasons shift through the year (Loader et al. 2011). Several studies have attempted to detect seasonality via source water variability as both a dating method in non-annual ring forming tropical trees, and a climate signal (Evans and Schrag 2004).
Kahmen et al. (2011) explored the mechanistic explanations for sensitivity of δ18O to climate in tropical tree rings. Using the mechanistic Péclet-modified Craig-Gordon model (PMGG) they reported that both air temperature and air humidity influenced tree that δ18O, integrated via VPD, along an aridity gradient of Metrosideros polymorpha in Hawaii. Xylem water δ18O decreased along the gradient whilst leaf and stem δ18O increased, suggesting that evaporative enrichment dominated the source water signal in Hawaii’s coastal island environment.
In tropical rainforest environments of Central Guyana, Pons and Helle (2011) found δ18O cellulose patterns mimicked the source water annual isotope cycle well, with minima in both rainfall and cellulose at the start of the area’s primary wet season. They tested their hypothesis with dual isotope measurements, which revealed that cellulose δ13C also showed low moisture stress at the same time as the minima in cellulose δ18O. Moreover, these revealing studies were successfully carried out in tropical trees with anatomically indistinct tree rings (Pons and Helle 2011).
In South America, in the western Amazon basin, Brienen and Helle (2012) reported a lower influence of temperature and vapor pressure variations on tree-ring δ18O records from Cedrela odorata. Tree-ring δ18O correlated well with δ18O in precipitation, regional precipitation variability during the wet season and streamflow from Amazonian rivers. Non time stable relationships with ENSO parameters were also observed, as well as an increase in δ18O values over the twentieth century, shared with ice core records. Baker et al. (2015) reinforced the dominance of a source water signal in the Brienen and Helle (2012) δ18O Cedrela record via correlations with other δ18O tree-ring records from low altitude rainforest tree species, and with high altitude Polylepis tarapacana in the Bolivian Altiplano. The δ18O signature of Polylepis tarapacana chronologies along the South American Altiplano revealed a strong signal of December to March precipitation associated to the South American Summer Monsoon (Rodríguez-Catón et al. 2021). In Southern Ecuador, ENSO variability has also been shown to be recorded in δ18O tree-ring records of Cedrela montana (Volland et al. 2016). Good agreement with wet season precipitation was found in high-resolution δ18O records of Tachigali myrmecophila in Manaus, Brazil, in Cedrela odorata at 12 °S in the Amazonian and a 150-year Polylepis tarapacana tree-ring δ18O record from the Bolivian Altiplano at 22 °S (Ballantyne et al. 2011).
In Costa Rica variations in the tree-ring δ18O records from 16-year old Hyeronima alcorneides were found to be sensitive to tropical precipitation amount, especially during ENSO events (Evans et al. 2006). The δ18O cycles measured in two Pouteria trees, which did not have visible tree rings, and were assigned a calendar year via a radiocarbon age-depth model, showed enriched δ18O during warm ENSO wet seasons (Anchukaitis and Evans 2010). Annual cyclicity in δ18O has also been revealed in ring-less mangrove Rhizophora mucronata which also recorded sensitivity to the ENSO event in 1997 (Verheyden et al. 2004a).
In Central Java in Indonesia, Schollaen et al. (2013) reported that δ18O chronologies from rainforest teak (Tectona grandis) recorded precipitation signals during the dry and the rainy season. The δ18O teak tree-ring record also showed strong sensitivity to ENSO events driven by warmer sea surface temperatures over the central Pacific (Schollaen et al. 2015). In Indonesia and Thailand, high-resolution δ18O records from cross dated tree rings of Tectona grandis, and ring-less Samanea saman grown at the same locality, show high correlations with instrumental data, reflecting the seasonal cycles of rainfall and air humidity (Poussart et al. 2004).
Tropical oxygen isotope dendroclimatology, to date, has revealed coherent correlations between various hydroclimate and source water signals and δ18O in tree-rings, often in trees with indistinct growth rings (e.g. Sano et al. 2009). These examples highlight opportunities to extend our understanding of past climate from trees to many parts of the tropics, from remote to highly populated areas where climate change may have direct and serious implications. Despite these promising single site studies, the environmental and physiological mechanisms controlling tropical isotopic signals between the atmosphere and soil, through the stem and canopy to the wood and into the cellulose of the tree-ring are highly complex, and more so in the tropics. The interplay between isotopic signals carried in the source water and those produced at the leaf level and during downstream enrichment and photosynthetic processes are still not well understood (Gessler et al. 2014). The mechanistic interpretation of δ18O in tropical trees remains therefore often problematic. Additional relevant discussions can also be found in Chaps. 13 and 22.
4 Conclusions
The contributions of stable isotope dendroclimatology have increased tenfold, over recent decades, thanks to better analytical systems allowing greater sample throughput and replication, improved understandings of the mechanistic controls over the isotope signal and increased geographical and species coverage in example studies. Contributions to our understanding of the carbon–water-climate relationship in trees are now available from long, well replicated stable isotope tree-ring series across the old world, with pockets of emerging studies in previously underrepresented geographical areas. As global average temperatures continue to rise and we move into a ‘1.5-degree world’ with increasing climate extremes, the additional glimpses into our pre anthropogenic climate that we obtain from tree-ring isotope records becomes ever more important. Coupled with the ability to explore how trees and woodland respond to changing climate and CO2 levels in the atmosphere, the isotope dendro proxies are now a vital part of the palaeoenvironmental toolbox.
References
Allen ST, Kirchner JW, Braun S, Siegwolf RTW, Goldsmith GR (2019) Seasonal origins of soil water used by trees. Hydrol Earth Syst Sci 23:1199–1210
Anchukaitis KJ et al (2008) Stable isotope chronology and climate signal calibration in neotropical montane cloud forest trees. J Geophys Res American Geophysical Union (AGU) 113(G3). https://doi.org/10.1029/2007jg000613
Anchukaitis KJ, Evans MN (2010) Tropical cloud forest climate variability and the demise of the Monteverde golden toad. Proc Natl Acad Sci 107(11):5036–5040. https://doi.org/10.1073/pnas.0908572107
Anchukaitis KJ, Wilson R, Briffa KR, Buntgen U, Cook ER, D’Arrigo R, Davi N, Esper J, Frank D, Gunnarson BE, Hegerl G, Helama S, Klesse S, Krusic PJ, Linderholm HW, Myglan V, Osborn TJ, Zhang P, Rydval M, Schneider L, Schurer A, Wiles G, Zorita E (2017) Last millennium Northern Hemisphere summer temperatures from tree rings: Part II, spatially resolved reconstructions. Quat Sci Rev 163:1–22
Anderson WT et al (1998) Oxygen and carbon isotopic record of climatic variability in treering cellulose (Picea abies): an example from central Switzerland (1913–1995). J Geophys Res Atmos (Blackwell Publishing Ltd) 103(D24):31625–31636. https://doi.org/10.1029/1998JD200040
Andreu-Hayles L et al (2011) Long treeringchronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests. Glob Change Biol 17(6):2095–2112. https://doi.org/10.1111/j.1365-2486.2010.02373.x
Andreu-Hayles L et al (2017) 400 Years of summer hydroclimate from stable isotopes in Iberian trees. Clim Dyn (Springer Verlag) 49(1–2):143–161. https://doi.org/10.1007/s00382-016-3332-z
Andreu-Hayles L et al (2019) A high yield cellulose extraction system for small whole wood samples and dual measurement of carbon and oxygen stable isotopes. Chem Geol (Elsevier) 504(November 2017):53–65. https://doi.org/10.1016/j.chemgeo.2018.09.007
Araguás-Araguás L, Froehlich K, Rozanski K (2000) Deuterium and oxygen-18 isotope composition of precipitation and atmospheric moisture. Hydrol Process 14(8):1341–1355. https://doi.org/10.1002/1099-1085(20000615)14:8%3c1341::AID-HYP983%3e3.0.CO,2-Z
Baker JCA et al (2015) Oxygen isotopes in treerings show good coherence between species and sites in Bolivia. Glob Planet Change (Elsevier (BV)) 133:298–308. https://doi.org/10.1016/j.gloplacha.2015.09.008
Bale RJ et al (2011) An annually resolved bristlecone pine carbon isotope chronology for the last millennium. Quat Res 76(1):22–29. https://doi.org/10.1016/j.yqres.2011.05.004
Ballantyne AP et al (2011) Regional differences in South American monsoon precipitation inferred from the growth and isotopic composition of tropical trees, earth interactions. Earth Interact Am Meteorol Soc 15(5):1–35. https://doi.org/10.1175/2010ei277.1
Barbour M, Walcroft AS, Farquhar GD (2002) Seasonal variation in δ13C and δ18O of cellulose from growth rings of Pinus radiata. Plant Cell Environ 25:1483–1499
Barbour MM (2007) Stable oxygen isotope composition of plant tissue: a review. Funct Plant Biol 34:83–94
Battipaglia G, Cherubini P, Saurer M, Siegwolf RTW, Strumia S, Cotrufo MF (2007) Volcanic explosive eruptions of the Vesuvio decrease tree-ring growth but not photosynthetic rates in the surrounding forests. Glob Change Biol 13(6):1122–1137. https://doi.org/10.1111/j.1365-2486.2007.01350.x
Battipaglia G et al (2013) Special issue: WSE symposium: wood growth under environmental changes: the need for a multidisciplinary approach. Tree Physiol (oxford University Press (OUP)) 34(8):787–791. https://doi.org/10.1093/treephys/tpu076
Bégin C et al (2015) Assessing treeringcarbon and oxygen stable isotopes for climate reconstruction in the Canadian northeastern boreal forest. Palaeogeogr Palaeoclimatol Palaeoecol 423:91–101. https://doi.org/10.1016/j.palaeo.2015.01.021
Belmecheri S, Maxwell RS, Taylor AH, Davis KJ, Freeman KH, Munger WJ (2014) Treering δ13C tracks flux tower ecosystem productivity estimates in a NE temperate forest. Environ Res Lett (IOP Publishing) 9(7):074011. https://doi.org/10.1088/1748-9326/9/7/074011
Berlage HP (1931) On the relationship between thickness of treerings of Djati (teak) trees and rainfall on Java. Tectona 24:939–953
Bert D, Leavitt SW, Dupouey J-L (1997) Variations of wood Δ13C and water-use efficiency of Abies alba during the last century. Ecology 78(5):1588–1596. https://doi.org/10.1890/0012-9658(1997)078[1588:VOWCAW]2.0.CO,2
Biondi F (2001) A 400-year treeringchronology from the tropical treeline of North America. AMBIO J Humn Environ American Bryol Lichenol Soc 30(3):162. https://doi.org/10.1639/0044-7447(2001)030[0162:aytrcf]2.0.co,2
Birks SJ, Edwards TWD (2009) Atmospheric circulation controls on precipitation isotope-climate relations in western Canada. Tellus B Chem Phys Meteorol (informa UK, Limited) 61(3):566–576. https://doi.org/10.1111/j.1600-0889.2009.00423.x
Boucher O, Randall D, Artaxo P, Bretherton C, Feingold G, Forster P, Kerminen V-M, Kondo Y, Liao H, Lohmann U et al (2013) Clouds and aerosols. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge and New York, NY, pp 571–657
Bradley RS (1985) Quaternary paleoclimatology: methods of paleoclimatic reconstruction. Allen & Unwin, Boston
Bräuning A (2006) Tree-ring evidence of ‘Little Ice Age’ glacier advances in Southern Tibet. The Holocene 16:369–380
Brendel O, Iannetta PPM, Stewart D (2000) A rapid and simple method to isolate pure alpha-cellulose. Phytochem Anal 11(1):7–10. https://doi.org/10.1002/(SICI)1099-1565(200001/02)11:1%3c7::AID-PCA488%3e3.0.CO,2-U
Breshears DD, Myers OB et al (2009) Tree die-off in response to global change-type drought: mortality insights from a decade of plant water potential measurements. Front Ecol Environ 7:185–189. https://doi.org/10.1890/080016
Brienen R, Helle G (2012) Oxygen isotopes in tree rings are a good proxy for Amazon precipitation and El Niño-Southern oscillation variability. PNAS 1–6. https://doi.org/10.1073/pnas.1205977109
Brienen RJW et al (2017) Tree height strongly affects estimates of water-use efficiency responses to climate and CO2 using isotopes. Nature Commun (Springer, US) 8(1):288. https://doi.org/10.1038/s41467-017-00225-z
Brienen RJW, Schöngart J, Zuidema PA (2016) Treerings in the tropics: insights into the ecology and climate sensitivity of tropical trees, pp 439–461. https://doi.org/10.1007/978-3-319-27422-5_20
Brodribb TJ, McAdam SAM, Jordan GJ, Martins SCV (2014) Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc Natl Acad Sci 111(40):14489–14493. https://doi.org/10.1073/pnas.1407930111
Brookman TH (2014) Stable isotope dendroclimatology of New Zealand kauri (Agathis australis (D. Don) Lindl.) and cedar (Libocedrus bidwilliii Hook. F.), University of Canterbury, Christchurch, New Zealand
Buckley BM et al (2010) Climate as a contributing factor in the demise of Angkor, Cambodia. Proc Natl Acad Sci 107(15):6748–6752. https://doi.org/10.1073/pnas.0910827107
Buhay WM, Edwards TWD (1995) Climate in southwestern ontario, Canada, between ad 1610 and 1885 inferred from oxygen and hydrogen isotopic measurements of wood cellulose from trees in different hydrologic settings. Quat Res 44(3):438–446. https://doi.org/10.1006/qres.1995.1089
Büntgen U, Kolář T, Rybníček M, Koňasová E, Trnka M, Ač A, Krusic PJ, Esper J, Treydte K, Reinig F, Kirdyanov A, Herzig F, Urban O (2020) No evidence of age trends in oak stable isotopes. Paleooceanogr Paleoclimatol. https://doi.org/10.1029/2019PA003831
Burk R, Stuiver M (1981) Oxygen isotope ratios in trees reflect mean annual temperature and humidity. Science 211(4489):1417–1419. https://doi.org/10.1126/science.211.4489.1417
Campbell R et al (2011) Blue intensity in pinus sylvestris treerings: a manual for a new palaeoclimate proxy. Tree-Rring Res 127–134
Cernusak LA, Farquhar GD, Pate JS (2005) Environmental and physiological controls over oxygen and carbon isotope composition of Tasmanian blue gum, Eucalyptus globulus. Tree Physiol. Oxford University Press (OUP) 25(2):129–146. https://doi.org/10.1093/treephys/25.2.129
Cernusak LA, English NB (2015) Beyond tree-ring widths: stable isotopes sharpen the focus on climate responses of temperate forest trees. Tree Physiol 35:1–3. https://doi.org/10.1093/treephys/tpu115
Chiang JCH (2009) The tropics in paleoclimate, annual review of earth and planetary sciences. Ann Rev 37(1):263–297. https://doi.org/10.1146/annurev.earth.031208.100217
Churakova OV et al (2019) Siberian treeringand stable isotope proxies as indicators of temperature and moisture changes after major stratospheric volcanic eruptions. Clim past. Copernicus GmbH 15(2):685–700. https://doi.org/10.5194/cp-15-685-2019
Clark DA, Clark DB, Oberbauer SF (2013) Field-quantified responses of tropical rainforest aboveground productivity to increasing CO2 and climatic stress, 1997–2009. J Geophys Res Biogeosei (american Geophysical Union (AGU)) 118(2):783–794. https://doi.org/10.1002/jgrg.20067
Corlett RT (2011) Impacts of warming on tropical lowland rainforests. Trends Ecol Evolut (Elsevier (BV)) 26(11):606–613. https://doi.org/10.1016/j.tree.2011.06.015
Coster C (1927) Zur Anatomie und Physiologie der Zuwachszonen- und Jahresringbildung in den Tropen. Ann Jard Bot 37:49–160. https://scholar.google.com/scholar?oi=gsb40&q=Coster%2CC.%2C. Zur Anatomie und Physiologie der Zuwachszonen- und Jahresringbildung in den Tropen. Ann Jard Bot Buitenzong 37%2C 49-160&lookup=0&hl=en. Accessed 31 Oct 2019
Craig H (1954) Carbon-13 variations in sequoia rings and the atmosphere. Science 119(3083)
Craig H, Gordon LI (1965) Deuterium and oxygen-18 variations in the ocean and the marine atmosphere. In: Tongiorgi E (ed) Proceedings of a conference on stable isotopes in oceanographic studies and paleotemperatures. Lischi and Figli, Pisa, Italy, pp 9–130
Csank AZ, Fortier D, Leavitt SW (2013) Annually resolved temperature reconstructions from a late Pliocene-early Pleistocene polar forest on Bylot Island, Canada. Palaeogeogr Palaeoclimatol Palaeoecol (Elsevier (BV)) 369:313–322. https://doi.org/10.1016/j.palaeo.2012.10.040
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
D’Arrigo R, Wilson R, Anchukaitis KJ (2013) Volcanic cooling signal in treering temperature records for the past millennium. J Geophys Res Atmos (Blackwell Publishing Ltd.) 118(16):9000–9010. https://doi.org/10.1002/jgrd.50692
D’Arrigo R et al (2006a) The reconstructed Indonesian warm pool sea surface temperatures from treerings and corals: linkages to Asian monsoon drought and El Niño-Southern Oscillation. Paleoceanography (American Geophysical Union (AGU)) 21(3). https://doi.org/10.1029/2005pa001256
D’Arrigo R, Wilson R, Jacoby G (2006b) On the long-term context for late twentieth century warming. J Geophys Res Atmos (Blackwell Publishing Ltd.) 111(3). https://doi.org/10.1029/2005JD006352
Daux V et al (2011) Can climate variations be inferred from treeringparameters and stable isotopes from Larix decidua? Juvenile effects, budmoth outbreaks, and divergence issue. Earth Planet Sci Lett (Elsevier (BV)) 309(3–4):221–233. https://doi.org/10.1016/j.epsl.2011.07.003
Daux V, Michelot-Antalik A, Lavergne A, Pierre M, Stievenard M, Bréda N, Damesin C (2018) Comparisons of the performance of δ13C and δ18O of Fagus sylvatica, Pinus sylvestris, and Quercus petraea in the record of past climate variations. J Geophys Res Biogeosci 123:1145–1160. https://doi.org/10.1002/2017JG004203
Dekker SC et al (2016) Spatial and temporal variations in plant water-use efficiency inferred from treering, eddy covariance and atmospheric observations. Earth Syst Dyn (Copernicus (GmbH)) 7(2):525–533. https://doi.org/10.5194/esd-7-525-2016
Dessler AE (2010) A determination of the cloud feedback from climate variations over the past decade. Science 330(6010):1523–1527. https://doi.org/10.1126/science.1192546
Dongmann G, Nurnberg HW, Forstel H, Wagener K (1974) Enrichment of H218O in leaves of transpiring plants. Radiat Environ Biophys 11:41–52
Dorado-Liñán I, Büntgen U, González-Rouco F, Zorita E, Montávez JP, Gómez-Navarro JJ, Brunet M, Heinrich I, Helle G, Gutiérrez E (2012) Estimating 750 years of temperature variations and its uncertainties in the Pyrenees by tree-ring reconstructions and climate simulations. Clim past 8:919–933
Dorado-Liñán I, Zorita E, González-Rouco JF, Heinrich I, Campello F, Andreu-Hayles L, Muntán E, Gutiérrez E (2015) Eight-hundred years of summer temperature variations in the southeast of the Iberian Peninsula reconstructed from tree rings. Clim Dyn 44(1–2):75–93
Dorado-Liñán I et al (2011) Pooled versus separate measurements of treeringstable isotopes. Sci Total Environ (Elsevier (BV)) 409(11):2244–2251. https://doi.org/10.1016/j.scitotenv.2011.02.010
Dorado-Liñán I et al (2016) Changes in surface solar radiation in Northeastern Spain over the past six centuries recorded by treering δ13C. Clim Dyn (Springer Verlag) 47(3–4):937–950. https://doi.org/10.1007/s00382-015-2881-x
Duffy JE et al (2017) Short-lived juvenile effects observed in stable carbon and oxygen isotopes of UK oak trees and historic building timbers. Chem Geol 472(September):1–7. https://doi.org/10.1016/j.chemgeo.2017.09.007
Duffy JE et al (2019) Absence of age-related trends in stable oxygen isotope ratios from Oak treerings. Glob Biogeochem Cycles (american Geophysical Union (AGU)). https://doi.org/10.1029/2019gb006195
Edwards TWD, Fritz P (1986) Assessing meteoric water composition and relative humidity from 18O and 2H in wood cellulose: paleoclimatic implications for southern Ontario, Canada. Appl Geochem 1:715–723
Edwards TWD et al (2008) Climatic and hydrologic variability during the past millennium in the eastern Rocky Mountains and northern Great Plains of western Canada. Quat Res 70(2):188–197. https://doi.org/10.1016/j.yqres.2008.04.013
Ehleringer JR, Hall AE, Farquhar GD (1993) Preface. In: Stable isotopes and plant carbon-water relations. Elsevier, p xix. https://doi.org/10.1016/b978-0-08-091801-3.50005-2
Ellsworth PV, Anderson WT, Sonninen E, Barbour MM, Sternberg LSL (2013) Reconstruction of source water using the d18O of treering phenylglucosazone: a potential tool in paleoclimate studies. Dendrochronologia 31:153–158. https://doi.org/10.1016/j.dendro.2012.10.004
Epstein S, Krishnamurthy RV (1990) Environmental information in the isotopic record in trees. Philos Trans R Soc A Math Phys Eng Sci (The Royal Society 330(1615):427–439. https://doi.org/10.1098/rsta.1990.0023
Epstein S, Yapp C (1977) Isotope tree thermometers. Nature 266:477–478. https://doi.org/10.1038/266477a0
Epstein S, Yapp C (1976) Climatic implications of the D/H ratio of hydrogen in CH groups in tree cellulose. Earth Planet Sci Lett 30:255–261. http://www.sciencedirect.com/science/article/pii/0012821X76902521. Accessed on 26 November 2015
Esper J et al (2010) Low-frequency noise in δ13C and δ18O treering data: a case study of Pinus uncinata in the Spanish Pyrenees. Global Biogeochem Cycles 24(2):1–11. https://doi.org/10.1029/2010GB003772
Esper J et al (2014) Northern European summer temperature variations over the common era from integrated treeringdensity records. J Quat Sci 29(5):487–494. https://doi.org/10.1002/jqs.2726
Esper J et al (2015) Long-term summer temperature variations in the Pyrenees from detrended stable carbon isotopes. Geochronometria (walter De Gruyter GmbH) 42(1):53–59. https://doi.org/10.1515/geochr-2015-0006
Esper J, Frank D (2009) Divergence pitfalls in treeringresearch. Clim Change 94:261–266. https://doi.org/10.1007/s10584-009-9594-2
Esper J, Holzkamper S, Büntgen U, Schone B, Keppler F, Hartl C, St. George S, Riechelmann DFC, Treydte K (2018) Site-specific climatic signals in stable isotope records from Swedish pine forests. Trees-Struct Funct 32:855–869
Etien N, Daux V et al (2008a) Summer maximum temperature in northern France over the past century: instrumental data versus multiple proxies (tree-ring isotopes, grape harvest dates and forest fires). Clim Change 94:429–456
Etien N, Daux V, Masson-Delmotte V, Stievenard M, Bernard V, Durost S, Guillemin MT, Mestre O, Pierre M (2008b) A bi-proxy reconstruction of Fontainebleau (France) growing season temperature from A.D. 1596 to 2000. Clim past 4:91–106
Evans MN et al (2006) A forward modeling approach to paleoclimatic interpretation of treeringdata. J Geophys Res (American Geophysical Union (AGU)) 111(G3). https://doi.org/10.1029/2006jg000166
Evans MN, Schrag DP (2004) A stable isotope-based approach to tropical dendroclimatology. Geochim Cosmochim Acta (Elsevier (BV)) 68(16):3295–3305. https://doi.org/10.1016/j.gca.2004.01.006
Farmer JG, Baxter MS (1974) Atmospheric carbon dioxide levels as indicated by the stable isotope record in wood. Nature 247(5439):273. https://www.nature.com/articles/247273a0. Accessed on 28 October 2019
Farquhar GD et al (1989a) Carbon isotope fractionation and plant water-use efficiency. In: Stable isotopes in ecological research. Springer, New York, pp 21–40. https://doi.org/10.1007/978-1-4612-3498-2_2
Farquhar GD, Lloyd J (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. Stable isotopes and plant carbon-water relations. Academic Press, San Diego, pp 47–70
Farquhar GD, Ehleringer JR, Hubick KT (1989b) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537. https://doi.org/10.1146/annurev.pp.40.060189.002443
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Ferrio JP, Voltas J (2005) Carbon and oxygen isotope ratios in wood constituents of Pinus halepensis as indicators of precipitation, temperature and vapour pressure deficit. Tellus B Chem Phys Meteorol (informa UK Limited) 57(2):164–173. https://doi.org/10.3402/tellusb.v57i2.16780
Flato G, Marotzke J, Abiodun B, Braconnot P, Chou SC, Collins W, Cox P, Driouech F, Emori S, Eyring V, Forest C, Gleckler P, Guilyardi E, Jakob C, Kattsov V, Reason C, Rummukainen M (2013) Evaluation of climate models. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Doschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of working Group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, pp 741–882. https://doi.org/10.1017/CBO9781107415324.020
Francey RJ, Farquhar GD (1982) An explanation of 13C/12C variations in treerings. Nature 297(5861):28–31. https://doi.org/10.1038/297028a0
Frank D et al (2010) A noodle, hockey stick, and spaghetti plate: a perspective on high-resolution paleoclimatology. Wiley Interdiscip Rev Clim Change (Wiley-Blackwell) 1(4):507–516. https://doi.org/10.1002/wcc.53
Frank DC et al (2015) Water-use efficiency and transpiration across European forests during the anthropocene. Nat Clim Chang 5(6):579–583. https://doi.org/10.1038/nclimate2614
Franks PJ et al (2013) Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century. New Phytologist (Wiley) 197(4):1077–1094. https://doi.org/10.1111/nph.12104
Fritts HC (1976) Tree rings and climate. Academic Press, London, New York and San Francisco, p 567
Fromm J (2013) Cellular aspects of wood formation. Plant Cell Monographs. https://doi.org/10.1007/978-3-642-36491-4
Gagen M et al (2007) Exorcising the ‘segment length curse’: summer temperature reconstruction since AD 1640 using non-detrended stable carbon isotope ratios from pine trees in northern Finland. The Holocene 17(4):435–446
Gagen M et al (2008) Do treering δ13C series from Pinus sylvestris in northern Fennoscandia contain long-term non-climatic trends? Chem Geol 252:42–51. https://doi.org/10.1016/j.chemgeo.2008.01.013
Gagen M, Zorita E et al (2011a) Cloud response to summer temperatures in Fennoscandia over the last thousand years. Geophys Res Lett 38:1–5. https://doi.org/10.1029/2010GL046216
Gagen M, Finsinger W et al (2011b) Evidence of changing intrinsic water-use efficiency under rising atmospheric CO2 concentrations in Boreal Fennoscandia from subfossil leaves and treering δ13C ratios. Global Change Biol (Blackwell Publishing Ltd) 17(2):1064–1072. https://doi.org/10.1111/j.1365-2486.2010.02273.x
Gagen M et al (2012) A rapid method for the production of robust millennial length stable isotope treering series for climate reconstruction. Global Planet Change 82–83:96–103. https://doi.org/10.1016/j.gloplacha.2011.11.006
Gagen MH et al (2016) North Atlantic summer storm tracks over Europe dominated by internal variability over the past millennium. Nat Geosci (Nature Publishing Group) 9(8):630–635. https://doi.org/10.1038/ngeo2752
Gagen M, McCarroll D, Edouard J-L (2004) The effect of site conditions on pine treering width, density and δ13C series. Arct Antarct Alp Res 36(2):166–171
Gagen M, McCarroll D, Edouard JL (2006) Combining ring width, density and stable carbon isotope proxies to enhance the climate signal in treerings: an example from the southern French Alps. Clim Change 78:363–379. https://doi.org/10.1007/s10584-006-9097-3
Gebrekirstos A et al (2009) Stable carbon isotope ratios in treerings of co-occurring species from semi-arid tropics in Africa: patterns and climatic signals. Global Planet Change 66(3–4):253–260. https://doi.org/10.1016/j.gloplacha.2009.01.002
Gennaretti F et al (2017) Bayesian multiproxy temperature reconstruction with black spruce ring widths and stable isotopes from the northern Quebec taiga. Clim Dyn (Springer Verlag) 49(11–12):4107–4119. https://doi.org/10.1007/s00382-017-3565-5
St. George S, Esper J (2019) ‘Concord and discord among Northern Hemisphere paleotemperature reconstructions from treerings. Quat Rev (Elsevier Ltd) 203:278–281. https://doi.org/10.1016/j.quascirev.2018.11.013
Gessler A et al (2014) Stable isotopes in treerings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol 00:1–23. https://doi.org/10.1093/treephys/tpu040
Giguère-Croteau C et al. (2019) North America’s oldest boreal trees are more efficient water users due to increased [CO2], but do not grow faster. Proc Natl Acad Sci U S A (National Academy of Sciences) 116(7):2749–2754. https://doi.org/10.1073/pnas.1816686116
Giuggiola A, Ogee J, Gessler A, Rigling A, Bugmann H, Treydte K (2016) Improvement of water and light availability after thinning at a xeric site: which matters more? A dual isotope approach. New Phytol 210:108–121
Gouirand I et al (2008) On the spatiotemporal characteristics of Fennoscandian treeringbased summer temperature reconstructions. Theor Appl Climatol (Springer Wien) 91(1–4):1–25. https://doi.org/10.1007/s00704-007-0311-7
Granda E et al (2017) Aged but withstanding: maintenance of growth rates in old pines is not related to enhanced water-use efficiency. Agric Forest Meteorol (Elsevier (BV)) 243:43–54. https://doi.org/10.1016/j.agrformet.2017.05.005
Gray J, Thompson P (1977) Climatic information from 18O/16O ratios of cellulose in tree rings. Nature 262:481–482
Green JW (1963) Methods of carbohydrate chemistry, Whistler RL (ed), 3rd edn. Academic Press, New York, NY
Grießinger J, Bräuning A, Helle G, Thomas A, Schleser G (2011) Late Holocene Asian summer monsoon variability reflected by δ18O in tree-rings from Tibetan junipers. Geophys Res Lett 38. https://doi.org/10.1029/2010GL045988
Grießinger J et al (2018) Imprints of climate signals in a 204 year δ18O treering record of Nothofagus pumilio from Perito Moreno Glacier, Southern Patagonia (50°S). Front Earth Sci (Frontiers Media (SA)) 6. https://doi.org/10.3389/feart.2018.00027
Gu L et al (2003) Response of a deciduous forest to the Mount Pinatubo eruption: enhanced photosynthesis. Science 299(5615):2035–2038. https://doi.org/10.1126/science.1078366
Hafner P et al (2014) A 520 year record of summer sunshine for the eastern European Alps based on stable carbon isotopes in larch treerings. Clim Dyn (Springer Verlag) 43(3–4):971–980. https://doi.org/10.1007/s00382-013-1864-z
Harlow BA, Marshall JD, Robinson AP (2006) A multi-species comparison of δ13C from whole wood, extractive-free wood and holocellulose. Tree Physiol (oxford University Press) 26(6):767–774. https://doi.org/10.1093/treephys/26.6.767
Hartl-Meier C, Zang C, Büntgen U, Esper J, Rothe A, Göttlein A, Dirnböck T, Treydte K (2015) Uniform climate sensitivity in tree-ring stable isotopes across species and sites in a mid-latitude temperate forest. Tree Physiol 35(1):4–15. https://doi.org/10.1093/treephys/tpu096 Epub 2014 Dec 2 PMID: 25466725
Heinrich I et al (2008) Hydroclimatic variation in Far North Queensland since 1860 inferred from treerings. Palaeogeogr Palaeoclimatol Palaeoecol (Elsevier (BV)) 270(1–2):116–127. https://doi.org/10.1016/j.palaeo.2008.09.002
Heinrich I et al (2013) Winter-to-spring temperature dynamics in Turkey derived from treerings since AD 1125. Clim Dyn 41(7–8):1685–1701. https://doi.org/10.1007/s00382-013-1702-3
Helama S et al (2002) The supra-long Scots pine treeringrecord for Finnish Lapland: Part 2, interannual to centennial variability in summer temperatures for 7500 years. Holocene 12(6):681–687. https://doi.org/10.1191/0959683602hl581rp
Helama S et al (2015) Age-related trends in subfossil treering δ13C data. Chem Geol (Elsevier (BV)) 416:28–35. https://doi.org/10.1016/j.chemgeo.2015.10.019
Helama S, Arppe L, Timonen M, Mielikaïnen K, Oinonen M (2018) A 7.5 chronology of stable carbon isotopes from tree rings with implications for their use in palaeo-cloud reconstruction. Global Planet Change 170:20–33
Hereş AM et al (2014) Drought-induced mortality selectively affects Scots pine trees that show limited intrinsic water-use efficiency responsiveness to raising atmospheric CO2. Funct Plant Biol (CSIRO Publishing) 41(3):244. https://doi.org/10.1071/fp13067
Holm JA, Kueppers LM, Chambers JQ (2017) Novel tropical forests: response to global change. New Phytologist (Wiley) 213(3):988–992. https://doi.org/10.1111/nph.14407
Holmes JA, Zhang J, Chen F, Qiang M, (2007) Paleoclimatic implications of an 850-year oxygen-isotope record from the northern Tibetan Plateau. Geophys Res Lett 24. https://doi.org/10.1029/2007GL032228
Holzkämper S et al (2012) Comparison of stable carbon and oxygen isotopes in Picea glauca treerings and Sphagnum fuscum moss remains from subarctic Canada. Quat Res (Cambridge University Press (CUP)) 78(2):295–302. https://doi.org/10.1016/j.yqres.2012.05.014
Hunter MO et al (2013) Tree height and tropical forest biomass estimation. Biogeosciences (Copernicus (GmbH)) 10(12):8385–8399. https://doi.org/10.5194/bg-10-8385-2013
Jacoby GC, D’Arrigo RD (1995) Treering width and density evidence of climatic and potential forest change in Alaska. Glob Biogeochem Cycles 9(2):227–234
Johnstone JA, Roden JS, Dawson TE (2013) Oxygen and carbon stable isotopes in coast redwood tree rings respond to spring and summer climate signals. JGR Biogeosci 118:1438–1450
Kahmen A et al (2011) Cellulose δ18O is an index of leaf-to-air vapor pressure difference (VPD) in tropical plants. Proc Natl Acad Sci 108(5):1981–1986. https://doi.org/10.1073/pnas.1018906108
Klippel L, Krusic PJ, Konter O, St. George S, Trouet V, Esper J (2019) A 1200+ year reconstruction of temperature extremes for the northeastern Mediterranean region. Int J Climatol 39:2336–2350
Kirdyanov AV et al (2008) Climate signals in treeringwidth, density and δ13C from larches in Eastern Siberia (Russia). Chem Geol 252(1–2):31–41. https://doi.org/10.1016/j.chemgeo.2008.01.023
Körner C (2009) Responses of humid tropical trees to rising CO2. Ann Rev Ecol Evol Syst (Ann Rev) 40(1):61–79. https://doi.org/10.1146/annurev.ecolsys.110308.120217
Kress A et al (2010) A 350 year drought reconstruction from Alpine treering stable isotopes. Global Biogeochem Cycles 24(2):1–16. https://doi.org/10.1029/2009GB003613
Labuhn I et al (2014) Tree age, site and climate controls on treering cellulose δ18O: a case study on oak trees from south-western France. Dendrochronologia (Elsevier (GmbH)) 32(1):78–89. https://doi.org/10.1016/j.dendro.2013.11.001
Labuhn I, Daux V, Girardclos O, Stievenard M, Pierre M, Masson-Delmotte V (2016) French summer droughts since 1326AD: a reconstruction based on treering cellulose δ18O. Clim past Discuss 12:1101–1117. https://doi.org/10.5194/cpd-11-5113-2015
Lavergne A et al (2016) Are the oxygen isotopic compositions of Fitzroya cupressoides and Nothofagus pumilio cellulose promising proxies for climate reconstructions in northern Patagonia? J Geophys Res Biogeosci (American Geophysical Union (AGU)) 121(3):767–776. https://doi.org/10.1002/2015jg003260
Lavergne A, Daux V, Villalba R, Pierre M, Stievenard M, Srur AM (2017a) Improvement of isotope-based climate reconstructions in Patagonia through a better understanding of climate influences on isotopic fractionation in tree rings. Earth Planet Sci Lett 459:372–380
Lavergne A, Gennaretti F, Risi C, Daux V, Boucher E, Savard MM, Naulier M, Villalba R, Bégin C, Guiot J (2017b) Modelling tree-ring cellulose δ18O variations of two temperature-sensitive tree species from North and South America. Clim Past Discuss 13:1515–1526. https://www.clim-past-discuss.net/cp-2017-93/
Lavergne A et al (2018) Past summer temperatures inferred from dendrochronological records of fitzroya cupressoides on the eastern slope of the northern patagonian andes. J Geophys Res (Blackwell Publishing Ltd) 123(1):32–45. https://doi.org/10.1002/2017JG003989
Lavergne A et al (2019) Observed and modelled historical trends in the water-use efficiency of plants and ecosystems. Glob Change Biol (Wiley). https://doi.org/10.1111/gcb.14634
Leavitt SW, Long A (1991) Seasonal stable-carbon isotope variability in treerings: possible paleoenvironmental signals. Chem Geol Isotope Geosci Sect (Elsevier (BV)) 87(1):59–70. https://doi.org/10.1016/0168-9622(91)90033-s
Leavitt SW, Long A (1986) Stable-carbon isotope variability in tree foliage and wood. Ecology 67(4):1002–1010
Leavitt SW, Long A (1988) Stable carbon isotope chronologies from trees in the southwestern United States. Global Biogeochem Cycles 2(3):189–198. https://doi.org/10.1029/GB002i003p00189
Leavitt SW, Long A (1989a) Drought indicated in carbon-13/carbon-12 ratios of southwestern treerings. JAWRA J Am Water Resour Assoc 25(2):341–347. https://doi.org/10.1111/j.1752-1688.1989.tb03070.x
Leavitt SW, Long A (1989b) The atmospheric δ13C record as derived from 56 pinyon trees at 14 sites in the Southwestern United States. Radiocarbon (cambridge University Press (CUP)) 31(03):469–474. https://doi.org/10.1017/s0033822200012054
Leonelli G, Battipaglia G, Cherubini P, Saurer M, Siegwolf RTW, Maugeri M, Stenni B, Fusco S, Maggi V, Pelfin M (2017) Larix decidua δ18O tree-ring cellulose mainly reflects the isotopic signature of winter snow in a high-altitude glacial valley of the European Alps. Sci Total Environ 579:230–237
Lévesque M et al (2014) Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions. New phytologist 203(1):94–109. https://doi.org/10.1111/nph.12772
Levesque M, Andreu-Hayles L, Smith WK, Williams AP, Hobi ML, Allred BW, Pederson N (2019) Tree-ring isotopes capture interannual vegetation productivity dynamics at the biome scale. Nat Commun 10:1–10. https://doi.org/10.1038/s41467-019-08634-y
Li Z-H et al (2011) Micro-scale analysis of treering δ18O and δ13C on α-cellulose spline reveals high-resolution intra-annual climate variability and tropical cyclone activity. Chem Geol (Elsevier (BV)) 284(1–2):138–147. https://doi.org/10.1016/j.chemgeo.2011.02.015
Libby LM et al (1976) Isotopic tree thermometers. Nature 261(5558):284–288. https://doi.org/10.1038/261284a0
Libby LM (1972) Multiple thermometry in paleo climate and historic climate. Tech Rep
Libby LM, Pandolfi LJ (1974) Temperature dependence of isotope ratios in treerings. Proc Natl Acad Sci U S Am 71(6):2482–2486. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=388483&tool=pmcentrez&rendertype=abstract
Lin YS et al (2015) Optimal stomatal behaviour around the world. Nat Clim Change (Nature Publishing Group) 5(5):459–464. https://doi.org/10.1038/nclimate2550
Linderholm HW et al (2014) Fennoscandia revisited: a spatially improved treeringreconstruction of summer temperatures for the last 900 years. Clim Dyn. https://doi.org/10.1007/s00382-014-2328-9
Lipp J et al (1991) Stable isotopes in treering cellulose and climatic change. Tellus 322–330. https://doi.org/10.1034/j.1600-0889.1991.t01-2-00005.x
Liu Y et al (2004) A preliminary seasonal precipitation reconstruction from treeringstable carbon isotopes at Mt. Helan, China, since AD 1804. Glob Planet Change 229–239. https://doi.org/10.1016/j.gloplacha.2004.01.009
Liu Y, Wang R, Leavitt SW, Song H, Linderholm HW, Li Q, An Z (2012) Individual and pooled tree-ring stable-carbon isotope series in Chinese pine from the Nan Wutai region, China: common signal and climate relationships. Chem Geol 330–331:17–26. https://doi.org/10.1016/j.chemgeo.2012.08.008
Liu Y et al (2014) Treeringstable carbon isotope-based May-July temperature reconstruction over Nanwutai, China, for the past century and its record of 20th century warming. Quat Sci Rev (Elsevier Ltd) 93(July):67–76. https://doi.org/10.1016/j.quascirev.2014.03.023
Liu Y et al (2018) Treeringstable carbon isotope-based April–June relative humidity reconstruction since ad 1648 in Mt. Tianmu, China. Clim Dyn (Springer Verlag) 50(5–6):1733–1745. https://doi.org/10.1007/s00382-017-3718-6
Lloyd J, Farquhar GD (2008) Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos Trans R Soc B Biol Sci (The Royal Society) 363(1498):1811–1817. https://doi.org/10.1098/rstb.2007.0032
Loader N et al (1997) An improved technique for the batch processing of small wholewood samples to α-cellulose. Chem Geol 136:313–317
Loader NJ, Heller G, Los SO, Lehmkuhl F, Schleser GH (2010) Twentieth-century summer temperature variability in the southern Altai Mountains: carbon and oxygen isotope study of tree-rings. The Holocene 20:1149–1156
Loader NJ et al (2011) Recent trends in the intrinsic water-use efficiency of ringless rainforest trees in Borneo. Philos Trans R Soc B: Biol Sci 366:3330–3339. https://doi.org/10.1098/rstb.2011.0037
Loader NJ et al (2013) Stable carbon isotopes from Torneträsk, northern Sweden provide a millennial length reconstruction of summer sunshine and its relationship to Arctic circulation. Quat Sci Rev 62:97–113. https://doi.org/10.1016/j.quascirev.2012.11.014
Loader NJ et al (2016) Measurements of hydrogen, oxygen and carbon isotope variability in Sphagnum moss along a micro-topographical gradient in a southern Patagonian peatland. J Quat Sci 31(4):426–435. https://doi.org/10.1002/jqs.2871
Loader NJ et al (2017) Inter-annual carbon isotope analysis of treerings by laser ablation. Chem Geol (Elsevier (BV)) (2016). https://doi.org/10.1016/j.chemgeo.2017.06.021
Loader NJ et al (2019) Treering dating using oxygen isotopes: a master chronology for central England. J Quat Sci (Wiley) 34(6):475–490. https://doi.org/10.1002/jqs.3115
Loader NJ, Robertson I, McCarroll D (2003) Comparison of stable carbon isotope ratios in the whole wood, cellulose and lignin of Oak treerings. Palaeogeogr Palaeoclimatol Palaeoecol 196:395–407
Lorrey AM et al (2016) Stable oxygen isotope signatures of early season wood in New Zealand kauri (Agathis australis) treerings: prospects for palaeoclimate reconstruction. Dendrochronologia (Elsevier (BV)) 40:50–63. https://doi.org/10.1016/j.dendro.2016.03.012
Luterbacher J et al (2016) European summer temperatures since Roman times. Environ Res Lett (IOP Publishing) 11(2):024001. https://doi.org/10.1088/1748-9326/11/2/024001
Mann ME, Bradley RS, Hughes MK (1999) Northern hemisphere temperatures during the past millennium: inferences, uncertainties, and limitations. Geophys Res Lett 26(6):759–762. https://doi.org/10.1029/1999GL900070
Marshall JD, Monserud RA (1996) Homeostatic gas-exchange parameters inferred from 13C/12C in treerings of conifers. Oecologia (Springer Verlag) 105(1):13–21. https://doi.org/10.1007/BF00328786
Martínez-Sancho E et al (2018) Increased water-use efficiency translates into contrasting growth patterns of Scots pine and sessile oak at their southern distribution limits. Glob Change Biol (Wiley) 24(3):1012–1028. https://doi.org/10.1111/gcb.13937
Masson-Delmotte V et al (2005) Changes in European precipitation seasonality and in drought frequencies revealed by a four-century-long treeringisotopic record from Brittany, western France. Clim Dyn (Springer Science and Business Media (LLC)) 24(1):57–69. https://doi.org/10.1007/s00382-004-0458-1
McCarroll D et al (2002) Blue reflectance provides a surrogate for latewood density of high-latitude pine treerings. Arct Antarct Alp Res 34(4):450–453. https://doi.org/10.1080/15230430.2002.12003516
McCarroll D et al (2009) Correction of treering stable carbon isotope chronologies for changes in the carbon dioxide content of the atmosphere. Geochim Cosmochim Acta 73(6):1539–1547. https://doi.org/10.1016/j.gca.2008.11.041
McCarroll D et al (2013) A 1200-year multiproxy record of tree growth and summer temperature at the northern pine forest limit of Europe. The Holocene 23:471–484. https://doi.org/10.1177/0959683612467483
McCarroll D, Loader NJ (2004) Stable isotopes in treerings. Quat Sci Rev 23:771–801. https://doi.org/10.1016/j.quascirev.2003.06.017
McCarroll D, Pawellek F (1998) Stable carbon isotope ratios of latewood cellulose in Pinus sylvestris from northern Finland: variability and signal-strength. The Holocene 8:675–684. https://doi.org/10.1191/095968398675987498
McCarroll D, Pawellek F (2001) Stable carbon isotope ratios of Pinus sylvestris from northern Finland and the potential for extracting a climate signal from long Fennoscandian chronologies. The Holocene 11(5):517–526
Medlyn BE et al (2017) How do leaf and ecosystem measures of water-use efficiency compare? New Phytologist (Wiley) 216(3):758–770. https://doi.org/10.1111/nph.14626
Meier WJH, Aravena JC, Jaña R, Braun MH, Hochreuther P, Soto-Rogel P, Grießinger J (2020) A tree-ring δ18O series from southernmost Fuego-Patagonia is recording flavors of the Antarctic oscillation. Glob Planet Change 195:103302
De Micco V et al (2019) From xylogenesis to treerings: wood traits to investigate tree response to environmental changes. IAWA J (Brill) 40(2):155–182. https://doi.org/10.1163/22941932-40190246
De Mil T et al (2017) Cambial activity in the understory of the Mayombe forest, DR Congo. Trees Struct Funct (Springer Verlag) 31(1):49–61. https://doi.org/10.1007/s00468-016-1454-x
Naulier M et al (2014) Carbon and oxygen isotopes of lakeshore black spruce trees in northeastern Canada as proxies for climatic reconstruction. Chem Geol 374–375(May 2014):37–43. https://doi.org/10.1016/j.chemgeo.2014.02.031
Naulier M et al (2015) A millennial summer temperature reconstruction for northeastern Canada using oxygen isotopes in subfossil trees. Clim Past (Copernicus (GmbH)) 11(9):1153–1164. https://doi.org/10.5194/cp-11-1153-2015
Neukom R et al (2019) No evidence for globally coherent warm and cold periods over the preindustrial common era. Nature 571(7766):550–554. https://doi.org/10.1038/s41586-019-1401-2
Ohashi S et al (2009) Detecting invisible growth rings of trees in seasonally dry forests in Thailand: isotopic and wood anatomical approaches. Trees (Springer Science and Business Media (LLC)) 23(4):813–822. https://doi.org/10.1007/s00468-009-0322-3
Ohashi S et al (2015) Seasonal variations in the stable oxygen isotope ratio of wood cellulose reveal annual rings of trees in a Central Amazon terra firme forest. Oecologia (Springer Science and Business Media (LLC)) 180(3):685–696. https://doi.org/10.1007/s00442-015-3509-x
PAGES 2k Consortium (2013) Continental-scale temperature variability during the past two millennia. Nat Geosci 6(5). https://doi.org/10.1038/ngeo1797
PAGES 2k Consortium (2017) A global multiproxy database for temperature reconstructions of the common era. Scientific Data. The Author(s), 4:170088. https://doi.org/10.1038/sdata.2017.88
Pan Y et al (2011) A large and persistent carbon sink in the world’s forests. Science (American Association for the Advancement of Science (AAAS)) 333(6045):988–993. https://doi.org/10.1126/science.1201609
Pauly M, Helle G, Miramont C, Büntgen U, Treydte K, Reinig F, Guibal F, Sivan O, Heinrich I, Riedel F, Kromer B (2018) Subfossil trees suggest enhanced Mediterranean hydroclimate variability at the onset of the Younger Dryas. Sci Rep 8(1):1–8
Pauly M, Helle G, Büntgen U, Wacker L, Treydte K, Reinig F, Turney C, Nievergelt D, Kromer B, Friedrich M, Sookdeo A, Heinrich I, Riedel F, Brauer A (2020) An annual-resolution stable isotope record from Swiss subfossil pine trees growing in the late Glacial. Quat Sci Rev. https://doi.org/10.1016/j.quascirev.2020.106550
Payomrat P et al (2018) Treeringstable carbon isotope-based June–September maximum temperature reconstruction since AD 1788, north-west Thailand. Tellus Ser B Chem Phys Meteorol (Taylor and Francis Ltd.) 70(1). https://doi.org/10.1080/16000889.2018.1443655
Pearman GI, Francey RJ, Fraser PJB (1976) Climatic implications of stable carbon isotopes in tree rings. Nature 260:771–772
Peñuelas J, Canadell JG, Ogaya R (2011) Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob Ecol Biogeogr (Wiley) 20(4):597–608. https://doi.org/10.1111/j.1466-8238.2010.00608.x
Pons TL, Helle G (2011) Identification of anatomically non-distinct annual rings in tropical trees using stable isotopes. Trees (Springer Science and Business Media (LLC)) 25(1):83–93. https://doi.org/10.1007/s00468-010-0527-5
Porter TJ et al (2009) Climatic signals in δ13C and δ18O of treerings from White Spruce in the Mackenzie Delta Region, Northern Canada. Arct Antarct Alp Res (Informa (UK) Limited) 41(4):497–505. https://doi.org/10.1657/1938-4246-41.4.497
Porter TJ et al (2013) Spring-summer temperatures since {AD} 1780 reconstructed from stable oxygen isotope ratios in white spruce treerings from the Mackenzie Delta, northwestern Canada. Clim Dyn (Springer Science and Business Media (LLC)) 42(3–4):771–785. https://doi.org/10.1007/s00382-013-1674-3
Poussart PF, Evans MN, Schrag DP (2004) Resolving seasonality in tropical trees: multi-decade, high-resolution oxygen and carbon isotope records from Indonesia and Thailand. Earth Planet Sci Lett (Elsevier (BV)) 218(3–4):301–316. https://doi.org/10.1016/s0012-821x(03)00638-1
Poussart PF, Schrag DP (2005) Seasonally resolved stable isotope chronologies from northern Thailand deciduous trees. Earth Planet Sci Lett (Elsevier (BV)) 235(3–4):752–765. https://doi.org/10.1016/j.epsl.2005.05.012
Pumijumnong N, Eckstein D (2011) Reconstruction of pre-monsoon weather conditions in northwestern Thailand from the treeringwidths of Pinus merkusii and Pinus kesiya. Trees (Springer Science and Business Media (LLC)) 25(1):125–132. https://doi.org/10.1007/s00468-010-0528-4
Qin C, Yang B, Bräuning A, Grießinger J, Wernicke J (2015) Drought signals in tree-ring stable oxygen isotope series of Qilian juniper from the arid northeastern Tibetan Plateau. Glob Planet Change 125:48–59
Raffalli-Delerce G, Masson-Delmotte V, Dupouey JL, Stievenard M, Breda N, Moisselin JM (2004) Reconstruction of summer droughts using tree-ring cellulose isotopes: a calibration study with living oaks from Brittany (Western France). Tellus B Chem Phys Meteorol 56:160–174. https://doi.org/10.1111/j.1600-0889.2004.00086.x
Rahman M et al (2019) Trends in tree growth and intrinsic water-use efficiency in the tropics under elevated CO2 and climate change. Trees (Springer Science and Business Media (LLC)) 33(3):623–640. https://doi.org/10.1007/s00468-019-01836-3
Ramesh R, Bhattacharya SK, Gopalan K (1986) Climatic correlations in the stable isotope records of silver fir (Abies pindrow) trees from Kashmir, India. Earth Planet Sci Lett 79(1–2):66–74. https://doi.org/10.1016/0012-821X(86)90041-5
Rayback SA, Henry GHR (2006) Reconstruction of summer temperature for a Canadian high arctic site from retrospective analysis of the dwarf shrub, Cassiope tetragona. Arct Antarct Alp Res 38(2):228–238. https://doi.org/10.1657/1523-0430(2006)38[228:ROSTFA]2.0.CO,2
Reynolds-Henne CE, Siegwolf RTW, Henne S, Treydte KS, Esper J, Saurer M (2007) Temporal stability of climate-isotope relationships in tree rings of oak and pine (Ticino, Switzerland). Glob Biogeochem Cycles 21(GB4009):2007. https://doi.org/10.1029/2007GB002945
Rinne KT et al (2005) On the purification of α-cellulose from resinous wood for stable isotope (H, C and O) analysis. Chem Geol 222:75–82
Rinne KTK, Loader NJN, Switsur VR, Waterhouse JS (2013) 400-year May–August precipitation reconstruction for Southern England using oxygen isotopes in tree rings. Quat Sci Rev 60:13–25. https://doi.org/10.1016/j.quascirev.2012.10.048
Robert EMR et al (2011) Mangrove growth rings: fact or fiction? Trees (Springer Science and Business Media (LLC)) 25(1):49–58. https://doi.org/10.1007/s00468-010-0487-9
Robertson I, Waterhouse JS, Barker AC, Carter AHC, Switsur VR (2001) Oxygen isotope ratios of oak in east England: implications for reconstructing the isotopic composition of precipitation. Earth Planet Sci Lett 191:21–31
Robertson I, Rolfe J et al (1997) Signal strength and climate relationships in 13C/12C ratios of treering cellulose from oak in southwest Finland. Geophys Res Lett (American Geophysical Union) 24(12):1487–1490. https://doi.org/10.1029/97GL01293
Roden JS, Ehleringer JR (2007) Summer precipitation influences the stable oxygen and carbon isotopic composition of treeringcellulose in Pinus ponderosa. Tree Physiol 27(4):491–501. https://doi.org/10.1093/treephys/27.4.491
Roden JS, Johnstone JA, Dawson TE (2011) Regional and watershed-scale coherence in the stable-oxygen and carbon isotope ratio time series in tree rings of coast redwood (Sequoia sempervirens). Tree-Ring Res 67:71–86. https://doi.org/10.3959/2010-4.1
Rodríguez-Catón, M., Andreu-Hayles, L., Morales, M.S., Daux, V., Christie, D.A., Coopman, R.E., Alvarez, C., Rao, M.P., Aliste, D., Flores, F. & Villalba, R. (2021) Different climate sensitivity for radial growth, but uniform for tree-ring stable isotopes along an aridity gradient in Polylepis tarapacana, the world's highest elevation tree species. Tree Physiology, 41, 1353–1371
Roig FA, Siegwolf R, Boninsegna JA (2006) Stable oxygen isotopes (δ18O) in Austrocedrus chilensis treerings reflect climate variability in northwestern Patagonia, Argentina. Int J Biometeorol (Springer Science and Business Media (LLC)) 51(2):97–105. https://doi.org/10.1007/s00484-006-0049-4
Rozanski K, Araguas-Araguas GL (1993) Isotopic patterns in modern global precipitation. Geophys Monogr Ser. https://doi.org/10.1029/GM078p0001
Rozendaal DMA, Zuidema PA (2011) Dendroecology in the tropics: a review. Trees Struct Funct 3–16. https://doi.org/10.1007/s00468-010-0480-3
Sano M, Buckley BM, Sweda T (2009) Treeringbased hydroclimate reconstruction over northern Vietnam from Fokienia hodginsii: eighteenth century mega-drought and tropical Pacific influence. Clim Dyn (Springer Science and Business Media (LLC)) 33(2–3):331–340. https://doi.org/10.1007/s00382-008-0454-y
Santini F et al (2018) Scarce population genetic differentiation but substantial spatiotemporal phenotypic variation of water-use efficiency in Pinus sylvestris at its western distribution range. Eur J Forest Res (Springer Science and Business Media (LLC)) 137(6):863–878. https://doi.org/10.1007/s10342-018-1145-9
Sass-Klaassen U et al (2016) A tree-centered approach to assess impacts of extreme climatic events on forests. Front Plant Sci (Frontiers Media (SA)) 7. https://doi.org/10.3389/fpls.2016.01069
Saurer M, Cherubini P, Siegwolf R (2000) Oxygen isotopes in tree rings of Abies alba: the climatic significance of interdecadal variations. J Geophys Res 105:12461–12470
Saurer M, Cherubini P, Reynolds-Henna CE, Treydte KS, Anderson WT, Siegwolf RTW (2008) An investigation of the common signal in treering stable isotope chronologies at temperate sites. J Geophys Res 113:1–11. https://doi.org/10.1029/2008jg000689
Saurer M, Borella S, Schweingruber F et al (1997) Stable carbon isotopes in tree rings of beech: climatic versus site-related influences. Trees 11:291–297. https://doi.org/10.1007/s004680050087
Saurer M et al (2014) Spatial variability and temporal trends in water-use efficiency of European forests. Glob Change Biol 20(12). https://doi.org/10.1111/gcb.12717
Saurer M, Siegwolf RTW, Schweingruber FH (2004) Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob Change Biol (Wiley) 10(12):2109–2120. https://doi.org/10.1111/j.1365-2486.2004.00869.x
Savard MM, Daux V (2020) An overview on isotopic divergences–causes for instability of tree-ring isotopes and climate correlations. Clim past 16:1223–1243. https://doi.org/10.5194/cp-16-1223-2020
Schifman LA et al (2012) Carbon isotope variation in shrub willow (Salix spp.) ring-wood as an indicator of long-term water status, growth and survival. Biomass Bioenerg 36:316–326. https://doi.org/10.1016/j.biombioe.2011.10.042
Schleser GH et al (1999) Isotope signals as climate proxies: the role of transfer functions in the study of terrestrial archives. Quat Sci Rev (Elsevier Ltd) 18(7):927–943. https://doi.org/10.1016/S0277-3791(99)00006-2
Schneider L et al (2015) Revising midlatitude summer temperatures back to A.D. 600 based on a wood density network. Geophys Res Lett (Wiley) 42(11):4556–4562. https://doi.org/10.1002/2015GL063956@10.1002/(ISSN)1944-8007.2015EDHIGHLIGHTS
Schollaen K et al (2013) {ENSO} flavors in a treering δ18O record of Tectona grandis from Indonesia. Clim Past (Copernicus (GmbH)) 11(10):1325–1333. https://doi.org/10.5194/cp-11-1325-2015
Schollaen K, Karamperidou C, Krusic P, Cook E, Helle G (2015) ENSO flavors in a tree-ring delta O-18 record of Tectona grandis from Indonesia. Clim past 11:1325–1333
Schongart J et al (2004) Teleconnection between tree growth in the Amazonian floodplains and the El Nino-Southern Oscillation effect. Glob Change Biol (Wiley) 10(5):683–692. https://doi.org/10.1111/j.1529-8817.2003.00754.x
Schöngart J et al (2002) Phenology and stem-growth periodicity of tree species in Amazonian floodplain forests. J Trop Ecol (Cambridge University Press (CUP)) 18(4):581–597. https://doi.org/10.1017/s0266467402002389
Schubert BA, Timmermann A (2015) Reconstruction of seasonal precipitation in Hawai’i using high-resolution carbon isotope measurements across treerings. Chem Geol (Elsevier (BV)) 417:273–278. https://doi.org/10.1016/j.chemgeo.2015.10.013
Seftigen K, Linderholm HW, Loader NJ, Liu Y, Young GHF (2011) The influence of climate on 13C/12C and 18O/16O ratios in tree ring cellulose of Pinus sylvestris L. growing in the central Scandinavian Mountains. Chem Geol 286:84–93
Shestakova TA et al (2019) Spatio-temporal patterns of tree growth as related to carbon isotope fractionation in European forests under changing climate. Glob Ecol Biogeogr (Blackwell Publishing Ltd). https://doi.org/10.1111/geb.12933
Sidorova OV et al (2013) The application of treerings and stable isotopes for reconstructions of climate conditions in the Russian Altai. Clim Change 120:153–167. https://doi.org/10.1007/s10584-013-0805-5
Silva LCR et al (2015) Isotopic and nutritional evidence for species- and site-specific responses to N deposition and elevated CO2 in temperate forests. J Geophys Res Biogeosci (American Geophysical Union (AGU)) 120(6):1110–1123. https://doi.org/10.1002/2014jg002865
Silva LCR, Anand M (2013) Probing for the influence of atmospheric CO2 and climate change on forest ecosystems across biomes. Glob Ecol Biogeogr Edited by b. Shipley (Wiley) 22(1):83–92. https://doi.org/10.1111/j.1466-8238.2012.00783.x
Skomarkova MV, Vaganova EA, Mund M, Knohl A, Linke P, Boerner A, Schulze ED (2006) Inter-annual and seasonal variability of radial growth, wood density and carbon isotope ratios in tree rings of beech (Fagus sylvatica) growing in Germany and Italy. Trees 20(5):571–586
van der Sleen P et al (2014) Understanding causes of tree growth response to gap formation: ∆13C-values in treerings reveal a predominant effect of light. Trees (Springer Science and Business Media (LLC)) 28(2):439–448. https://doi.org/10.1007/s00468-013-0961-2
van der Sleen P, Zuidema PA, Pons TL (2017) Stable isotopes in tropical treerings: theory, methods and applications. Funct Ecol Edited by r. Oliveira (Wiley) 31(9):1674–1689. https://doi.org/10.1111/1365-2435.12889
Smerdon JE, Pollack HN (2016) Reconstructing earth’s surface temperature over the past 2000 years: the science behind the headlines. Wiley Interdiscip Rev Clim Change (Wiley-Blackwell) 746–771. https://doi.org/10.1002/wcc.418
Song L et al (2016) Water use patterns of Pinus sylvestris var. mongolica trees of different ages in a semiarid sandy lands of Northeast China. Environ Exp Bot (Elsevier (BV)) 129:94–107. https://doi.org/10.1016/j.envexpbot.2016.02.006
Sprenger M, Seeger S, Blume T, Weiler M (2016) Travel times in the vadose zone: variability in space and time. Water Resour Res 52:5727–5754. https://doi.org/10.1002/2015WR018077
Stahle DW (1999) Useful strategies for the development of tropical treering chronologies. IAWA J (Brill) 20(3):249–253. https://doi.org/10.1163/22941932-90000688
Steppe K, Sterck F, Deslauriers A (2015) Diel growth dynamics in tree stems: linking anatomy and ecophysiology. Trends Plant Sci (Elsevier (BV)) 20(6):335–343. https://doi.org/10.1016/j.tplants.2015.03.015
Sternberg LDSL O’Reilly (2009) Oxygen stable isotope ratios of tree-ring cellulose: the next phase of understanding. New Phytologist 181:553–562. https://doi.org/10.1111/j.1469-8137.2008.02661.x
Stoffel M et al (2015) Estimates of volcanic-induced cooling in the Northern Hemisphere over the past 1,500 years. Nat Geosci (Nature Publishing Group) 8(10):784–788. https://doi.org/10.1038/ngeo2526
Szejner P et al (2016) Latitudinal gradients in treering stable carbon and oxygen isotopes reveal differential climate influences of the North American Monsoon system. J Geophys Res Biogeosci (Blackwell Publishing Ltd) 121(7):1978–1991. https://doi.org/10.1002/2016JG003460
Therrell MD et al (2006) Treeringreconstructed rainfall variability in Zimbabwe. Clim Dyn (Springer Science and Business Media (LLC)) 26(7–8):677–685. https://doi.org/10.1007/s00382-005-0108-2
Treydte KS et al (2006) The twentieth century was the wettest period in northern Pakistan over the past millennium. Nature 440(April):1179–1182. https://doi.org/10.1038/nature04743
Treydte K, Frank D et al (2007) Signal strength and climate calibration of a European tree-ring isotope network. Geophys Res Lett 34:L24302. https://doi.org/10.1029/2007GL031106
Treydte KS et al (2009) Impact of climate and CO2 on a millennium-long treeringcarbon isotope record. Geochim Cosmochim Acta (Elsevier Ltd) 73(16):4635–4647. https://doi.org/10.1016/j.gca.2009.05.057
Trouet V, Esper J, Beeckman H (2010) Climate/growth relationships of Brachystegia spiciformis from the miombo woodland in south central Africa. Dendrochronologia (Elsevier (BV)) 28(3):161–171. https://doi.org/10.1016/j.dendro.2009.10.002
Verheyden A et al (2004a) Annual cyclicity in high-resolution stable carbon and oxygen isotope ratios in the wood of the mangrove tree Rhizophora mucronata. Plant Cell Environ (Wiley) 27(12):1525–1536. https://doi.org/10.1111/j.1365-3040.2004.01258.x
Verheyden A et al (2004b) Growth rings, growth ring formation and age determination in the mangrove Rhizophora mucronata. Ann Bot 94(1):59–66. https://doi.org/10.1093/aob/mch115
Verheyden A et al (2005) Comparison between δ13C of α-cellulose and bulk wood in the mangrove tree Rhizophora mucronata: implications for dendrochemistry. Chem Geol 219(1–4):275–282. https://doi.org/10.1016/j.chemgeo.2005.02.015
Volland F, Pucha D, Bräuning A (2016) Hydro-climatic variability in southern Ecuador reflected by treeringoxygen isotopes. Erdkunde 70(1):69–82. https://doi.org/10.3112/erdkunde.2016.01.05
Voltas J et al (2013) A retrospective, dual-isotope approach reveals individual predispositions to winter-drought induced tree dieback in the southernmost distribution limit of Scots pine. Plant Cell Environ 36(8):1435–1448. https://doi.org/10.1111/pce.12072
Waterhouse JS et al (2004) Northern European trees show a progressively diminishing response to increasing atmospheric carbon dioxide concentrations. Quat Sci Rev 23(7–8):803–810. https://doi.org/10.1016/j.quascirev.2003.06.011
Welker JM, Rayback S, Henry GHR (2005) Arctic and North Atlantic Oscillation phase changes are recorded in the isotopes (δ18O and δ13C) of Cassiope tetragona plants. Glob Change Biol 11:997–1002
Wernicke J, Grießinger J, Hochreuther P, Bräuning A (2015) Variability of summer humidity during the past 800 years on the eastern Tibetan Plateau inferred from δ18O of tree-ring cellulose. Clim past 11:327–337
Wilson AT, Grinsted MJ (1977) 12C/13C in cellulose and lignin as palaeothermometers. Nature 265(5590):133. https://www.nature.com/articles/265133a0. Accessed on:28 October 2019
Wilson AT, Grinsted MJ (1978) The possibilities of deriving past climate information from stable isotope studies on treerings. N Z Dep Sci Ind Res Bull 220:61–66. https://inis.iaea.org/search/search.aspx?orig_q=RN:9394183. Accessed on 1 November 2019
Wilson R et al (2014) Blue Intensity for dendroclimatology: the BC blues: a case study from British Columbia, Canada. Holocene (SAGE Publications Ltd) 24(11):1428–1438. https://doi.org/10.1177/0959683614544051
Wilson R et al (2016) Last millennium northern hemisphere summer temperatures from treerings: Part I: The long term context. Quat Sci Rev. https://doi.org/10.1016/j.quascirev.2015.12.005
Woodley EJ et al (2012) Estimating uncertainty in pooled stable isotope time-series from treerings. Chem Geol 294–295:243–248. https://doi.org/10.1016/j.chemgeo.2011.12.008
Worbes M (1999) Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo forest reserve in Venezuela. J Ecol (Wiley) 87(3):391–403. https://doi.org/10.1046/j.1365-2745.1999.00361.x
Worbes M (2002) One hundred years of treeringresearch in the tropics - a brief history and an outlook to future challenges. Dendrochronologia (Elsevier (GmbH)) 20(1–2):217–231. https://doi.org/10.1078/1125-7865-00018
Worbes M, Junk WJ (1999) How old are tropical trees? The persistence of a Myth. IAWA J (Brill) 20(3):255–260. https://doi.org/10.1163/22941932-90000689
Wright SJ, Muller-Landau HC, Schipper JAN (2009) The future of tropical species on a warmer planet. Conserv Biol (Wiley) 23(6):1418–1426. https://doi.org/10.1111/j.1523-1739.2009.01337.x
Xu C, Buckley BM, Wang S-YS, An W, Li Z, Nakatsuka T, Guo Z (2021) Oxygen isotopes in tree rings from greenland: a new proxy of NAO. Atmosphere 12:39
Xu G, Liu X, Sun W, Szejner P, Zeng X, Yoshimura K, Trouet V (2020) Seasonal divergence between soil water availability and atmospheric moisture recorded in intra-annual tree-ring δ18O extremes. Environ Res Lett 15:094036
Yapp CJ, Epstein S (1977) Climatic implications of D/H ratios of meteoric water over North America (9500–22,000 B.P.) as inferred from ancient wood cellulose CH hydrogen. Earth Planet Sci Lett 34(3):333–350. https://doi.org/10.1016/0012-821X(77)90043-7
Young GHF et al (2010) A 500-year record of summer near-ground solar radiation from treeringstable carbon isotopes. The Holocene 20(3):315–324
Young GHF et al (2012a) Central England temperature since AD 1850: the potential of stable carbon isotopes in British oak trees to reconstruct past summer temperatures. J Quat Sci 27(6):606–614
Young GHF et al (2012b) Changes in atmospheric circulation and the Arctic Oscillation preserved within a millennial length reconstruction of summer cloud cover from northern Fennoscandia. Clim Dyn 39(1–2):495–507. https://doi.org/10.1007/s00382-011-1246-3
Young GHF, Bale RJ, Loader NJ, Mccarroll D, Nayling N, Vousden N (2012c) Central England temperature since AD 1850: the potential of stable carbon isotopes in British oak trees to reconstruct past summer temperatures. J Quat Sci 27:606–614
Young GHF et al (2015) Oxygen stable isotope ratios from British oak treerings provide a strong and consistent record of past changes in summer rainfall. Clim Dyn. https://doi.org/10.1007/s00382-015-2559-4
Young GHF et al (2019) Cloud cover feedback moderates fennoscandian summer temperature changes over the past 1,000 years. Geophys Res Lett (Blackwell Publishing Ltd) 46(5):2811–2819. https://doi.org/10.1029/2018GL081046
Zhu J, Poulsen CJ, Tierney JE (2019) Simulation of Eocene extreme warmth and high climate sensitivity through cloud feedbacks. Sci Adv (American Association for the Advancement of Science (AAAS)) 5(9):eaax1874. https://doi.org/10.1126/sciadv.aax1874
Zuidema PA et al (2013) Tropical forests and global change: filling knowledge gaps. Trends Plant Sci (Elsevier (BV)) 18(8):413–419. https://doi.org/10.1016/j.tplants.2013.05.006
Zuidema PA, Brienen RJW, Schöngart J (2012) Tropical forest warming: looking backwards for more insights. Trends Ecol Evol (Elsevier (BV)) 27(4):193–194. https://doi.org/10.1016/j.tree.2011.12.007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 © The Author(s)
About this chapter
Cite this chapter
Gagen, M. et al. (2022). Climate Signals in Stable Isotope Tree-Ring Records. In: Siegwolf, R.T.W., Brooks, J.R., Roden, J., Saurer, M. (eds) Stable Isotopes in Tree Rings. Tree Physiology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-92698-4_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-92698-4_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92697-7
Online ISBN: 978-3-030-92698-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)