Abstract
Although the fields of dendrochronology and light stable-isotope mass spectrometry emerged at different times in the first half of the 20th Century, their convergence with the earliest measurements of isotope composition of tree rings is now ca. 70 years old. Much of the early stable isotope analysis (including on wood) explored natural variation of isotopes in the environment, but those researchers making the measurements were already contemplating the role of the isotope composition of the source substrates (e.g., water and CO2), biochemical fractionation, and environment as contributors to final tree-ring isotope values. Growing interest in tree-ring isotopes was heavily motivated by paleoclimate or paleoatmosphere reconstruction, but this new field rapidly developed to generate greatly improved mechanistic understanding along with expanded applications to physiology, ecology, pollution, and more. This chapter primarily charts the historical progression in tree-ring C-H-O isotope studies over those seven decades, but it also identifies potential productive emerging and future directions.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
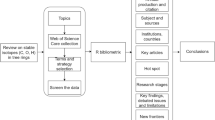
The roots of stable-isotope dendrochronology extend back to the middle of the 20th Century during the convergence of the maturing discipline dendrochronology with development of powerful isotope analytical tools. By that time, methods and principles of tree-ring studies (see Chap. 2) had already been developed and refined for about 50 years, largely based on the year-to-year variability in size of rings, but to which an arsenal of other tree-ring measurements has progressively been added over the next 50 years (e.g., wood density, cell size, and dendrochemistry including isotopes). Additionally, measurement of isotope ratios by mass spectrometers became established by the middle of the century, providing a new tool for quantitative research. A large (and rapidly growing) number of stable-isotope dendrochronology investigations have been conducted since the commingling of the two scientific fields, as related to reconstruction and understanding of weather/climate, ecology, and physiology. We can view any stable-isotope series derived from tree rings as a rich and layered story that has been transcribed by physiological, physicochemical and biochemical processes in response to a wide range of influences from climate to atmospheric chemistry and pollution to ecology (Fig. 1.1).
Schematic representation of interplay between environmental factors and ecophysiology that produces tree-ring C-H-O isotope composition. Environment can influence the ecophysiological, biochemical, and physicochemical mechanisms responsible for producing the isotope composition and consequently isotope dendrochronology can be used to infer environment and ecological events affecting ecophysiology (e.g., insect outbreaks). Tree rings allow identification of changes through time, but if a network of sites is sampled, the tree-ring isotope results can also reveal changes in space (isoscapes)
The dramatic growth of this field from the 1980s into the 2000s is illustrated in the burgeoning number of papers presented at international tree-ring conferences (Fig. 1.2). Several useful overviews are available in the literature related to tree-ring isotope methodology, theory, and applications, such as McCarroll and Loader (2004), Robertson et al. (2008) and Managave and Ramesh (2012). This chapter describes the historical trajectory of studies and their findings from measurements of stable-isotope composition of tree rings. It is not exhaustive, but fortunately further details and more recent advancements and applications are covered in many of the other chapters in this volume.
Growth in numbers of light-stable-isotope tree-ring papers (oral and poster) presented at major international tree-ring conferences. Other major meetings, such as AGU, EGU, INQUA, AAG, and regional tree-ring meetings in Asia, Europe and the Americas often also feature tree-ring isotope papers in various sessions
2 Origins
The development of the isotope-ratio mass spectrometer (IRMS) opened the door to isotope measurements on tree rings (and many other materials). Key to the development of IRMS technology was Alfred O.C. Nier at the University of Minnesota, who in the late 1940s‒early 1950s produced the “double-focusing” mass spectrometer, which used both electrostatic and magnetic focusing of ion beams containing the isotopes of interest, an advance that is the basis of most modern instrumentation (Prohaska 2015). Harold C. Urey at the University of Chicago then added a dual inlet system into the design for alternately admitting aliquots of reference gas and unknown gas into the mass spectrometer (Brand et al. 2015). His newly configured mass spectrometer was applied to measurement of stable isotope composition of various materials, in collaboration with renowned geochemists in the early stages of their careers, such as Samuel Epstein and Harmon Craig. Urey was able to discern the importance of temperature effects on isotope fractionation in carbonate-water equilibria, which established the potential of isotope “thermometers” as a novel tool in paleoclimatology (Epstein et al. 1951; Fairbridge and Gornitz 2009).
Craig’s Ph.D. research sought to characterize natural carbon isotope variability in the carbon system (Craig 1953) through extensive δ13C analyses of hot-spring gases, carbon in sedimentary, igneous and metamorphic rocks, terrestrial and marine organic carbon, and a variety of freshwater and marine carbonate rocks. Among the organic carbon samples, he analyzed 22 “modern” wood samples (AD 1892–1950) from four continents and 16 “fossil” wood samples primarily from N. America (15 of which were radiocarbon-dated by Willard Libby of the University of Chicago at 2300 to >25,000 years old). In the modern wood, no patterns in isotope composition related to species, age, geographical location or elevation were detected. The δ13C of the fossil wood fell within the range of modern wood, –22.5 to –27.3‰. Overall, Craig was cognizant that changes in δ13C of CO2 could influence the isotope composition of wood but thought any such changes were being “randomly masked” by other factors, probably local environment effects. He was also aware of interannual variability in isotopic composition among rings within individual trees.
In his Science paper the following year, Craig (1954) interpreted the δ13C data in Craig (1953) as indicating that δ13C of atmospheric CO2 has not varied by more than 2‰ over the last 25,000 years and may have actually been fairly constant over millions of years. However, the real novelty of the new paper was that it communicated the first-ever δ13C analyses of annual growth rings of a calendar-dated giant sequoia from the Sierra Nevada mountains of California. One or two tree rings in each century were analyzed from the period from 1072 B.C. to A.D. 1649. The δ13C values steadily increased ca. 2‰ in the first 150 years of the record, with various upward and downward deviations thereafter. Coincidentally, in long-lived kauri trees in New Zealand, Jansen (1962) found a similar increase in tree-ring δ13C over the first 200 years of the tree’s 800-year life. The sequoia isotope variability did not seem to be related to precipitation (although the tree may have been growing in a landscape position unlikely to be limited by moisture), and variability of atmospheric δ13C of CO2 was discounted. Presaging advancements to come later in the 20th Century, Craig concluded that effects of environmental conditions (e.g., light, temperature, precipitation) on fractionation during photosynthesis and respiration were the primary contributors to the isotope variation observed.
This pioneering tree-ring isotope work in the 1950s exclusively involved stable-carbon isotopes, presumably because the C isotope composition could be readily measured on CO2 gas after the wood samples were combusted. Analysis of H and O isotopes in tree-ring cellulose would have to wait until the 1970s when pretreatment and off-line vacuum methodologies were developed for analyzing only non-exchangeable H (by nitration of cellulose) and procedures for analyzing O isotopes in organic matter (by mercury chloride or nickel pyrolysis) were applied to tree rings (see Epstein et al. 1977).
3 Advances
3.1 20th Century Spin Up
Over the next several decades of isotope dendrochronology, efforts were largely focused on the use of isotopes in tree rings as paleothermometers, along the line of the great success with isotopic composition of marine forams to understand glacial-interglacial temperature history over the last few million years (e.g., Emiliani 1955; Emiliani and Geiss 1959). These tree-ring isotope efforts began in earnest in the 1970s with a surge of papers attempting to quantify temperature coefficients. For carbon isotopes, the early studies by Libby and Pandolfi (1974), Pearman et al. (1976), Wilson and Grinsted (1977), Tans (1978), Farmer (1979), and Harkness and Miller (1980) found both positive and negative temperature coefficients with tree-ring δ13C. Additionally, the 1970s saw attempts to use tree-ring δ13C as a measure of changes in δ13C of atmospheric CO2 (Freyer and Wiesberg 1973; Stuiver 1978; Freyer 1979a, 1981), in an effort to extend the record of regular direct measurements at Mauna Loa and the South Pole, which had just begun at Mauna Loa in the late 1950s (Keeling et al. 1979). Such records are critical to understanding alteration of the global carbon cycle by anthropogenic inputs to the atmosphere of carbon from fossil fuels and land-use change. The above studies did not analyze a common standard wood component nor did they establish a minimum number of trees to be sampled to ensure the δ13C records were representative, which probably also contributes to some of the variability among published results. Variability of isotopes within and between trees and among species was later addressed by Ramesh et al. (1985), Leavitt and Long (1986), and Leavitt (2010). The impetus for developing tree-ring δ13C records as a proxy for long-term changes in δ13C of atmospheric CO2 faded by the late 1980s as reliable isotopic measurements on atmospheric gas trapped in ice cores became established (e.g., Friedli et al. 1986) and the role of environmental influences complicating interpretation of tree-ring δ13C relative to atmospheric δ13C was more fully appreciated.
The development of a plant carbon-isotope fractionation model in the early 1980s (Vogel 1980; Farquhar 1982; see also Chap. 9) provided both the theoretical background with which to better explore the potential of isotope dendrochronology and new insights into the source of the isotope-environment relationships (Francey and Farquhar 1982). Consequently, the next wave of tree-ring δ13C papers usually refined their analysis and interpretations with respect to this model and the reality that a multitude of environmental parameters could actually influence plant δ13C (i.e., δ13 of atmospheric CO2, temperature, light, moisture, humidity) (e.g., Freyer and Belacy 1983; Peng et al. 1983; Stuiver et al. 1984; Stuiver and Braziunas 1987; Leavitt and Long 1988, 1989). The model provides a basis for understanding differences between δ13C records as well as for selection of tree locations to best capture variability of an environmental parameter of interest.
Meanwhile, H and O isotopes of water in tree rings were also being explored in greater depth in the 1970s. For example, Schiegl (1974) was the first to identify a positive correlation between temperature and δ2H in tree rings (of spruce), which was predicted based on the established positive relationship between temperature and δ2H and δ18O of precipitation (Dansgaard 1964), i.e., source water for photosynthates formed in leaves. The whole-wood analysis of Schiegl (1974) left some room for uncertainty because of the suite of compounds in whole wood as well as the fact that analysis of whole wood would include both non-exchangeable and exchangeable hydrogen, the latter unlikely to represent the original composition when the wood was formed. Epstein and Yapp (1976) and Epstein et al. (1976) circumvented these problems by analyzing the cellulose component of wood and removing the exchangeable H atoms by replacing them with nitrate. Tree-ring δ18O studies in these early decades also found positive correlations of δ18O with temperature, but again no specific wood constituent was being analyzed by all studies (e.g., Gray and Thompson 1976; Libby and Pandolfi 1979; Burk and Stuiver 1981). These various early studies identified climate relationships by either spatial gradient analysis of isotope composition of tree rings and mean climate of different sites, or by isotope analysis of tree-ring series compared to inter-annual climate variation, and the results were not always the same. For both δ2H and δ18O, some studies were also identifying significant correlations with direct water-related parameters such as precipitation and humidity (e.g., Burk and Stuiver 1981; Edwards et al. 1985; Krishnamurthy and Epstein 1985; Ramesh et al. 1986).
With expanded awareness of likely environmental and physiological influences on the isotopic composition of meteoric water and water being fixed in plants, including temperature, evaporation, humidity, and biochemical fractionation by enzymes, increasingly sophisticated models for plant δ2H and δ18O slowly began to emerge in the 1980s (e.g., Burk and Stuiver 1981; Edwards et al. 1985; Edwards and Fritz 1986; Saurer et al. 1997). A critical advance in model refinement was supported by the experimental work of Roden and Ehleringer (1999a, b, 2000) in the 1990s examining the acquisition of leaf and tree-ring isotope values under controlled environmental conditions including source water isotope composition and humidity. This culminated in the advanced mechanistic model for tree-ring δ2H and δ18O developed by Roden et al. (2000) (see also Chaps. 10 and 11). This work exposed the primary contributions of both isotopic composition of source water (taken up by the roots) and evaporation in the leaves (along with the biochemical fractionation) toward influencing the composition of the rings. This supported results of previous empirical δ2H and δ18O tree-ring studies, which depending on dominant influence, could be alternately reflecting (a) source water isotope composition (and thus temperature related to atmospheric condensation processes) or (b) moisture variables such as evaporation, relative humidity, vapor pressure deficit, rainfall. Further refinements have been made to account for the Péclet effect, the consequence of net convective and diffusive water movement in the leaf (Barbour et al. 2004), and to improve understanding of post-photosynthetic processes influencing isotopes (Gessler et al. 2014).
3.2 21st Century Expansion
With these models established for stable C, H and O isotopes in tree rings, climate‒tree-ring isotope studies in the 2000s could now be planned and interpreted based on refined understanding of the environmental controls on fractionation processes. The wave of these studies was fortuitously aided by the timely development of continuous flow-through technology for gases produced by elemental analyzers and streamed with He carrier gas into the isotope-ratio mass spectrometers (Brenna et al. 1997; see also Chaps. 6 and 7), which resulted in faster analysis. The elemental analyzers contributed to this reduction in analysis time by producing the gases needed for analysis in minutes compared to the previous need for lengthy production and purification of the gases on separate (“off-line”) vacuum-line systems. This new instrumentation also allowed analysis on samples of as little as tens of micrograms instead of several milligrams commonly needed with the previous generation of mass spectrometers.
In the last two decades, tree-ring isotopes (particularly of oxygen and carbon) have been used to explore, identify and reconstruct various elements of temporal climate variability. For example, variability and impact of large-scale climate modes, such as ENSO (El Nino-Southern Oscillation) have been studied with δ18O in the Amazon basin, N. America, and Asia, (e.g., Li et al. 2011; Brienen et al. 2012; Sano et al. 2012; Xu et al. 2013a, b; Labotka et al. 2015; Liu et al. 2017). Many of these studies were based on precipitation/evaporation links to δ18O, but others utilized δ18O in tree rings as related to rainfall and drought (e.g., Treydte et al. 2006; Rinne et al. 2013; Young et al. 2015), humidity (e.g., Wright and Leavitt 2006; An et al. 2013; Labuhn et al. 2016), monsoon variability (e.g., Grießinger et al. 2011; Szejner et al. 2016), and even tropical cyclones (e.g., Miller et al. 2006). Tree-ring δ18O itself has likewise been used to better explore ecophysiological aspects of the models (e.g., Gessler et al. 2013).
Stable-carbon isotopes have likewise been an important component of a sweeping range of tree-ring projects. Some of these studies have sought climatological information, inferring temperature and precipitation (e.g., Barber et al. 2004; Liu et al. 2008, 2014) and even sunlight/sunshine (e.g., Ogle et al. 2005; Young et al. 2010; Gagen et al. 2011; Loader et al. 2013; Hafner et al. 2014) because according to the plant carbon isotope fractionation models, the amount of sunlight can influence rate of photosynthesis, which in turn would affect the ratio of intercellular to ambient CO2 (ci/ca) and thus δ13C. Tree-ring carbon isotopes have been used in ecological studies related to destructive insect outbreaks (e.g., Haavik et al. 2008; Hultine et al. 2013), some of which also use δ18O (Kress et al. 2009; Weidner et al. 2010). Many other studies are more ecophysiological in nature, inferring water-use efficiency and stomatal response (e.g., Hietz et al. 2005; Peñuelas et al. 2008; Rowell et al. 2009; Wang et al. 2012; Xu et al. 2013a, b, 2018; Saurer et al. 2014; Frank et al. 2015; van der Sleen et al. 2015) and productivity (Belmecheri et al. 2014). Also, McDowell et al. (2010) identified trees most susceptible to mortality in drought with carbon isotopes that showed their reduced ability to regulate the difference between atmospheric and intercellular leaf CO2 during drought. Tree-ring δ13C has been used to infer past atmospheric CO2 concentration (Zhao et al. 2006), and atmospheric CO2 concentration itself has been considered as a subtle influence in accurately modeling tree-ring δ13C (McCarroll et al. 2009). Wang et al. (2019) found evidence from tree-ring isotopes that rising atmospheric CO2 is improving water-use efficiency and thereby decreasing strength of relationships of ring width with moisture on the Tibetan Plateau.
Some studies are more focused on plant physiology and biochemistry related to assimilation and translocation processes (e.g., Kagawa et al. 2005; Eglin et al. 2008; Eilmann et al. 2010; Bryukhanova et al. 2011; Rinne et al. 2015). Tree-ring δ13C and δ18O have also helped to identify growth rings in tropical trees where they may not be clearly visible (e.g., Evans and Schrag 2004; Anchukaitis et al. 2008; Ohashi et al. 2009), and tree-ring δ13C studies have even reconstructed snow (Liu et al. 2011) and sea level (Yu et al. 2004). Finally, other applications not involving environment or ecophysiology include assessing tree-ring isotopes as a means of crossdating (e.g., Leavitt et al. 1985; Roden 2008) and as an aid in determining provenience of wood (Kagawa and Leavitt 2010).
Tree-ring δ13C has also been used to investigate aspects of plant nutrition (e.g., Bukata and Kyser 2008; Walia et al. 2010; Silva et al. 2015) and particularly pollution (e.g., Battipaglia et al. 2010; Rinne et al. 2010), which has been a fertile and growing application of tree-ring isotopes. Pollutants may impact processes such as stomatal conductance or photosynthesis, and the C-H-O isotopic composition may reflect that alteration, often accompanied by tree-ring width decline (see also Chap. 24). One of the earliest investigations was of SO2 pollution from a coal-fired foundry by Freyer (1979b), who found altered tree-ring δ13C. Reduced ring size and less negative δ13C were also found in tree rings in the vicinity of a SO2-emitting copper smelter in Utah (Martin and Sutherland 1990). Likewise, δ13C in tree rings was elevated up to ca. 100 km downwind from a copper smelter, and the anomaly originated at the time the smelter opened and was presumed to be a consequence of activation of stomatal closure (Savard et al. 2004); tree-ring δ2H also seem to be altered by this pollution (Savard et al. 2005). δ15N in tree rings has been found shifted as a consequence of automobile NOx pollution (Doucet et al. 2012) and δ15N shifts along with δ18O and δ13C in tree rings, which indicate increased iWUE near an oil refinery, are consistent with NOx pollution (Guerrieri et al. 2010). Choi et al. (2005) also found such an increase in iWUE in an area of elevated NOx in S. Korea. Tree-ring isotope evidence for ozone pollution has also been found (Novak et al. 2007), and pollution effects have been identified as reducing climate sensitivity of tree-ring isotopes (e.g., Leonelli et al. 2012; Boettger et al. 2014).
4 Emerging Directions
Several directions in tree-ring isotopes have seen growing interest over the last couple of decades and are promising for future investigations. For example, seasonal variations of isotope composition in tree rings (e.g., Helle and Schleser 2004; Monson et al. 2018; see also Chaps. 7, 14 and 15) can be related to both environmental conditions during the growing season as well as late in the previous season when photosynthates may be stored. The resolution of analysis may vary from 2 to 3 earlywood-latewood subdivisions (e.g., Szejner et al. 2016) to numerous microtomed subdivisions a few tens of microns wide (e.g., Evans and Schrag 2004; Helle and Schleser 2004). Computer-controlled milling (Dodd et al. 2008) and laser dissection have been used to separate small subdivisions in lieu of microtoming (e.g., Schollaen et al. 2014) for isotope analysis, and laser ablation holds hope for more rapid analysis of these seasonal isotope variations (e.g., Vaganov et al. 2009; Loader et al. 2017) with the products of ablation admitted to a combustion interface connected to the mass spectrometer.
Other analytical advances applied to tree rings include compound-specific isotope analysis and simultaneous analysis of C and O isotopes (dual-isotope analysis, see Chap. 7). Additionally, interpreting different aspects of the environment with multiple isotopes has been suggested and applied. For example, Scheidegger et al. (2000) described how under some circumstances δ18O and δ13C from the same tree ring may separately reflect humidity and rates of carbon fixation, respectively, although complete independence is not likely (Roden and Siegwolf 2012; see also Chap. 16). Isotopomers (isotope abundance in different intramolecular positions of the glucose repeat units in cellulose, see also Chap. 7) are also regarded as carrying different environmental signals. Sternberg et al. (2006) concluded that different biosynthetic pathways contribute to heterogeneous distribution of isotope composition, and identified O bonded to the carbon-2 position as carrying the isotopic composition of the original source water. O on other positions then carry cumulative influence of source water composition and humidity, from which source water composition could then be subtracted to attain a more accurate proxy of humidity (Sternberg 2009). Augusti and Schleucher (2007) similarly found H isotope differences depending on position in repeating glucose molecule in cellulose so that physiology and climate could be independently inferred from position-specific isotope analysis. Large, position-specific differences in C-isotope composition were recently identified in cellulose, interpreted as post-photosynthetic shifts in metabolic branching, which may be influenced by environment (Wieloch et al. 2018).
It may be possible to fold the plant isotope fractionation models (Farquhar et al. 1982; Roden et al. 2000) into mechanistic computer models of tree-ring formation, which use physiological processes and environmental conditions during the growing season (e.g., moisture, temperature, and sunlight) to produce photosynthates, activate cambium, and then divide, expand and mature cells in order to replicate observed growth rings (see Chap. 26). Some models operate at the cell level with short time steps and dozens of tunable input parameters, such as the ‘TREERING’ model (Fritts et al. 1999) and the ‘Vaganov-Shashkin’ (VS) model (Vaganov et al. 2011). The model of Hölttä et al. (2010) also operates on very short time steps to simulate whole-tree growth. The simpler ‘VS-Lite’ model (Tolwinski-Ward et al. 2011) has fewer tunable input parameters and operates on monthly climate data to produce wood increments rather than cell growth, and it has gained greater traction for routine applications. An early effort to add isotope algorithms into such models was made by Hemming et al. (2001), who inserted carbon isotope fractionation (of Farquhar et al. 1982) into the TREERING model. Gessler et al. (2014) reviews the extensive catalog of processes (e.g., physicochemical, metabolic, fractionation, transport, storage) within tree tissues, which can contribute to the final carbon and oxygen isotope composition in tree rings and must be considered in interpretation and modeling of tree-ring isotopes. Babst et al. (2018) describe the state of mechanistic tree-ring growth models, including scaling from tree-ring series to whole tree, and potential integration with dynamic global vegetation models (DGVMs) (see also Chap. 26). Because DGVMs are an important tool for improved understanding of current and future global ecology, carbon and water cycles, forest productivity, carbon sequestration, etc., implementation of accurate tree-ring isotope subroutines would provide another layer of control and validation of many aspects of the Earth system.
Finally, spatial mapping of isotope composition in what are known as “isoscapes” is of growing interest as related to fields of ecology, hydrology, climate, geology, forensics, etc. (Bowen 2010). Tree-ring isotopes can play a particularly important role in developing these isoscapes because tree rings can add the temporal component to identify changes in isoscapes through time. A number of tree-ring isoscapes related to climate have been developed in the southwestern USA, Europe, Siberia, and particularly notable in China where a rapid expansion of tree-ring isotope chronologies has been ongoing (Saurer et al. 2002; Leavitt et al. 2010; del Castillo et al. 2013). Gori et al. (2018) have recently explored δ18O and δ2H isoscapes as a tool for provenancing wood cut during logging operations.
5 Conclusions
Isotope dendrochronology has been around for ca. 70 years, but the explosion in the number and diversity of studies over the last 30 years has been breathtaking. This has been driven by advancements in analytical equipment as well as growing recognition of problems for which isotope composition of tree rings may provide resolution, particularly when knowing changes through time is also important. Tree-ring isotopes provide insights into physiological and biochemical processes from leaves to site of wood formation, and changes in isotope values can be used to infer changes in environmental conditions, such as climate parameters.
The history, advances and findings presented here are intended to provide a solid and useful chronology of events, but this summary is not exhaustive. The contents of this book can fill in more of those gaps.
References
An W, Liu X, Leavitt SW, Ren J, Xu G, Zeng X, Wang W, Qin D, Ren J (2013) Relative humidity history on the Batang-Litang Plateau of western China since 1755 reconstructed from tree-ring δ18O and δD. Clim Dyn 42:2639–2654. https://doi.org/10.1007/s00382-013-1937-z
Anchukaitis KJ, Evans MN, Wheelwright NT, Schrag DP (2008) Stable isotope chronology and climate signal calibration in neotropical cloud forest trees. J Geophys Res 113:G03030. https://doi.org/10.1029/2007JG000613
Augusti A, Schleucher J (2007) The ins and outs of stable isotopes in plants. New Phytol 174:473–475
Babst F, Bodesheim P, Charney N, Friend AD, Girardin MP, Klesse S, Moore DJP, Seftigen K, Björklund J, Bouriaud O, Dawson A, DeRose RJ, Dietze MC, Eckes AH, Enquist B, Frank DC, Mahecha MD, Poulter B, Evans MEK (2018) When tree-rings go global: challenges and opportunities of retro- and prospective insight. Quat Sci Rev 197:1–20
Barber VA, Juday GP, Finney BP, Wilmking M (2004) Reconstruction of summer temperatures in interior Alaska from tree-ring proxies: evidence for changing synoptic climate regimes. Clim Change 63:91–120
Barbour MM, Roden JS, Farquhar GD, Ehleringer JR (2004) Expressing leaf water and cellulose oxygen isotope ratios as enrichment above source water reveals evidence of a Péclet effect. Oecologia 138:426–435
Battipaglia G, Marzaioli F, Lubritto C, Altieri S, Strumia S, Cherubini P, Cotrufo MF (2010) Traffic pollution affects tree-ring width and isotopic composition of Pinus pinea. Sci Total Environ 408(3):586–593
Belmecheri S, Maxwell RS, Taylor AH, Davis KJ, Freeman KH, Munger WJ (2014) Tree-ring δ13C tracks flux tower ecosystem productivity estimates in a NE temperate forest. Environ Res Lett 9. https://doi.org/10.1088/1748-9326/9/7/074011
Boettger T, Haupt M, Friedrich M, Waterhouse JS (2014) Reduced climate sensitivity of carbon, oxygen and hydrogen stable isotope ratios in tree-ring cellulose of silver fir (Abies alba Mill.) influenced by background SO2 in Franconia (Germany, Central Europe). Environ Pollut 185:281–294
Bowen GJ (2010) Isoscapes: spatial pattern in isotopic biogeochemistry. Ann Rev Earth Planet Sci 38:161–187. https://doi.org/10.1146/annurev-earth-040809-152429
Brand WA, Douthitt CB, Fourel F, Maia R, Rodrigues C, Maguas C, Prohaska T (2015) Gas source isotope ratio mass spectrometry (IRMS). In: Prohaska T, Irrgeher J, Zitek A, Jakubowski N (eds) Sector field mass spectrometry for elemental and isotopic analysis [New developments in mass spectrometry, no 3], Chap 16. The Royal Society of Chemistry, Cambridge, UK, pp 500–549
Brenna JT, Corso TN, Tobias HJ, Caimi RJ (1997) High-precision continuous-flow isotope ratio mass spectrometry. Mass Spectrom Rev 16(5):227–258
Brienen RJW, Helle G, Pons TL, Guyot JL, Gloor M (2012) Oxygen isotopes in tree rings are a good proxy for Amazon precipitation and El Niño-Southern Oscillation variability. Proc Nat Acad Sci USA 109(42):16957–16962
Bryukhanova MV, Vaganov EA, Wirth C (2011) Influence of climatic factors and reserve assimilates on the radial growth and carbon isotope composition in tree rings of deciduous and coniferous species. Contemp Probl Ecol 4(2):126–132. https://doi.org/10.1134/S1995425511020020
Bukata AR, Kyser TK (2008) Tree-ring elemental concentrations in oak do not necessarily passively record changes in bioavailability. Sci Total Environ 390(1):275–286
Burk RL, Stuiver M (1981) Oxygen isotope ratios in trees reflect mean annual temperature and humidity. Science 211:1417–1419
Choi WJ, Lee S-M, Chang SX, Ro H-M (2005) Variations of δ13C and δ15N in Pinus densiflora tree-rings and their relationship to environmental changes in eastern Korea. Water Air Soil Pollut 164:173–187
Craig H (1953) The geochemistry of the stable carbon isotopes. Geochim Cosmochim Acta 3:53–92
Craig H (1954) Carbon-13 variations in sequoia rings and the atmosphere. Science 119:141–143
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
del Castillo J, Aguilera M, Voltas J, Ferrio JP (2013) Isoscapes of tree-ring carbon-13 perform like meteorological networks in predicting regional precipitation patterns. J Geophys Res Biogeosci 118(1):352–360
Dodd JP, Patterson WP, Holmden C, Brasseur JM (2008) Robotic micromilling of tree-rings: a new tool for obtaining subseasonal environmental isotope records. Chem Geol 252:21–30
Doucet A, Savard MM, Bégin C, Smirnoff A (2012) Tree-ring δ15N values to infer air quality changes at regional scale. Chem Geol 320(321):9–16
Edwards TWD, Aravena R, Fritz P, Morgan AV (1985) Interpreting paleoclimate from 18O and 2H in plant cellulose: comparison with evidence from fossil insects and relict permafrost in southwestern Ontario. Can J Earth Sci 22:1720–1726
Edwards TWD, Fritz P (1986) Assessing meteoric water composition and relative humidity from 18O and 2H in wood cellulose: paleoclimatic implications for southern Ontario, Canada. Appl Geochem 1:715–723
Eglin T, Maunoury-Danger F, Fresneau C, Lelarge C, Pollet B, Lapierre C, Francois C, Damesin C (2008) Biochemical composition is not the main factor influencing variability in carbon isotope composition of tree rings. Tree Physiol 28(11):1619–1628
Eilmann B, Buchmann N, Siegwolf R, Saurer M, Cherubini P, Rigling A (2010) Fast response of Scots pine to improved water availability reflected in tree-ring width and δ13C. Plant Cell Environ 33:1351–1360
Emiliani C (1955) Pleistocene temperatures. J Geol 63:538–578
Emiliani C, Geiss J (1959) On glaciations and their causes. Int J Earth Sci 46:576–601
Epstein S, Buchsbaum R, Lowenstam HA, Urey HC (1951) Carbon-water isotopic temperature scale. Bull Geol Soc Am 62:417–426
Epstein S, Thompson P, Yapp CJ (1977) Oxygen and hydrogen isotopic ratios in plant cellulose. Science 198:1209–1215
Epstein S, Yapp CJ (1976) Climatic implications of the D/H ratio of hydrogen in C-H groups in tree cellulose. Earth Planet Sci Lett 30:252–261
Epstein S, Yapp CJ, Hall JH (1976) The determination of the D/H ratio of non-exchangeable hydrogen in cellulose extracted from aquatic and land plants. Earth Planet Sci Lett 30:241–251
Evans MN, Schrag DP (2004) A stable isotope-based approach to tropical dendroclimatology. Geochim Cosmochim Acta 68(16):3295–3305. https://doi.org/10.1016/j.gca.2004.01.006
Fairbridge R, Gornitz V (2009) History of paleoclimatology—biographies. In: Gornitz V (ed) Encyclopedia of paleoclimatology and ancient environments. Springer, New York, pp 428‒437
Farmer JG (1979) Problems in interpreting tree-ring δ13C records. Nature 279:229–231
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Francey RJ, Farquhar GD (1982) An explanation of 13C/12C variations in tree rings. Nature 297:28–31
Frank DC, Poulter B, Saurer M, Esper J, Huntingford C, Helle G, Treydte K, Zimmerman NE, Schleser GH, Ahlstrom A, Ciais P (2015) Water-use efficiency and transpiration across European forests during the Anthropocene. Nat Clim Chang 5:579–583
Freyer HD (1979a) On the 13C record in tree rings. Part 1. 13C variations in northern hemispheric trees during the last 150 years. Tellus 31:124–137
Freyer HD (1979b) On the 13C record in tree rings. Part 2. Registration of microenvironmental CO2 and anomalous pollution effect. Tellus 31:308–312
Freyer HD (1981) Recent 13C/12C trends in atmospheric CO2 and tree rings. Nature 293:679–680
Freyer HD, Belacy N (1983) 13C/12C records in Northern Hemispheric trees during the past 500 years- anthropogenic impact and climate superpositions. J Geophys Res 88:6844–6852
Freyer HD, Wiesberg L (1973) 13C-decrease in modern wood due to the large-scale combustion of fossil fuels. Naturwissenschaften 60:517–518
Friedli H, Lotscher H, Oeschger H, Siegenthaler U, Stauffer B (1986) Ice core record of the 13C/12C ratio of atmospheric carbon dioxide in the past two centuries. Nature 324:237–238
Fritts HC, Shashkin AV, Downes G (1999) A simulation model of conifer ring growth and cell structure. In: Wimmer R, Vetter RE (eds) Tree-ring analysis. Cambridge University Press, Cambridge, UK, pp 3–32
Gagen MH, Zorita E, McCarroll D, Young GHF, Grudd H, Jalkanen R, Loader NJ, Robertson I, Kirchhefer AJ (2011) Cloud response to summer temperatures in Fennoscandia over the last thousand years. Geophys Res Lett 38. https://doi.org/10.1029/2010GL046216
Gessler A, Brandes E, Keitel C, Boda S, Kayler ZE, Granier A, Barbour M, Farquhar GD, Treydte K (2013) The oxygen isotope enrichment of leaf-exported assimilates-Does it always reflect lamina leaf water enrichment? New Phytol 200:144–157
Gessler A, Ferrio JP, Hommel R, Treydte K, Werner RA, Monson RK (2014) Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol 34:796–818
Gori Y, Stradiotti A, Camin F (2018) Timber isoscapes. A case study in a mountain area in the Italian Alps. PloS One 13(2):e0192970. https://doi.org/10.1371/journal.pone.0192970
Gray J, Thompson P (1976) Climatic information from 18O/16O ratios of cellulose in tree rings. Nature 262:481–482
Grießinger J, Bräuning A, Helle G, Thomas A, Schleser G (2011) Late Holocene Asian summer monsoon variability reflected by δ18O in tree-rings from Tibetan junipers. Geophys Res Lett 38:L03701. https://doi.org/10.1029/2010GL045988
Guerrieri R, Siegwolf RTW, Saurer M, Ripullone F, Mencuccini M, Borghetti M (2010) Anthropogenic NOx emissions alter the intrinsic water-use efficiency (WUEi) for Quercus cerris stands under Mediterranean climate conditions. Environ Pollut 158:2841–2847
Haavik L, Stephen F, Fierke M, Salisbury V, Leavitt SW, Billings S (2008) Tree-ring δ13C and historic growth patterns as indicators of northern red oak (Quercus rubra Fagaceae) susceptibility to red oak borer (Enapholodes rufulus (Haldeman) (Coleoptera: Cerambycidae)). For Ecol Manage 255:1501–1509
Hafner P, McCarroll D, Robertson I, Loader N, Gagen M, Young G, Bale R, Sonninen E, Levanič T (2014) A 520 year record of summer sunshine for the eastern European Alps based on stable carbon isotopes in larch tree rings. Clim Dyn 43:971–980. https://doi.org/10.1007/s00382-013-1864-z
Harkness DD, Miller BF (1980) Possibility of climatically induced variations in the 14C and 13C enrichment patterns as recorded by a 300-year-old Norwegian pine. Radiocarbon 22:291–298
Helle G, Schleser GH (2004) Beyond CO2-fixation by Rubisco—an interpretation of 13C/12C variations in tree rings from novel intra-seasonal studies on broad-leaf trees. Plant Cell Environ 27:367–380
Hemming D, Fritts H, Leavitt SW, Wright W, Long A, Shashkin A (2001) Modelling tree-ring δ13C. Dendrochronologia 19(1):23–38
Hietz P, Wanek W, Dünisch O (2005) Long-term trends in cellulose δ13C and water-use efficiency of tropical Cedrela and Swietenia from Brazil. Tree Physiol 25:745–752
Hölttä T, Mäkinen H, Nöjd P, Mäkelä A, Nikinmaa E (2010) A physiological model of softwood cambial growth. Tree Physiol 30:1235e1252
Hultine KR, Dudley TL, Leavitt SW (2013) Herbivory-induced mortality increases with radial growth in an invasive riparian phreatophyte. Ann Bot 11(6):1197–1206. https://doi.org/10.1093/aob/mct077
Jansen HS (1962) Depletion of carbon-13 in young kauri trees. Nature 196:84–85
Kagawa A, Leavitt SW (2010) Stable carbon isotopes of tree rings as a tool to pinpoint timber geographic origin. J Wood Sci. https://doi.org/10.1007/s10086-009-1085-6. Special Issue “Wood Science and Technology for Mitigation of Global Warming”
Kagawa A, Sugimoto A, Yamashita K, Abe H (2005) Temporal photosynthetic carbon isotope signatures revealed in a tree ring through 13CO2 pulse-labelling. Plant, Cell Environ 28:906–915
Keeling CD, Mook WG, Tans PP (1979) Recent trends in the 13C/12C ratio of atmospheric carbon dioxide. Nature 277:121–123
Kress A, Saurer M, Buntgen U, Treydte KS, Bugmann H, Siegwolf RTW (2009) Summer temperature dependency of larch budmoth outbreaks revealed by Alpine tree-ring isotope chronologies. Oecologia 160(2):353–365
Krishnamurthy RV, Epstein S (1985) Tree ring D/H ratio from Kenya, East Africa and its palaeoclimatic significance. Nature 317:160–162
Labotka DM, Grissino-Mayer HD, Mora CI, Johnson EJ (2015) Patterns of moisture source variability and climate oscillations in the Southeastern United States: a four century seasonally resolved tree-ring oxygen isotope record. Clim Dyn 46:2145–2154. https://doi.org/10.1007/s00382-015-2694-y
Labuhn I, Daux V, Girardclos O, Stievenard M, Pierre M, Masson-Delmotte V (2016) French summer droughts since 1326 CE: a reconstruction based on tree ring cellulose δ18O. Climate of the past 12:1101–1117. https://doi.org/10.5194/cp-12-1101-2016
Leavitt SW (2010) Tree-ring C-H-O isotope variability and sampling. Sci Total Environ 408:5244–5253
Leavitt SW, Long A (1986) Stable-carbon isotope variability in tree foliage and wood. Ecology 67:1002–1010
Leavitt SW, Long A (1988) Stable carbon isotope chronologies from trees in the southwestern United States. Global Biogeochem Cycles 2:189–198
Leavitt SW, Long A (1989) Drought indicated in carbon-13/carbon-12 ratios of southwestern tree rings. Water Resour Bull 25:341–347
Leavitt SW, Long A, Dean JS (1985) Tree-ring dating through pattern-matching of stable-carbon isotope time series. Tree-Ring Bulletin 45:1–9
Leavitt SW, Treydte K, Liu Y (2010) Environment in time and space: opportunities from tree-ring isotope networks. In: West JB, Bowen GJ, Dawson TE, Tu KP (eds) Understanding movement, pattern, and processes on Earth through isotope mapping, Chap 6. Springer, Dordrecht, pp 113–135
Leonelli G, Battipaglia G, Siegwolf RTW, Saurer M, Morra di Cella U, Cherubini P, Pelfini M (2012) Climatic isotope signals in tree rings masked by air pollution: a case study conducted along the Mont Blanc Tunnel access road (Western Alps, Italy). Atmos Environ 61:169–179
Li Q, Nakatsuka T, Kawamura K, Liu Y, Song HM (2011) Hydroclimate variability in the North China Plain and its link with El Nino-Southern Oscillation since 1784 AD: Insights from tree-ring cellulose δ18O. J Geophys Res 116(D22):D22106
Libby LM, Pandolfi LJ (1974) Temperature dependence of isotope ratios in tree-rings. Proc Nat Acad Sci USA 71:2482‒2486
Libby LM, Pandolfi LJ (1979) Tree thermometers and commodities: historic climate indicators. Environ Int 2:317–333
Liu X, Shao X, Wang L, Liang E, Qin D, Ren J (2008) Response and dendroclimatic implications of δ13C in tree rings to increasing drought on the northeastern Tibetan Plateau. J Geophys Res 113:G03015. https://doi.org/10.1029/2007JG000610
Liu X, Zhao L, Chen T, Shao X, Liu Q, Hou S, Qin D, An W (2011) Combined tree-ring width and δ13C to reconstruct snowpack depth: a pilot study in the Gongga Mountain, west China. Theoret Appl Climatol 103:133–144
Liu Y, Cobb KM, Song H, Li Q, Li CY, Nakatsuka T, An Z, Zhou W, Cai Q, Li J, Leavitt SW, Sun C, Mei R, Shen C-C, Chan M-H, Sun J, Yan L, Lei Y, Ma Y, Li X, Chen D, Linderholm HW (2017) Recent enhancement of central pacific El Niño variability relative to last eight centuries. Nat Commun 8. https://doi.org/10.1038/ncomms15386
Liu Y, Wang Y, Li Q, Song H, Linderhlom HW, Leavitt SW, Wang R, An Z (2014) Tree-ring stable carbon isotope-based May-July temperature reconstruction over Nanwutai, China, for the past century and its record of 20th century warming. Quat Sci Rev 93:67–76. https://doi.org/10.1016/j.quascirev.2014.03.023
Loader NJ, McCarroll D, Barker S, Jalkanen R, Grudd H (2017) Inter-annual carbon isotope analysis of tree-rings by laser ablation. Chem Geol 466:323–326
Loader NJ, Young GHF, Grudd H, McCarroll D (2013) Stable carbon isotopes from Torneträsk, northern Sweden provide a millennial length reconstruction of summer sunshine and its relationship to Arctic circulation. Quat Sci Rev 62:97–113
Managave SR, Ramesh R (2012) Isotope dendroclimatology: a review with a special emphasis on tropics. In: Baskaran M (ed) Handbook of environmental isotope geochemistry. Springer, The Netherlands, pp 811–834
Martin B, Sutherland EK (1990) Air pollution in the past recorded in width and composition of stable carbon isotopes of annual growth rings of Douglas-fir. Plant Cell Environ 13:839–3844
McCarroll D, Gagen M, Loader NJ, Robertson I, Anchukaitis KJ, Los S, Young GHF, Jalkanen R, Kirchhefer A, Waterhouse JS (2009) Correction of tree ring stable carbon isotope chronologies for changes in the carbon dioxide content of the atmosphere. Geochim Cosmochim Acta 73:1539–1547
McCarroll D, Loader NJ (2004) Stable isotopes in tree rings. Quat Sci Rev 23:771–801
McDowell NG, Allen CD, Marshall L (2010) Growth, carbon-isotope discrimination, and drought drought-associated mortality across a Pinus ponderosa elevational transect. Glob Change Biol 16(1):399–415. https://doi.org/43110.1111/j.1365-2486.2009.01994.x
Miller DL, Mora CI, Grissino-Mayer HD, Mock CJ, Uhle ME, Sharp Z (2006) Tree ring isotope record of tropical cyclone activity. Proc Nat Acad Sci USA 103:14294‒14297
Monson RK, Szejner P, Belmecheri S, Morino KA, Wright WE (2018) Finding the seasons in tree ring stable isotope ratios. Am J Bot 105(5):819–821
Novak K, Cherubini P, Saurer M, Fuhrer J, Skelly JM, Kräuchi N, Schaub M (2007) Ozone air pollution effects on tree-ring growth, δ13C, visible foliar injury and leaf gas exchange in three ozone-sensitive woody plant species. Tree Physiol 27(7):941–949
Ogle N, Turney C, Kalin R, O'Donnell L, Butler C (2005) Palaeovolcanic forcing of short-term dendroisotopic depletion: the effect of decreased solar intensity on Irish oak. Geophys Res Lett 32(4). https://doi.org/10.1029/2004GL021623
Ohashi S, Okada N, Nobuchi T, Siripatanadilok S, Veenin T (2009) Detecting invisible growth rings of trees in seasonally dry forests in Thailand: isotopic and wood anatomical approaches. Trees-Struct Funct 23:813–822
Pearman GI, Francey RJ, Fraser PJ (1976) Climatic implications of stable carbon isotopes in tree rings. Nature 260:771–773
Peng TH, Broecker WS, Freyer HD, Trumbore S (1983) A deconvolution of the tree-ring based δ13C record. J Geophys Res 88:3609–3620
Peñuelas J, Hunt JM, Ogaya R, Jump AS (2008) Twentieth century changes of tree-ring δ13C at the southern range-edge of Fagus sylvatica: increasing water-use efficiency does not avoid the growth decline induced by warming at low altitudes. Glob Change Biol 14:1076–1088
Prohaska T (2015) History. In: Prohaska T, Irrgeher J, Zitek A, Jakubowski N (eds) Sector field mass spectrometry for elemental and isotopic analysis [New developments in mass spectrometry No. 3], Chap 2. The Royal Society of Chemistry, Cambridge, UK, pp 10–25
Ramesh R, Bhattacharya SK, Gopalan K (1985) Dendrochronological implications of isotope coherence in trees from Kashmir Valley, India. Nature 317(6040):802–804
Ramesh R, Bhattacharya SK, Gopalan K (1986) Climatic correlations in the stable isotope records of silver fir (Abies pindrow) trees from Kashmir, India. Earth Planet Sci Lett 79:66–74
Rinne ΚΤ, Loader ΝJ, Switsur VR, Treydte KS, Waterhouse JS (2010) Investigating the influence of sulphur dioxide (SO2) on the stable isotope ratios (δ13C and δ18O) of tree rings. Geochim Cosmochim Acta 74:2327–2339
Rinne KT, Loader NJ, Switsur VR, Waterhouse JS (2013) 400-year May–August precipitation reconstruction for Southern England using oxygen isotopes in tree rings. Quat Sci Rev 60:13–25. https://doi.org/10.1016/j.quascirev.2012.10.048
Rinne KT, Saurer M, Kirdyanov AV, Loader NJ, Bryukhanova MV, Werner RA, Siegwolf RT (2015) The relationship between needle sugar carbon isotope ratios and tree rings of larch in Siberia. Tree Physiol 35(11):1192–1205
Robertson I, Leavitt SW, Loader NJ, Buhay B (2008) Progress in isotope dendroclimatology. Chem Geol 252:EX1–EX4
Roden J (2008) Cross-dating of tree ring δ18O and δ13C time series. Chem Geol 252:72–79
Roden JS, Ehleringer JR (1999a) Hydrogen and oxygen isotope ratios of tree-ring cellulose for riparian trees grown long-term under hydroponic, controlled environmental environments. Oecologia 121:467–477
Roden JS, Ehleringer JR (1999b) Observations of hydrogen and oxygen isotopes in leaf water confirm the Craig-Gordon model under wide-ranging environmental conditions. Plant Physiol 120:1165–1173
Roden JS, Ehleringer JR (2000) Hydrogen and oxygen isotope ratios of tree ring cellulose for field-grown riparian trees. Oecologia 123:481–489
Roden JS, Lin G, Ehleringer JR (2000) A mechanistic model for interpretation of hydrogen and oxygen isotope ratios in tree-ring cellulose. Geochim Cosmochim Acta 64(1):21–35
Roden J, Siegwolf R (2012) Is the dual-isotope conceptual model fully operational? Tree Physiol 32:1179–1182
Rowell DM, Ades PK, Tausz M, Arndt SK, Adams MA (2009) Lack of genetic variation in tree ring δ13C suggests a uniform, stomatally-driven response to drought stress across Pinus radiata genotypes. Tree Physiol 29:191–198
Sano M, Xu C, Nakatsuka T (2012) A 300-year Vietnam hydroclimate and ENSO variability record reconstructed from tree ring δ18O. J Geophy Res 117(D12). https://doi.org/10.1029/2012JD017749
Saurer M, Aellen K, Siegwolf R (1997) Correlating δ13C and δ18O in cellulose of trees. Plant Cell Environ 20:1543–1550
Saurer M, Schweingruber F, Vaganov EA, Shiyatov SG, Siegwolf R (2002) Spatial and temporal oxygen isotope trends at the northern tree-line in Eurasia. Geophys Res Lett 29. https://doi.org/10.1029/2001GL013739
Saurer M, Spahni R, Frank DC, Joos F, Leuenberger M, Loader NJ, McCarroll D, Gagen M, Poulter B, Siegwolf RTW, Andreu-Hayles L, Boettger T, Dorado Liñán I, Fairchild IJ, Friedrich M, Gutierrez E, Haupt M, Hilasvuori E, Heinrich I, Helle G, Grudd H, Jalkanen R, Levanič T, Linderholm HW, Robertson I, Sonninen E, Treydte K, Waterhouse JS, Woodley EJ, Wynn PM, Young GHF (2014) Spatial variability and temporal trends in water-use efficiency of European forests. Glob Change Biol 20:3700–3712. https://doi.org/10.1111/gcb.12717
Savard MM, Begin C, Parent M, Smirnoff A, Marion J (2004) Effects of smelter sulfur dioxide emissions: a spatiotemporal perspective using carbon isotopes in tree rings. J Environ Qual 33:13–25
Savard MM, Bégin C, Smirnoff A, Marion J, Sharp Z, Parent M (2005) Fractionation change of hydrogen isotopes in trees due to atmospheric pollutants. Geochim Cosmochim Acta 69:3723–3731
Scheidegger Y, Saurer M, Bahn M, Siegwolf R (2000) Linking stable oxygen and carbon isotopes with stomatal conductance and photosynthetic capacity: a conceptual model. Oecologia 125:350–357
Schiegl WE (1974) Climatic significance of deuterium abundance in growth rings of Picea. Nature 251:582–584
Schollaen K, Heinrich I, Helle G (2014) UV-laser-based microscopic dissection of tree rings—a novel sampling tool for δ13C and δ18O studies. New Phytolol 201(3):1045–1055
Silva LCR, Gómez-Guerrero A, Doane TA, Horwath WR (2015) Isotopic and nutritional evidence for species- and site-specific responses to N deposition and elevated CO2 in temperate forests. J Geophys Res Biogeosci 120:1110–1123. https://doi.org/10.1002/2014JG002865
Sternberg LSL (2009) Oxygen stable isotope ratios of tree-ring cellulose: the next phase of understanding. New Phytol 181:553–562
Sternberg L, Pinzon MC, Anderson WT, Jahren H (2006) Variation in oxygen isotope fractionation during cellulose synthesis: intramolecular and biosynthetic effects. Plant Cell Environ 29:1881–1889
Stuiver M (1978) Atmospheric carbon dioxide and carbon reservoir changes. Science 129:253–258
Stuiver M, Burk RL, Quay PD (1984) 13C/12C ratios and the transfer of biospheric carbon to the atmosphere. J Geophys Res 89:11731–11748
Stuiver M, Braziunas TF (1987) Tree cellulose 13C/12C isotope ratios and climate change. Nature 328:58–60
Szejner P, Wright WE, Babst F, Belmecheri S, Trouet V, Leavitt SW, Ehleringer JR, Monson RK (2016) Latitudinal gradients in tree-ring stable carbon and oxygen isotopes reveal differential climate influences of the North American Monsoon System. J Geophys Res Biogeosci 121(7):1978–1991. https://doi.org/10.1002/2016JG003460
Tans PP (1978) Carbon-13 and carbon-14 in trees and the atmospheric CO2 increase. Ph.D. Dissertation, State University of Groningen, The Netherlands
Tolwinski-Ward SE, Evans MN, Hughes MK, Anchukaitis KJ (2011) An efficient forward model of the climate controls on interannual variation in tree-ring width. Clim Dyn 36(11–12):2419–2439. https://doi.org/10.1007/s00382-010-0945-5
Treydte KS, Schleser GH, Helle G, Frank DC, Winiger M, Haug GH, Esper J (2006) The twentieth century was the wettest period in northern Pakistan over the past millennium. Nature 440:1179–1182
Vaganov EA, Anchukaitis KJ, Evans MN (2011) How well understood are the processes that create dendroclimatic records? A mechanistic model of the climatic control on conifer tree-ring growth dynamics. In: Hughes M, Swetnam T, Diaz H (eds) Dendroclimatology. Developments in paleoenvironmental research, vol 11. Springer, Dordrecht, pp 37–75
Vaganov EA, Schulze E-D, Skomarkova MV, Knohl A, Brand WA, Roscher C (2009) Intra-annual variability of anatomical structure and δ13C values within tree rings of spruce and pine in alpine, temperate and boreal Europe. Oecologia 161:729–745
van der Sleen P, Groenendijk P, Vlam M, Anten NPR, Boom A. Bongers F, Pons TL, Terburg G, Zuidema PA (2015) No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat Geosci 8:24–28
Vogel JC (1980) Fractionation of the carbon isotopes during photosynthesis. In: Sitzungsberichte der Heidelberger Akademie der Wissenschaften (Mathematisch-Naturwissenschaftliche Klasse) Jahrgang 1980. Sprinter, Berlin, Heidelberg, pp 111–135
Walia A, Guy RD, White B (2010) Carbon isotope discrimination in western hemlock and its relationship to mineral nutrition and growth. Tree Physiol 30:728–740
Wang W, Liu X, An W, Xu G, Zeng X (2012) Increased intrinsic water-use efficiency during a period with persistent decreased tree radial growth in northwestern China: causes and implications. For Ecol Manage 275:14–22
Wang W, Liu X, Xu G, Treydte K, Shao X, Qin D, Wang G, McDowell NG (2019) CO2 fertilization confounds tree-ring records of regional hydroclimate at northeastern Qinghai-Tibetan Plateau. Earth Space Sci 6:730–740. https://doi.org/10.1029/2018EA000529
Weidner K, Heinrich I, Helle G, Löffler J, Neuwirth B, Schleser GH, Vos H (2010) Consequences of larch budmoth outbreaks on the climatic significance of ring width and stable isotopes of larch. Trees-Struct Funct 24:399–409
Wieloch T, Ehlers I, Yu J, Frank D, Grabner M, Gessler A, Schleucher J (2018) Intramolecular 13C analysis of tree rings provides multiple plant ecophysiology signals covering decades. Sci Rep 8:5048. https://doi.org/10.1038/s41598-018-23422-2
Wilson AT, Grinsted MJ (1977) 12C/13C in cellulose and lignin as paleothermometers. Nature 265:133–135
Wright WE, Leavitt SW (2006) Boundary layer humidity reconstruction for a semiarid location from tree ring cellulose δ18O. J Geophys Res 111:D18105. https://doi.org/10.1029/2005JD006806
Xu G, Liu X, Belmecheri S, Chen T, Wu G, Wang B, Zeng X, Wang W (2018) Disentangling contributions of CO2 concentration and climate to changes in intrinsic water-use efficiency in the arid boreal forest in China’s Altay mountains. Forests 9(10):642. https://doi.org/10.3390/f9100642
Xu C, Sano M, Nakatsuka T (2013a) A 400-year record of hydroclimate variability and local ENSO history in northern Southeast Asia inferred from tree-ring δ18O. Palaeogeogr Palaeoclimatol Palaeoecol 386:588–598
Xu G, Liu X, Qin D, Chen T, An W, Wang W, Wu G, Zeng X (2013b) Climate warming and increasing atmospheric CO2 have contributed to increased intrinsic water-use efficiency on the northeastern Tibetan Plateau since 1850. Trees-Struct Funct 27:465–475
Young GHF, Loader NJ, McCarroll D, Bale RJ, Demmler JC, Miles D, Nayling NT, Rinne KT, Robertson I, Watts C, Whitney M (2015) Oxygen stable isotope ratios from British oak tree-rings provide a strong and consistent record of past changes in summer rainfall. Clim Dyn 45:3609–3622. https://doi.org/10.1007/s00382-015-2559-4
Young GHF, McCarroll D, Loader NJ, Kirchhefer AJ (2010) A 500-year record of summer near-ground solar radiation from tree-ring stable carbon isotopes. Holocene 20:315–324
Yu KF, Zhao JX, Liu TS, Wang PX, Qian JL, Chen TG (2004) Alpha-cellulose δ13C variation in mangrove tree rings correlates well with annual sea level trend between 1982 and 1999. Geophys Res Lett 31:L11203. https://doi.org/10.1029/2004GL019450
Zhao X-Y, Qian J-L, Wang J, He Q-Y, Wang Z-L, Chen C-Z (2006) Using a tree ring δ13C annual series to reconstruct atmospheric CO2 concentration over the past 300 years. Pedosphere 16:371–379
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 © The Author(s)
About this chapter
Cite this chapter
Leavitt, S.W., Roden, J. (2022). Isotope Dendrochronology: Historical Perspective. In: Siegwolf, R.T.W., Brooks, J.R., Roden, J., Saurer, M. (eds) Stable Isotopes in Tree Rings. Tree Physiology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-92698-4_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-92698-4_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92697-7
Online ISBN: 978-3-030-92698-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)