Abstract
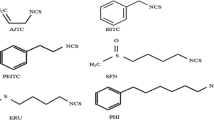
Naturally occurring isothiocyanates (ITCs), products of hydrolysis of glucosinolates (GSLs), attract great attention due to their well-defined indirect antioxidant and antitumor properties, which come as a result of their ability to regulate transcription factors, signaling pathways, cell cycle and apoptosis. Majority of studies on antioxidant activity of ITCs, in particular of those present in Brassica vegetables (sulforaphane, sulforaphene, erucin), indicate that some health-promoting effects might be connected rather with their indirect antioxidant mechanism of action. In this chapter several aspects of chemical and biological activity of ITCs and some parent GSLs are presented, with emphasis on chemical structure, reactivity of isothiocyanate moiety (–NCS) and the role of side chain during reactions with the reactive oxygen species and with model radicals used in common antioxidant assays. The literature survey indicates that at ambient temperatures ITCs are preventive antioxidants removing hydroperoxides and they are not radical trapping agents. However, chain-breaking character can be observed at elevated temperatures during oxidation of bulk phase lipids.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
Main abbreviations and acronyms: ABTS/ABTS+•, radical cation formed from 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic) acid; DPPH•, 2,2-diphenyl-1-picrylhydrazyl radical; ERN, erucin; ERS, erysolin; FRAP, ferric reducing antioxidant power; GL, glucosinolate(s); GSL-ERN, glucoerucin; GSL-RPS, glucoraphasatin; GSL-SFE, glucoraphenin; GSL-SFN, glucoraphanin; HAT: hydrogen atom transfer; ITC, isothiocyanate(s), ORAC, oxygen radical absorbance capacity; ROS, reactive oxygen species; RPS, raphasatin; SFE, sulforaphene; SFN, sulforaphane; SOD, superoxide dismutase; TEAC, Trolox Equivalent Antioxidant Capacity; TOSC, total oxidant scavenging capacity.
References
Agerbirk N, De Vos M, Kim JH, Jander G (2008) Indole glucosinolate breakdown and its biological effects. Phytochem Rev 8(1):101
Akbari E, Namazian M (2020) Sulforaphane: a natural product against reactive oxygen species. Comput Theor Chem 1183:112850
Amorati R, Valgimigli L (2015) Advantages and limitations of common testing methods for antioxidants. Free Radic Res 49(5):633–649
Baek SJ, McEntee MF, Legendre AM (2009) Review paper: cancer chemopreventive compounds and canine cancer. Vet Pathol 46(4):576–588
Barillari J, Canistro D, Paolini M, Ferroni F, Pedulli GF, Iori R, Valgimigli L (2005a) Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J Agric Food Chem 53(7):2475–2482
Barillari J, Cervellati R, Paolini M, Tatibouët A, Rollin P, Iori R (2005b) Isolation of 4-methylthio-3-butenyl glucosinolate from Raphanus sativus sprouts (kaiware daikon) and its redox properties. J Agric Food Chem 53(26):9890–9896
Barton DHR, Jaszberenyi JC, Theodorakis EA (1992) The invention of radical reactions. Part XXIII new reactions: Nitrile and thiocyanate transfer to carbon radicals from sulfonyl cyanides and sulfonyl isothiocyanates. Tetrahedron 48(13):2613–2626
Beard CI, Dailey BP (1949) The microwave spectra of CH3NCS and CH3SCN. J Am Chem Soc 71(3):929–936
Benati L, Leardini R, Minozzi M, Nanni D, Spagnolo P, Zanardi G (2000) Cascade radical reaction of 2-alkynyl-substituted aryl radicals with aryl isothiocyanates: A novel entry to benzothieno[2,3-b]quinolines through α-(arylsulfanyl)imidoyl radicals. J Org Chem 65(25):8669–8674
Benati L, Calestani G, Leardini R, Minozzi M, Nanni D, Spagnolo P, Strazzari S, Zanardi G (2003) Cascade radical reactions via α-(arylsulfanyl)imidoyl radicals: Competitive [4 + 2] and [4 + 1] radical annulations of alkynyl isothiocyanates with aryl radicals. J Org Chem 68(9):3454–3464
Bergmann E, Tschudnowsky M (1932) Die Dipolmente einiger organischer Stickstoffverbindungen. Z Phys Chem 17B(1):100–106
Billeter O (1875) Ueber organische Sulfocyanverbindungen. Ber Dtsch Chem Ges 8(1):462–466
Blažević I, Montaut S, Burčul F, Olsen CE, Burow M, Rollin P, Agerbirk N (2020) Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 169:112100
Block E (1992) The organosulfur chemistry of the genus Allium – Implications for the organic chemistry of sulfur. Angew Chem Int Ed Eng 31(9):1135–1178
Bonnesen C, Eggleston IM, Hayes JD (2001) Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res 61(16):6120–6130
Boutron F, Fremy E (1840) Untersuchung des schwarzen und weissen Senssamens. Justus Liebigs Ann Chem 34(2):230–232
Burčul F, Generalić Mekinić I, Radan M, Rollin P, Blažević I (2018) Isothiocyanates: cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J Enzyme Inhib Med Chem 33(1):577–582
Bussy A (1840) Untersuchungen über die Bildung des ätherischen Senföls. Justus Liebigs Ann Chem 34(2):223–230
Cabello-Hurtado F, Gicquel M, Esnault M-A (2012) Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem 132(2):1003–1009
Cedrowski J, Dąbrowa K, Krogul-Sobczak A, Litwinienko G (2020) A lesson learnt from food chemistry—elevated temperature triggers the antioxidant action of two edible isothiocyanates: erucin and sulforaphane. Antioxidants 9(11):1090
Cedrowski J, Dąbrowa K, Przybylski P, Krogul-Sobczak A, Litwinienko G (2021) Antioxidant activity of two edible isothiocyanates: Sulforaphane and erucin is due to their thermal decomposition to sulfenic acids and methylsulfinyl radicals. Food Chem 353:129213
Chang X, Lee K-E, Il Jeon S, Kim Y-J, Lee HK, Lee SW (2005) Bis(isothiocyanato)bis(phosphine) complexes of group 10 metals: reactivity toward organic isocyanides. Dalton Trans 23:3722–3731
Crich D, Quintero L (1989) Radical chemistry associated with the thiocarbonyl group. Chem Rev 89(7):1413–1432
Crooker K, Aliani R, Ananth M, Arnold L, Anant S, Thomas SM (2018) A review of promising natural chemopreventive agents for head and neck cancer. Cancer Prev Res (Philadelphia, PA) 11(8):441–450
Czochara R, Kusio J, Symonowicz M, Litwinienko G (2016) Fullerene C60 derivatives as high-temperature inhibitors of oxidative degradation of saturated hydrocarbons. Ind Eng Chem Res 55(37):9887–9894
Dadieu A (1931) Raman-Effekt und Konstitutions-Probleme, II. Mitteil.: Cyanverbindungen. Ber Dtsch Chem Ges (A and B Series) 64(2):358–361
Dadieu A, Kohlrausch KWF (1930) Raman-Effekt und Chemie. Ber Dtsch Chem Ges (A and B Series) 63(2):251–282
de Figueiredo SM, Filho SA, Nogueira-Machado JA, Caligiorne RB (2013) The anti-oxidant properties of isothiocyanates: a review. Recent Pat Endocr Metab Immune Drug Discov 7(3):213–225
Dousmanis GC, Sanders TM Jr, Townes CH, Zeiger HJ (1953) Structure of HNCS from microwave spectra. J Chem Phys 21(8):1416–1417
Drobnica L, Kristián P, Augustín J (1977) Chapter - The chemistry of the — NCS group. In: Patai S (ed) Cyanates and their thio derivatives. PATAI’S chemistry of functional groups, vol 2. John Wiley & Sons Ltd, Chichester, pp 1003–1221
Ettlinger MG, Lundeen AJ (1956) The structures of sinigrin and sinalbin; an enzymatic rearrangement. J Am Chem Soc 78(16):4172–4173
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56(1):5–51
Farag MA, Motaal AAA (2010) Sulforaphane composition, cytotoxic and antioxidant activity of crucifer vegetables. J Adv Res 1(1):65–70
Fenwick GR, Heaney RK, Mullin WJ, Van Etten CH (1983) Glucosinolates and their breakdown products in food and food plants. CRC Crit Rev Food Sci Nutr 18(2):123–201
Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Tercé F (2000) Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res 60(5):1426–1433
Gerlich G (1875) Ueber Pseudopropyl- und Allyrhodanür. Justus Liebigs Ann Chem 178(1):80–91
Gildmeister E (1913) The volatile oils, vol 1. John Wiley & Sons, New York, pp 1–432
Gill CI, Haldar S, Porter S, Matthews S, Sullivan S, Coulter J, McGlynn H, Rowland I (2004) The effect of cruciferous and leguminous sprouts on genotoxicity, in vitro and in vivo. Cancer Epidemiol Biomark Prev 13(7):1199–1205
Giudice A, Montella M (2006) Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. BioEssays 28(2):169–181
Goubeau J, Gott O (1940) Die Raman-Spektren einiger Rhodanverbindungen und die Struktur der Rhodanid-Gruppe. Ber Dtsch Chem Ges (A and B Series) 73(2):127–133
Greenwald P (2001) From carcinogenesis to clinical interventions for cancer prevention. Toxicology 166(1-2):37–45
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Hanschen FS, Lamy E, Schreiner M, Rohn S (2014) Reactivity and stability of glucosinolates and their breakdown products in foods. Angew Chem Int Ed 53(43):11430–11450
Herraiz T, Galisteo J (2004) Endogenous and dietary indoles: A class of antioxidants and radical scavengers in the ABTS assay. Free Radic Res 38(3):323–331
Hofmann AW (1868) Ueber die dem Senföl entsprechenden Isomeren der Schwefelcyanwasserstoffäther. Ber Dtsch Chem Ges 1(1):25–28
Hudlikar R, Wang L, Wu R, Li S, Peter R, Shannar A, Chou PJ, Liu X, Liu Z, Kuo HD, Kong AN (2020) Epigenetics/epigenomics and prevention of early stages of cancer by isothiocyanates. Cancer Prev Res (Philadelphia, PA) 14(2):151–164
International Agency for Research on Cancer, World Health Organization, Centre international de recherche sur le cancer (Lyon), & Kongress (2004) Chapter –- Glucosinolates, isothiocyanates and indoles. Cruciferous vegetables, isothiocyanates and indoles. In: IARC handbooks of cancer prevention, vol 9. IARC Press, Lyon, pp 13–23
Jakubíková J, Sedlák J, Mithen R, Bao Y (2005) Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol 69(11):1543–1552
Jin Y, Wang M, Rosen RT, Ho C-T (1999) Thermal degradation of sulforaphane in aqueous solution. J Agric Food Chem 47(8):3121–3123
Kam-Wing Lo K, Chun-Ming Ng D, Hui W-K, Cheung K-K (2001) Luminescent rhenium(I) polypyridine complexes with an isothiocyanate moiety–versatile labelling reagents for biomolecules. J Chem Soc Dalton Trans 18:2634–2640
Kassie F, Uhl M, Rabot S, Grasl-Kraupp B, Verkerk R, Kundi M, Chabicovsky M, Schulte-Hermann R, Knasmüller S (2003) Chemoprevention of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-induced colonic and hepatic preneoplastic lesions in the F344 rat by cruciferous vegetables administered simultaneously with the carcinogen. Carcinogenesis 24(2):255–261
Kelloff GJ, Boone CW, Steele VE, Fay JR, Lubet RA, Crowell JA, Sigman CC (1994) Mechanistic considerations in chemopreventive drug development. J Cell Biochem Suppl 20:1–24
Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC (2000) Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr 130(2S Suppl):467s–471s
Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN (2006) Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66(24):11580–11584
Kjær A (1960) Chapter - Naturally derived isothiocyanates (mustard oils) and their parent glucosides. In: Zechmeister L (ed) Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products/Progrés Dans la Chimie des Substances Organiques Naturelles, vol 18. Springer, Vienna, pp 122–176
Kjær A (1961) Chapter - Naturally occurring isothiocyanates and their parent glycosides. In: Kharasch N (ed) Organic sulfur compounds. Pergamon, New York, pp 409–420
Kjær A, Gmelin R (1955) Isothiocyanates XI. 4-methylthiobutyl isothiocyanate, a new naturally occurring mustard oil. Acta Chem Scand 9(3):542–544
Klaunig JE, Kamendulis LM (1999) Mechanisms of cancer chemoprevention in hepatic carcinogenesis: modulation of focal lesion growth in mice. Toxicol Sci 52(2 Suppl):101–106
Koelewijn P, Berger H (1972) Mechanism of the antioxidant action of dialkyl sulfoxides. Recl Trav Chim Pays-Bas 91(11):1275–1286
Landis-Piwowar KR, Iyer NR (2014) Cancer chemoprevention: current state of the art. Cancer Growth Metastasis 7:19–25
Leardini R, Nanni D, Pareschi P, Tundo A, Zanardi G (1997) α-(Arylthio)imidoyl radicals: [3 + 2] radical annulation of aryl isothiocyanates with 2-cyano-substituted aryl radicals. J Org Chem 62(24):8394–8399
Lee M-S (1996) Enzyme induction and comparative oxidative desulfuration of isothiocyanates to isocyanates. Chem Res Toxicol 9(7):1072–1078
Levi MS, Borne RF, Williamson JS (2001) A review of cancer chemopreventive agents. Curr Med Chem 8(11):1349–1362
Li Y, Tollefsbol TO (2010) Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Curr Med Chem 17(20):2141–2151
Ligen Z, Yuanfeng W, Yuke S, Lei Z, Mupunga J, Jianwei M, Shiwang L (2017) Broccoli seed extracts but not sulforaphane have strong free radical scavenging activities. Int J Food Sci Technol 52(11):2374–2381
Litwinienko G, Kasprzycka-Guttman T (2000) Study on the autoxidation kinetics of fat components by differential scanning calorimetry. 2. Unsaturated fatty acids and their esters. Ind Eng Chem Res 39(1):13–17
Litwinienko G, Daniluk A, Kasprzycka-Guttman T (2000) Study on autoxidation kinetics of fats by differential scanning calorimetry. 1. Saturated C12 - C18fatty acids and their esters. Ind Eng Chem Res 39(1):7–12
Lynett PT, Butts K, Vaidya V, Garrett GE, Pratt DA (2011) The mechanism of radical-trapping antioxidant activity of plant-derived thiosulfinates. Org Biomol Chem 9(9):3320–3330
Magesh S, Chen Y, Hu L (2012) Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev 32(4):687–726
Manesh C, Kuttan G (2003) Anti-tumour and anti-oxidant activity of naturally occurring isothiocyanates. J Exp Clin Cancer Res 22(2):193–199
Manson MM, Gescher A, Hudson EA, Plummer SM, Squires MS, Prigent SA (2000) Blocking and suppressing mechanisms of chemoprevention by dietary constituents. Toxicol Lett 112–113:499–505
Matteo M, Daniele N, Piero S (2007) Imidoyl radicals in organic synthesis. Curr Org Chem 11(15):1366–1384
Montaut S, Grandbois J, Rossi LS, Kamal S, Khouri J, Ménard MG, Joly HA (2012) Composition of Dithyrea wislizenii fruit extract and free-radical scavenging activity of its constituents. Can J Chem 90(8):652–659
Montaut S, Benson HJ, Kay M, Guido BS, Mahboob SS, Chénier J, Gasparetto J-L, Joly HA (2017) Probing the free-radical scavenging activity of the extract, the major glucosinolate and isothiocyanate of Eruca sativa Mill. and Lepidium densiflorum Schrad. seeds. J Food Compos Anal 61:52–58
Murata M, Yamashita N, Inoue S, Kawanishi S (2000) Mechanism of oxidative DNA damage induced by carcinogenic allyl isothiocyanate. Free Radic Biol Med 28(5):797–805
Nakamura Y, Kawakami M, Yoshihiro A, Miyoshi N, Ohigashi H, Kawai K, Osawa T, Uchida K (2002) Involvement of the mitochondrial death pathway in chemopreventive benzyl isothiocyanate-induced apoptosis. J Biol Chem 277(10):8492–8499
Nam AS, Chaligne R, Landau DA (2021) Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat Rev Genet 22(1):3–18
Nandini DB, Rao RS, Deepak BS, Reddy PB (2020) Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J Oral Maxillofac Pathol 24(2):405
Neoh TL, Yamamoto C, Ikefuji S, Furuta T, Yoshii H (2012) Heat stability of allyl isothiocyanate and phenyl isothiocyanate complexed with randomly methylated β-cyclodextrin. Food Chem 131(4):1123–1131
Nguyen VPT, Stewart J, Lopez M, Ioannou I, Allais F (2020) Glucosinolates: natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 25(19):4537
Nickisch R, Conen P, Gabrielsen SM, Meier MAR (2021) A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur. RSC Adv 11(5):3134–3142
Nuzzo S, Twamley B, Platts JA, Baker RJ (2016) Characterisation of isothiocyanic acid, HNCS, in the solid state: trapped by hydrogen bonding. Chem Commun 52(90):13296–13298
Okulicz M, Bialik I, Chichłowska J (2005) The time-dependent effect of gluconasturtiin and phenethyl isothiocyanate on metabolic and antioxidative parameters in rats. J Anim Physiol Anim Nutr 89(11–12):367–372
Olejnik A, Tomczyk J, Kowalska K, Grajek W (2010) The role of natural dietary compounds in colorectal cancer chemoprevention. Postep Hig Med Dosw 64:175–187
Papi A, Orlandi M, Bartolini G, Barillari J, Iori R, Paolini M, Ferroni F, Fumo MG, Pedulli GF, Valgimigli L (2008) Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (kaiware daikon) sprouts. J Agric Food Chem 56(3):875–883
Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L, Gamet-Payrastre L (2004) Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer 48(2):198–206
Peñéñory AB, Argüello JE, Puiatti M (2005) Novel model sulfur compounds as mechanistic probes for enzymatic and biomimetic oxidations. Eur J Org Chem 2005(1):114–122
Perschke W (1929) Über die Struktur der dreiatomigen Radikale der Rhodanwasserstoff- und Stickstoffwasserstoffsäure. Ber Dtsch Chem Ges (A and B Series) 62(11):3054–3056
Prasad AK, Mishra PC (2015) Mechanism of action of sulforaphane as a superoxide radical anion and hydrogen peroxide scavenger by double hydrogen transfer: a model for iron superoxide dismutase. J Phys Chem B 119(25):7825–7836
Prochazka Z (1959) Isolation of sulforaphane from hoary cress (Lepidium draba L.). Collect Czechoslov Chem Commun 24:2429–2430
Robiquet PJ, Boutron F (1831) Sur la sememce de moutarde. J Pharm Chim 17:279–282
Salah-Abbès JB, Abbès S, Abdel-Wahhab MA, Oueslati R (2010) In-vitro free radical scavenging, antiproliferative and anti-zearalenone cytotoxic effects of 4-(methylthio)-3-butenyl isothiocyanate from Tunisian Raphanus sativus. J Pharm Pharmacol 62(2):231–239
Satchell DPN, Satchell RS, Wassef WN (1990) The kinetics and mechanism of addition of water and alcohols to p-nitrophenyl isothiocyanate. The effects of added dimethyl sulphoxide. Z Naturforsch B 45(7):1032–1036
Schmid H, Karrer P (1948) Synthese der racemischen und der optisch aktiven Formen des Sulforaphans. Helv Chim Acta 31(6):1497–1505
Schneider W, Kaufmann H (1912) Untersuchungen über Senföle. II. Erysolin, ein Sulfonsenföl aus Erysimum perowskianum. Justus Liebigs Ann Chem 392(1):1–15
Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC (2002) Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis 23(12):2055–2061
Sharma S (1989) Isothiocyanates in heterocyclic synthesis. Sulfur Rep 8(5):327–454
Sita G, Hrelia P, Tarozzi A, Morroni F (2016) Isothiocyanates are promising compounds against oxidative stress, neuroinflammation and cell death that may benefit neurodegeneration in Parkinson’s disease. Int J Mol Sci 17(9):1454
Smith TK, Mithen R, Johnson IT (2003) Effects of Brassica vegetable juice on the induction of apoptosis and aberrant crypt foci in rat colonic mucosal crypts in vivo. Carcinogenesis 24(3):491–495
Smith TK, Lund EK, Parker ML, Clarke RG, Johnson IT (2004) Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis 25(8):1409–1415
Song D, Liang H, Kuang P, Tang P, Hu G, Yuan Q (2013) Instability and structural change of 4-methylsulfinyl-3-butenyl isothiocyanate in the hydrolytic process. J Agric Food Chem 61(21):5097–5102
Spencer GF, Daxenbichler E (1980) Gas chromatography−mass spectrometry of nitriles, isothiocyanates and oxazolidinethiones derived from cruciferous glucosinolates. J Sci Food Agric 31:359–367
Srinivasan P, Vadhanam MV, Arif JM, Gupta RC (2002) A rapid screening assay for antioxidant potential of natural and synthetic agents in vitro. Int J Oncol 20(5):983–986
Svehlíková V, Wang S, Jakubíková J, Williamson G, Mithen R, Bao Y (2004) Interactions between sulforaphane and apigenin in the induction of UGT1A1 and GSTA1 in CaCo-2 cells. Carcinogenesis 25(9):1629–1637
Talalay P, Dinkova-Kostova AT, Holtzclaw WD (2003) Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzym Regul 43(1):121–134
Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S (2002) Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62(18):5196–5203
Ulkowski M, Musialik M, Litwinienko G (2005) Use of differential scanning calorimetry to study lipid oxidation. 1. Oxidative stability of lecithin and linolenic Acid. J Agric Food Chem 53(23):9073–9077
Valgimigli L, Iori R (2009) Antioxidant and pro-oxidant capacities of ITCs. Environ Mol Mutagen 50(3):222–237
Valgimigli L, Ingold KU, Lusztyk J (1996) Solvent effects on the reactivity and free spin distribution of 2,2-diphenyl-1-picrylhydrazyl radicals 1. J Org Chem 61(22):7947–7950
Van Eylen D, Oey I, Hendrickx M, Van Loey A (2007) Kinetics of the stability of broccoli (Brassica oleracea Cv. Italica) myrosinase and isothiocyanates in broccoli juice during pressure/temperature treatments. J Agric Food Chem 55(6):2163–2170
Vanduchova A, Anzenbacher P, Anzenbacherova E (2019) Isothiocyanate from broccoli, sulforaphane, and its properties. J Med Food 22(2):121–126
Vineis P, Schatzkin A, Potter JD (2010) Models of carcinogenesis: an overview. Carcinogenesis 31(10):1703–1709
Visentin M, Tava A, Iori R, Palmieri S (1992) Isolation and identification for trans-4-(methylthio)-3-butenyl glucosinolate from radish roots (Raphanus sativus L.). J Agric Food Chem 40(9):1687–1691
Walter W, Bode K-D (1967) Syntheses of thiocarbamates. Angew Chem Int Ed Eng 6(4):281–293
Wertheim T (1844) Ueber das flüchtige Oel der Alliaria officinalis. Justus Liebigs Ann Chem 52(1):52–55
Will H (1844) Untersuchungen über die Constitution des ätherischen Oels des schwarzen Senfs. Justus Liebigs Ann Chem 52(1):1–51
Wu L, Noyan Ashraf MH, Facci M, Wang R, Paterson PG, Ferrie A, Juurlink BHJ (2004) Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc Natl Acad Sci U S A 101(18):7094–7099
Wu H, Liang H, Yuan Q, Wang T, Yan X (2010) Preparation and stability investigation of the inclusion complex of sulforaphane with hydroxypropyl-β-cyclodextrin. Carbohydr Polym 82(3):613–617
Wu Y, Mao J, Mei L, Liu S (2013) Kinetic studies of the thermal degradation of sulforaphane and its hydroxypropyl-β-cyclodextrin inclusion complex. Food Res Int 53(1):529–533
Wu Y, Mao J, You Y, Liu S (2014) Study on degradation kinetics of sulforaphane in broccoli extract. Food Chem 155:235–239
Ye L, Zhang Y (2001) Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis 22(12):1987–1992
Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH (2008) Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol 75(11):2214–2223
Yuan H, Yao S, You Y, Xiao G, You Q (2010) Antioxidant activity of isothiocyanate extracts from broccoli. Chin J Chem Eng 18(2):312–321
Zhang Y, Li J, Tang L (2005) Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radic Biol Med 38(1):70–77
Zhou JW, Wang M, Sun NX, Qing Y, Yin TF, Li C, Wu D (2019) Sulforaphane-induced epigenetic regulation of Nrf2 expression by DNA methyltransferase in human Caco-2 cells. Oncol Lett 18(3):2639–2647
Acknowledgements
Financial support from the National Science Centre, Poland (NCN grant OPUS No. 2018/31/B/ST4/02354 and NCN grant PRELUDIUM No. 2014/15/N/ST5/02939) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cedrowski, J., Grebowski, J., Litwinienko, G. (2022). Antioxidant Activity of Edible Isothiocyanates. In: Bravo-Diaz, C. (eds) Lipid Oxidation in Food and Biological Systems. Springer, Cham. https://doi.org/10.1007/978-3-030-87222-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-87222-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87221-2
Online ISBN: 978-3-030-87222-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)