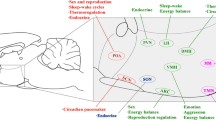
Abstract
The hypothalamus is an essential component of brain circuits that control critical physiological functions. It plays a particularly important role in regulating energy balance and feeding behaviors. Accumulating evidence suggests that perturbations in hypothalamic development greatly contribute to obesity and metabolic diseases in later life. This chapter will discuss the timelines during which hypothalamic neurons develop, paying particular attention to neurons producing agouti-related peptide/neuropeptide Y, pro-opiomelanocortin, and oxytocin, because of their documented role in feeding regulation. It will also describe hormonal, molecular, and cellular factors related to the development of these neuronal systems. Finally, it will review the role of genetic and nutritional factors in hypothalamic development.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AgRP:
-
agouti-related peptide
- AN:
-
accessory nucleus
- ARH:
-
arcuate nucleus of the hypothalamus
- ATG:
-
autophagy-related protein
- AVP:
-
arginine vasopressin
- BNST:
-
bed nucleus of the stria terminalis
- CTR:
-
calcitonin receptor
- DIO:
-
diet-induced obesity
- DMH:
-
dorsomedial nucleus of the hypothalamus
- E:
-
embryonic day
- ERK:
-
extracellular signal-regulated kinase
- GHSR:
-
growth hormone secretagogue receptor
- GLP1:
-
glucagon-like peptide 1
- GLP1-R:
-
glucagon-like peptide 1 receptor
- HFD:
-
high-fat diet
- LepR:
-
leptin receptor
- LHA:
-
lateral hypothalamic area
- MC4-R:
-
melanocortin 4 receptor
- MRI:
-
magnetic resonance imaging
- MTII:
-
melanotan II
- NPY:
-
neuropeptide Y
- OT:
-
oxytocin
- OTR:
-
oxytocin receptor
- P:
-
postnatal day
- POMC:
-
pro-opiomelanocortin
- PVH:
-
paraventricular nucleus of the hypothalamus
- PWS:
-
Prader-Willi Syndrome
- RAMP:
-
receptor activity-modifying protein
- SCN:
-
suprachiasmatic nucleus
- SON:
-
supraoptic nucleus
- STAT:
-
signal transducer and activator of transcription
- VMH:
-
ventromedial nucleus of the hypothalamus
- Y1R:
-
neuropeptide Y receptor 1
- αMSH:
-
alpha melanocyte-stimulating hormone
References
Abegg K, Hermann A, Boyle CN, Bouret SG, Lutz TA, Riediger T (2017) Involvement of amylin and leptin in the development of projections from the area postrema to the nucleus of the solitary tract. Front Endocrinol (Lausanne) 8:324
Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A (1999) Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev 13:2787–2800
Ackland J, Ratter S, Bourne GL, Rees LH (1983) Characterization of immunoreactive somatostatin in human fetal hypothalamic tissue. Regul Pept 5:95–101
Ahima RS, Hileman SM (2000) Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Pept 92:1–7
Ahima R, Prabakaran D, Flier J (1998) Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101:1020–1027
Alstein M, Whitnall MH, House S, Key S, Gainer H (1988) An immunochemical analysis of oxytocin and vasopressin prohormone processing in vivo. Peptides 9:87–105
Althammer F, Grinevich V (2017) Diversity of oxytocin neurons: beyond magno- and parvocellular cell types? J Neuroendocrinol
Altman J, Bayer SA (1986) The development of the rat hypothalamus. Adv Anat Embryol Cell Biol 100:1–178
Anthwal N, Pelling M, Claxton S, Mellitzer G, Collin C, Kessaris N, Richardson WD, Gradwohl G, Ang SL (2013) Conditional deletion of neurogenin-3 using Nkx2.1iCre results in a mouse model for the central control of feeding, activity and obesity. Dis Model Mech 6:1133–1145
Atasoy D, Betley JN, Su HH, Sternson SM (2012) Deconstruction of a neural circuit for hunger. Nature 488:172–177
Aujla PK, Naratadam GT, Xu L, Raetzman LT (2013) Notch/Rbpjκ signaling regulates progenitor maintenance and differentiation of hypothalamic arcuate neurons. Development 140:3511–3521
Bochukova EG, Lawler K, Croizier S, Keogh JM, Patel N, Strohbehn G, Lo KK, Humphrey J, Hokken-Koelega A, Damen L, Donze S, Bouret SG, Plagnol V, Farooqi IS (2018) A transcriptomic signature of the hypothalamic response to fasting and BDNF deficiency in Prader-Willi syndrome. Cell Rep 22:3401–3408
Bouret S, Simerly RB (2007) Development of leptin-sensitive circuits. J Neuroendocrinol 19:575–582
Bouret SG, Draper SJ, Simerly RB (2004a) Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24:2797–2805
Bouret SG, Draper SJ, Simerly RB (2004b) Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110
Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB (2008) Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7:179–185
Bouret SG, Bates SH, Chen S, Myers MG, Simerly RB (2012) Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci 32:1244–1252
Bray GA, York DA (1979) Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev 59
Brischoux F, Fellman D, Risold P-Y (2001a) Ontogenetic development of the diencephalic MCH neurons: a hypothalamic ‘MCH area’ hypothesis. Eur J Neurosci 13:1733–1744
Brischoux F, Fellmann D, Risold PY (2001b) Ontogenetic development of the diencephalic MCH neurons: a hypothalamic ‘MCH area’ hypothesis. Eur J Neurosci 13:1733–1744
Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M (2016) Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165:1762–1775
Bugnon C, Fellmann D, Bresson JL, Clavequin MC (1982) Immunocytochemical study of the ontogenesis of the CRH-containing neuroglandular system in the human hypothalamus. CR Acad Sci 294
Burford GD, Robinson IC (1982) Oxytocin, vasopressin and neurophysins in the hypothalamo-neurohypophysial system of the human fetus. J Endocrinol 95:403–408
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P (1995) Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269:546–549
Capuano CA, Leibowitz SF, BARR GA (1993) Effect of paraventricular injection of neuropeptide Y on milk and water intake of preweanling rat. Neuropeptides 24:177–182
Caqueret A, Boucher F, Michaud JL (2006) Laminar organization of the early developing anterior hypothalamus. Dev Biol 298:95–106
Caron E, Sachot C, Prevot V, Bouret SG (2010) Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol 518:459–476
Carreno G, Apps JR, Lodge EJ, Panousopoulos L, Haston S, Gonzalez-Meljem JM, Hahn H, Andoniadou CL, Martinez-Barbera JP (2017) Hypothalamic sonic hedgehog is required for cell specification and proliferation of LHX3/LHX4 pituitary embryonic precursors. Development 144:3289–3302
Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, More KJ, Breitbart RE (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495
Chen H, Simar D, Morris MJ (2009) Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS One 4:e6259
Coggeshall RE (1964) A study of diencephalic development in the albino rat. J Comp Neurol 122:241–269
Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM (2001) Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108:1113–1121
Coleman DL (1973) Effects of parabiosis of obese with diabetes and normal mice. Diabetologia 9:294–298
Coleman DL, Hummel KP (1969) Effects of parabiosis of normal with genetically diabetic mice. Diabetologia Am J Physiol:1298–1304
Coupe B, Ishii Y, Dietrich MO, Komatsu M, Horvath TL, Bouret SG (2012) Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab 15:247–255
Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove K, Strasburger G, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath T (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuits regulating energy homeostasis. Neuron 37:649–661
Croizier S, Franchi-Bernard G, Colard C, Poncet F, La Roche A, Risold P-Y (2010) A comparative analysis shows morphofunctional differences between the rat and mouse melanin-concentrating hormone systems. PLoS One 5:e15471
Croizier S, Amiot C, Chen X, Presse Fß, Nahon J-L, Wu JY, Fellmann D, Risold P-Y (2011) Development of posterior hypothalamic neurons enlightens a switch in the prosencephalic basic plan. PLoS One 6:e28574
Croizier S, Park S, Maillard J, Bouret SG (2018) Central dicer-miR-103/107 controls developmental switch of POMC progenitors into NPY neurons and impacts glucose homeostasis. elife 7
Daikoku S, Okamura Y, Kawano H, Tsuruo Y, Maegawa M, Shibasaki T (1984) Immunohistochemical study on the development of CRF-containing neurons in the hypothalamus of the rat. Cell Tissue Res 238:539–544
de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu S-M, Chua SC Jr (2005) Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115:3484–3493
Dearden L, Buller S, Furigo IC, Fernandez-Twinn DS, Ozanne SE (2020) Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways. Mol Metab 42:101079
Diaz C, Puelles L (2020) Developmental genes and malformations in the hypothalamus. Front Neuroanat 14:607111
Dongen VPAM, Nieuwenhuys R (1989) Diencephalon. In: Dubbeldam JL, Van Dongen PAM, Voogd J (eds) The central nervous system of vertebrates, vol 3. Springer, Berlin, pp 1844–1871
Drucker DJ (1998) Glucagon-like peptides. Diabetes 47:159–169
Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, Ciobanu AC, Triana del Rio R, Roth LC, Althammer F, Chavant V, Goumon Y, Gruber T, Petit-Demouliere N, Busnelli M, Chini B, Tan LL, Mitre M, Froemke RC, Chao MV, Giese G, Sprengel R, Kuner R, Poisbeau P, Seeburg PH, Stoop R, Charlet A, Grinevich V (2016) A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 89:1291–1304
Fuchs JL, Schwark HD (2004) Neuronal primary cilia: a review. Cell Biol Int 28:111–118
Geng X, Speirs C, Lagutin O, Inbal A, Liu W, Solnica-Krezel L, Jeong Y, Epstein DJ, Oliver G (2008) Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell 15:236–247
Gervais M, Labouèbe G, Picard A, Thorens B, Croizier S (2020) EphrinB1 modulates glutamatergic inputs into POMC-expressing progenitors and controls glucose homeostasis. PLoS Biol 18:e3000680
Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683
Glavas MM, Joachim SE, Draper SJ, Smith MS, Grove KL (2007) Melanocortinergic activation by melanotan II inhibits feeding and increases uncoupling protein 1 messenger ribonucleic acid in the developing rat. Endocrinology 148:3279–3287
Glendining KA, Jasoni CL (2019) Maternal high fat diet-induced obesity modifies histone binding and expression of Oxtr in offspring hippocampus in a sex-specific manner. Int J Mol Sci 20
Godefroy D, Dominici C, Hardin-Pouzet H, Anouar Y, Melik-Parsadaniantz S, Rostene W, Reaux-Le Goazigo A (2017) Three-dimensional distribution of tyrosine hydroxylase, vasopressin and oxytocin neurones in the transparent postnatal mouse brain. J Neuroendocrinol 29
Goldstone AP, Unmehopa UA, Bloom SR, Swaab DF (2002) Hypothalamic NPY and agouti-related protein are increased in human illness but not in Prader-Willi syndrome and other obese subjects. J Clin Endocrinol Metabol 87:927–937
Goldstone AP, Unmehopa UA, Swaab DF (2003) Hypothalamic growth hormone-releasing hormone (GHRH) cell number is increased in human illness, but is not reduced in Prader-Willi syndrome or obesity. 58:8
Goudsmit E, Neijmeijer-Leloux A, Swaab DF (1992) The human hypothalamo-neurohypophyseal system in relation to development, aging and Alzheimer’s disease. Prog Brain Res 93:237–247; discussion 247–238
Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL (2006) Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience 143:975–986
Greenwood MA, Hammock EA (2017) Oxytocin receptor binding sites in the periphery of the neonatal mouse. PLoS One 12:e0172904
Grinevich V, Desarménien MG, Chini B, Tauber M, Muscatelli F (2015) Ontogenesis of oxytocin pathways in the mammalian brain: late maturation and psychosocial disorders. Front Neuroanat 8
Grove KL, Allen S, Grayson BE, Smith MS (2003) Postnatal development of the hypothalamic neuropeptide Y system. Neuroscience 116:393–406
Gutnick A, Blechman J, Kaslin J, Herwig L, Belting HG, Affolter M, Bonkowsky JL, Levkowitz G (2011) The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary. Dev Cell 21:642–654
Haddad-Tóvolli R, Altirriba J, Obri A, Sánchez EE, Chivite I, Milà-Guasch M, Ramírez S, Gómez-Valadés AG, Pozo M, Burguet J, Velloso LA, Claret M (2020) Pro-opiomelanocortin (POMC) neuron translatome signatures underlying obesogenic gestational malprogramming in mice. Mol Metab 36:100963
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546
Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM (1997) Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. PNAS 94:8878–8883
Hammock EA, Levitt P (2013) Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse. Front Behav Neurosci 7:195
Hoffiz YC, Castillo-Ruiz A, Hall MAL, Hite TA, Gray JM, Cisternas CD, Cortes LR, Jacobs AJ, Forger NG (2021) Birth elicits a conserved neuroendocrine response with implications for perinatal osmoregulation and neuronal cell death. Sci Rep 11:2335
Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F (1993) Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics 91:398–402
Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS, Nollen NL, Savage CR (2006) Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity 14:1028–1037
Horvath TL, Sarman B, García-Cáceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Brönneke HS, Levin BE, Diano S, Cowley MA, Tschöp MH (2010) Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA 107:14875–14880
Hosoya T, Oda Y, Takahashi S, Morita M, Kawauchi S, Ema M, Yamamoto M, Fujii-Kuriyama Y (2001) Defective development of secretory neurones in the hypothalamus of Arnt2-knockout mice. Genes Cells 6:361–374
Hu Y, Li J, Zhu Y, Li M, Lin J, Yang L, Wang C, Lu Z (2020) Development and characterization of an Otp conditional loss of function allele. Genesis (New York: 2000) 58, e23370
Hyyppä M (1969) Differentiation of the hypothalamic nuclei during ontogenetic development in the rat. Z Anat Entwicklungsgesch 129:41–52
Ifft JD (1972) An autoradiographic study of the time of final division of neurons in rat hypothalamic nuclei. J Comp Neurol 144:193–204
Ishii Y, Bouret SG (2012) Embryonic birthdate of hypothalamic leptin-activated neurons in mice. Endocrinology 153:3657–3667
Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, Boulet S, Curtis MG, and Care, C.A.P.C.W.G.S.P.o.P (2006) Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care MMWR Recomm Rep 55, 1–23
Johnson MD, Bouret SG, Dunn-Meynell AA, Boyle CN, Lutz TA, Levin BE (2016) Early postnatal amylin treatment enhances hypothalamic leptin signaling and neural development in the selectively bred diet-induced obese rat. Am J Physiol Regul Integr Comp Physiol 311:R1032–r1044
Jurado MPMVaS (2020) Specification of oxytocinergic and vasopressinergic circuits in the developing mouse brain. BioRxiv https://doi.org/10.1101/2020.08.07.241364
Jurek B, Neumann ID (2018) The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev 98:1805–1908
Kamitakahara A, Bouyer K, Wang CH, Simerly R (2017) A critical period for the trophic actions of leptin on AgRP neurons in the arcuate nucleus of the hypothalamus. J Comp Neurol
Keyser A (1979) Development of the hypothalamus in mammals. (New York)
Khachaturian H, Alessi NE, Lewis ME, Munfakh N, Fitzsimmons MD, Watson SJ (1985) Development of hypothalamic opioid neurons: a combined immunocytochemical and [3H]thymidine autoradiographic study. Neuropeptides 5:477–480
Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ (1996) The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 10:60–69
Kirk SL, Samuelsson A-M, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW (2009) Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One 4:e5870
Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931–937
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Kokoeva MV, Yin H, Flier JS (2005) Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310:679–683
Kokoeva MV, Yin H, Flier JS (2007) Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol 505:209–220
Koutcherov Y, Mai JK, Ashwell KW, Paxinos G (2002) Organization of human hypothalamus in fetal development. J Comp Neurol 446:301–324
Kowalski, T.J., Liu, S.-M., Leibel, R.L., and Chua, S.C., Jr. (2001). Transgenic complementation of leptin-receptor deficiency: I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene diabetes 50, 425-435
Kozlov SV, Bogenpohl JW, Howell MP, Wevrick R, Panda S, Hogenesch JB, Muglia LJ, Van Gelder RN, Herzog ED, Stewart CL (2007) The imprinted gene Magel2 regulates normal circadian output. Nat Genet 39:1266–1272
Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HRC, McKinnon PJ, Solnica-Krezel L, Oliver G (2003) Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev 17:368–379
Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379:632–635
Lee S, Kozlov S, Hernandez L, Chamberlain SJ, Brannan CI, Stewart CL, Wevrick R (2000) Expression and imprinting of MAGEL2 suggest a role in Prader–Willi syndrome and the homologous murine imprinting phenotype. Hum Mol Genet 9:1813–1819
Lee S, Walker CL, Wevrick R (2003) Prader–Willi syndrome transcripts are expressed in phenotypically significant regions of the developing mouse brain. Gene Expr Patterns 3:599–609
Lee HJ, Macbeth AH, Pagani JH, Young WS 3rd (2009) Oxytocin: the great facilitator of life. Prog Neurobiol 88:127–151
Lee B, Lee S, Lee SK, Lee JW (2016) The LIM-homeobox transcription factor Isl1 plays crucial roles in the development of multiple arcuate nucleus neurons. Development 143:3763–3773
Lee B, Kim J, An T, Kim S, Patel EM, Raber J, Lee SK, Lee S, Lee JW (2018) Dlx1/2 and Otp coordinate the production of hypothalamic GHRH- and AgRP-neurons. Nat Commun 9:2026
Lee CH, Song DK, Park CB, Choi J, Kang GM, Shin SH, Kwon I, Park S, Kim S, Kim JY, Dugu H, Park JW, Choi JH, Min SH, Sohn JW, Kim MS (2020) Primary cilia mediate early life programming of adiposity through lysosomal regulation in the developing mouse hypothalamus. Nat Commun 11:5772
Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE (1997) Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273:R725–R730
Li C, Xu JJ, Hu HT, Shi CY, Yu CJ, Sheng JZ, Wu YT, Huang HF (2020) Amylin receptor insensitivity impairs hypothalamic POMC neuron differentiation in the male offspring of maternal high-fat diet-fed mice. Mol Metab 44:101135
Lu F, Kar D, Gruenig N, Zhang ZW, Cousins N, Rodgers HM, Swindell EC, Jamrich M, Schuurmans C, Mathers PH, Kurrasch DM (2013) Rax is a selector gene for mediobasal hypothalamic cell types. J Neurosci 33:259–272
Lutz TA, Coester B, Whiting L, Dunn-Meynell AA, Boyle CN, Bouret SG, Levin BE, Le Foll C (2018) Amylin selectively signals onto POMC neurons in the arcuate nucleus of the hypothalamus. Diabetes 67:805–817
Mai JK, Lensing-Hohn S, Ende AA, Safroniew MV (1997) Developmental organization of neurophysin neurons in the human brain. J Comp Neurol 385:477–489
Maillard J, Park S, Croizier S, Vanacker C, Cook JH, Prevot V, Tauber M, Bouret SG (2016) Loss of Magel2 impairs the development of hypothalamic Anorexigenic circuits. Hum Mol Genet 25:3208–3215
Makarenko IG, Ugrumov MV, Derer P, Calas A (2000) Projections from the hypothalamus to the posterior lobe in rats during ontogenesis: 1,1?-dioctadecyl-3,3,3?,3?-tetramethylindocarbocyanine perchlorate tracing study. J Comp Neurol 422:327–337
Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, Logan M, Placzek M (2006) Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev Cell 11:873–885
Marín O, Baker J, Puelles L, Rubenstein JLR (2002) Patterning of the basal telencephalon and hypothalamus is essential for guidance of cortical projections. Development 129:761–773
Matsumoto A, Arai Y (1976) Developmental changes in synaptic formation in the hypothalamic arcuate nucleus of female rats. Cell Tissue Res 14:143–156
McClellana KM, Parker KL, Tobet SA (2006) Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol 27:193–209
McMinn JE, Liu S-M, Liu H, Dragatsis I, Dietrich P, Ludwig T, Boozer CN, Chua SC Jr (2005) Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab 289:E403–E411
McNay DEG, Pelling M, Claxton S, Guillemot Fß, Ang S-L (2006) Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol Endocrinol 20:1623–1632
McNay DEG, Briançon N, Kokoeva MV, Maratos-Flier E, Flier JS (2012) Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest 122:142–152
Melnick I, Pronchuck N, Cowley MA, Grove KL, Colmers WF (2007) Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron 56:1103–1115
Mercer RE, Kwolek EM, Bischof JM, van Eede M, Henkelman RM, Wevrick R (2009) Regionally reduced brain volume, altered serotonin neurochemistry, and abnormal behavior in mice null for the circadian rhythm output gene Magel2. Am J Med Genet B Neuropsychiatr Genet 150B:1085–1099
Mercer RE, Michaelson SD, Chee MJS, Atallah TA, Wevrick R, Colmers WF (2013) Magel2 Is required for leptin-mediated depolarization of POMC neurons in the hypothalamic arcuate nucleus in mice. PLoS Genet 9:e1003207
Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, Guillon G, Lafitte D, Desarmenien MG, Tauber M, Muscatelli F (2015) An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biol Psychiatry 78:85–94
Michaud JL, DeRossi C, May NR, Holdener BC, Fan CM (2000) ARNT2 acts as the dimerization partner of SIM1 for the development of the hypothalamus. Mech Dev 90:253–261
Miller JL, Couch JA, Schmalfuss I, He G, Liu Y, Driscoll DJ (2007) Intracranial abnormalities detected by three-dimensional magnetic resonance imaging in Prader–Willi syndrome. Am J Med Genet A 143A:476–483
Miriam Altstein MHW, House S, Key S, Gainer H (1988) An immunochemical analysis of oxytocin and vasopressin prohormone processing in vivo. Peptides 9:87–105
Mistry A, Swick A, Romsos D (1999) Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Phys 277:R742–R747
Mountjoy KG, Wild JM (1998) Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Dev Brain Res 107:309–314
Nakai S, Kawano H, Yudate T, Nishi M, Kuno J, Nagata A, Jishage K, Hamada H, Fujii H, Kawamura K et al (1995) The POU domain transcription factor Brn-2 is required for the determination of specific neuronal lineages in the hypothalamus of the mouse. Genes Dev 9:3109–3121
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409:194–198
Nasif S, de Souza FS, González LE, Yamashita M, Orquera DP, Low MJ, Rubinstein M (2015) Islet 1 specifies the identity of hypothalamic melanocortin neurons and is critical for normal food intake and adiposity in adulthood. Proc Natl Acad Sci USA 112:E1861–E1870
Newmaster KT, Nolan ZT, Chon U, Vanselow DJ, Weit AR, Tabbaa M, Hidema S, Nishimori K, Hammock EAD, Kim Y (2020) Quantitative cellular-resolution map of the oxytocin receptor in postnatally developing mouse brains. Nat Commun 11:1885
Nilsson I, Johansen JE, Schalling M, Hˆkfelt T, Fetissov SO (2005a) Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Dev Brain Res 155:147–154
Nilsson I, Johansen JE, Schalling M, Hokfelt T, Fetissov SO (2005b) Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Dev Brain Res 155:147–154
Orquera DP, Tavella MB, de Souza FSJ, Nasif S, Low MJ, Rubinstein M (2019) The homeodomain transcription factor NKX2.1 is essential for the early specification of melanocortin neuron identity and activates Pomc expression in the developing hypothalamus. J Neurosci 39:4023–4035
Padilla SL, Carmody JS, Zeltser LM (2010) Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med 16:403–405
Park S, Aintablian A, Coupe B, Bouret SG (2020a) The endoplasmic reticulum stress-autophagy pathway controls hypothalamic development and energy balance regulation in leptin-deficient neonates. Nat Commun 11:1914
Park S, Jang A, Bouret SG (2020b) Maternal obesity-induced endoplasmic reticulum stress causes metabolic alterations and abnormal hypothalamic development in the offspring. PLoS Biol 18:e3000296
Pelleymounter M, Cullen M, Baker M, Hecht R, Winters D, Boone T, F, C. (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543
Pelling M, Anthwal N, McNay D, Gradwohl G, Leiter AB, Guillemot F, Ang SL (2011) Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev Biol 349:406–416
Peng CY, Mukhopadhyay A, Jarrett JC, Yoshikawa K, Kessler JA (2012) BMP receptor 1A regulates development of hypothalamic circuits critical for feeding behavior. J Neurosci 32:17211–17224
Piao H, Hosoda H, Kangawa K, Murata T, Narita K, Higuchi T (2008) Ghrelin stimulates milk intake by affecting adult type feeding behaviour in postnatal rats. J Neuroendocrinol 20:330–334
Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL (2004) Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304:110–115
Prader A, Labhart A, Willi H (1956) Ein syndrom von adipositas, kleinwuchskryptorchismus und oligophrenie nach myatonieartigem zustand im neugeborenenalter. Scweiz Med Wochenschr 86:1260–1261
Pravdivyi I, Ballanyi K, Colmers WF, Wevrick R (2015) Progressive postnatal decline in leptin sensitivity of arcuate hypothalamic neurons in the Magel2-null mouse model of Prader–Willi syndrome. Hum Mol Genet 24:4276–4283
Proulx K, Richard D, Walker C-D (2002) Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology 143:4683–4692
Quarta C, Fisette A, Xu Y, Colldén G, Legutko B, Tseng YT, Reim A, Wierer M, De Rosa MC, Klaus V, Rausch R, Thaker VV, Graf E, Strom TM, Poher AL, Gruber T, Le Thuc O, Cebrian-Serrano A, Kabra D, Bellocchio L, Woods SC, Pflugfelder GO, Nogueiras R, Zeltser L, Grunwald Kadow IC, Moon A, García-Cáceres C, Mann M, Treier M, Doege CA, Tschöp MH (2019) Functional identity of hypothalamic melanocortin neurons depends on Tbx3. Nat Metab 1:222–235
Rakic P, Cameron RS, Komuro H (1994) Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol 4:63–69
Risold PY, Croizier S, Legagneux K, Brischoux F, Fellmann D, Griffond B (2009) The development of the MCH system. Peptides 30:1969–1972
Rozo AV, Babu DA, Suen PA, Groff DN, Seeley RJ, Simmons RA, Seale P, Ahima RS, Stoffers DA (2017) Neonatal GLP1R activation limits adult adiposity by durably altering hypothalamic architecture. Mol Metab 6:748–759
Saper CB, Swanson LW, Cowan WM (1979) An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol 183:689–706
Sauer FC (1935) Mitosis in the neural tube. J Comp Neurol 62:377–405
Sawchenko PE (1998) Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol 402:435–441
Sawchenko PE, Swanson LW (1983) The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218:121–144
Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F (2010) A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet 19:4895–4905
Schmidt I, Fritz A, Scholch C, Schneider D, Simon E, Plagemann A (2001) The effect of leptin treatment on the development of obesity in overfed suckling Wistar rats. Int J Obes Relat Metab Disorders 25:1168–1174
Schonemann MD, Ryan AK, McEvilly RJ, O'Connell SM, Arias CA, Kalla KA, Li P, Sawchenko PE, Rosenfeld MG (1995) Development and survival of the endocrine hypothalamus and posterior pituitary gland requires the neuronal POU domain factor Brn-2. Genes Dev 9:3122–3135
Shapira NA, Lessig MC, He AG, James GA, Driscoll DJ, Liu Y (2005) Satiety dysfunction in Prader-Willi syndrome demonstrated by fMRI. J Neurol Neurosurg Psychiatry 76:260–262
Shimada M, Nakamura T (1973) Time of neuron origin in mouse hypothalamic nuclei. Exp Neurol 41:163–173
Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J, Blackshaw S (2010) A genomic atlas of mouse hypothalamic development. Nat Neurosci 13:767–775
Silverman A-J, Goldstein R, Gadde CA (1980) The ontogenesis of neurophysin-containing neurons in the mouse hypothalamus. Peptides 1:27–44
Steculorum SM, Bouret SG (2011) Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology 152:4171–4179
Steculorum SM, Collden G, Coupe B, Croizier S, Andrews Z, Jarosch F, Klussmann S, Bouret SG (2015) Ghrelin programs development of hypothalamic feeding circuits. J Clin Invest 125:846–858
Sun L, Lizneva D, Ji Y, Colaianni G, Hadelia E, Gumerova A, Ievleva K, Kuo TC, Korkmaz F, Ryu V, Rahimova A, Gera S, Taneja C, Khan A, Ahmad N, Tamma R, Bian Z, Zallone A, Kim SM, New MI, Iqbal J, Yuen T, Zaidi M (2019) Oxytocin regulates body composition. Proc Natl Acad Sci USA 116:26808–26815
Sussel L, Marin O, Kimura S, Rubenstein JL (1999) Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126:3359–3370
Swaab DF, Purba JS, Hofman MA (1995) Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metabol 80:573–579
Swanson LW, Kuypers HG (1980) The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194:555–570
Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y (2000) Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev 99:143–148
Tamborski S, Mintz EM, Caldwell HK (2016) Sex differences in the embryonic development of the central oxytocin system in mice. J Neuroendocrinol 28
Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274:1123–1133
Tolle V, Zizzari P, Tomasetto C, Rio MC, Epelbaum J, Bluet-Pajot MT (2001) In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology 73:54–61
Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ (1989) Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. J Neurosci 9:1764–1773
Tschöp M, Smiley DL, Heiman ML (2000) Ghrelin induces adiposity in rodent. Nature:908–913
Vaidyanathan R, Hammock EA (2017) Oxytocin receptor dynamics in the brain across development and species. Dev Neurobiol 77:143–157
van der Klaauw AA, Croizier S, Mendes de Oliveira E, Stadler LKJ, Park S, Kong Y, Banton MC, Tandon P, Hendricks AE, Keogh JM, Riley SE, Papadia S, Henning E, Bounds R, Bochukova EG, Mistry V, O'Rahilly S, Simerly RB, Minchin JEN, Barroso I, Jones EY, Bouret SG, Farooqi IS (2019) Human Semaphorin 3 variants link melanocortin circuit development and energy balance. Cell 176:729–742.e718
van Eerdenburg FJ, Rakic P (1994) Early neurogenesis in the anterior hypothalamus of the rhesus monkey. Brain Res Dev Brain Res 79:290–296
Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC, Predel R, Kloppenburg P, Horvath TL, Brüning JC (2014) Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 156:495–509
Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY (2020) The maternal microbiome modulates fetal neurodevelopment in mice. Nature 586:281–286
Wang W, Lufkin T (2000) The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev Biol 227:432–449
Wasinski F, Furigo IC, Teixeira PDS, Ramos-Lobo AM, Peroni CN, Bartolini P, List EO, Kopchick JJ, Donato J Jr (2020) Growth hormone receptor deletion reduces the density of axonal projections from hypothalamic arcuate nucleus neurons. Neuroscience 434:136–147
Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H (2001) Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet 38:792–798
Widmer H, Amerdeil H, Fontanaud P, Desarménien MG (1997) Postnatal maturation of rat hypothalamoneurohypophysial neurons: evidence for a developmental decrease in calcium entry during action potentials. J Neurophysiol 77:260–271
Wu TJ, Gibson MJ, Rogers MC, Silverman AJ (1997) New observations on the development of the gonadotropin-releasing hormone system in the mouse. J Neurobiol 33:983–998
Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JLR (2009) Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y+, proopiomelanocortin+, and tyrosine hydroxylase+ neurons in neonatal and adult mice. J Comp Neurol 517:37–50
Yoshimura R, Kimura T, Watanabe D, Kiyama H (1996) Differential expression of oxytocin receptor mRNA in the developing rat brain. Neurosci Res 24:291–304
Zhang Y, Proenca R, Maffel M, Barone M, Leopold L, Friedman JM (1994) Position cloning of the mouse obese gene and its human homologue. Nature 372:425
Zhou Q, Wang S, Anderson DJ (2000) Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 25:331–343
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bouret, S.G., Muscatelli, F. (2021). Ontogenesis of Hypothalamic Neurons in Mammals. In: Grinevich, V., Dobolyi, Á. (eds) Neuroanatomy of Neuroendocrine Systems. Masterclass in Neuroendocrinology, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-030-86630-3_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-86630-3_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86629-7
Online ISBN: 978-3-030-86630-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)