Abstract
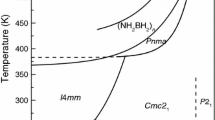
The paper presents the characteristics behavior of Ammonia Borane (NH3BH3), which is an encouraging solid-state hydrogen storage material having theoretical 19.6 weight % hydrogen content. Ammonia Borane decomposes thermally between 373 to 473 K temperatures, and the limitations associated with the decomposition is slow kinetics with a warm-up period of 20 min. With the addition of the Silicon nanoparticle approach, the ball milling process was used to enhance the kinetics and suppress the warm-up period during the isothermal decomposition. The isothermal decomposition curve for silicon added ball-milled Ammonia Borane represents an enhancement in hydrogen uptake of about 9 wt % compared to the pure crystalline powder sample of Ammonia Borane. Fourier-transform infrared spectroscopy (FTIR) and Transmission electron microscopy (TEM) spectroscopy techniques validated the hydrogen released characteristics from the Ammonia Borane.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Manoharan Y, Hosseini S, Butler B (2019) Review: hydrogen fuel cell vehicles; current status and future prospect. Appl Sci 9(11):2296
Abe JO, Popoola API, Ajenifuji E (2019) Hydrogen energy, economy and storage: review and recommendation. Int J Hydrogen Energy 44(29):15072–15086
Gangal A, Sharma P (2013) Kinetic analysis and modeling of thermal decomposition of Amonia Borane. Int J Chem Kinet 452–61
Zhitao X, Chaw KY, Guotao W, Ping C, Wendy S, Karmarkar A, Thomas A, Martin OJ, Simon RJ, Peter PE, William IFD (2018) High-Capacity hydrogen storage in lithium and Sodium Amidoboranes. Nat Mater 7:138–141
Frueh S, Kellett R, Mallery C, Molter T, Willis WS, King’ondu C, Suib S L, (2011) Pyrolytic decomposition of Ammonia Borane to Boron Nitride. Inorg Chem 50:783–792
Shore SG, Parry RW (1955) The cryastalline compound Ammonia Borane, 1H3NBH3. J Am Chem Soc 77:6084–6085
Gangal A, Kale P, Edla R, Manna J, Sharma P (2012) Study of kinetics and thermal decomposition of ammonia borane in presence of silicon nanoparticles. Int J Hydrogen Energy 37(8):6741–6748
Aneesh C (2013) Gangal (2013) Ammonia Borane as Hydrogen Storage Material. Thesis, Department of Energy Science and Engineering, IIT Bombay
Rosalind D (2016) Lithium Amide Halides for Hydrogen Storage, Thesis, Centre for Hydrogen and Fuel Cell Research, School of Chemical Engineering, University of Birmingham (2016).
Kalamkar R, Gangal A, Yakkundi V (2017) Development of experimental setup for measurement of stored hydrogen in solids by volumetric method. In: Pawar P, Ronge B, Balasubramaniam R, Seshabhattar S (eds) Techno-Societal 2016. ICATSA 2016. Springer, Cham. 569–577
Gangal AC, Edla R et al (2015) Effect of misch metal nanoparticles on thermal decomposition of Ammonia Borane, J Res Nanotechnol
Bor-Yih Yu (2013) Introduction to Aspen Plus, PSE Laboratory, Department of Chemical Engineering, Nation Taiwan University
Kalamkar R, Gangal A, Yakkundi V (2018) Hydrogen storage characteristics of mixture of Lithium Amide and Lithium Hydride using Severt’s type apparatus. In: Pawar P et al (eds), Techno-Societal 2018, Springer, Cham
Kalamkar R, Gangal A, Yakkundi V (2020) Fabrication and analysis of apparatus for measuring stored renewable hydrogen energy in metal hydrides. RAM 2020. SVNIIT, Gujarat
Edla R, Gangal A (2014) Kinetics and the thermal decomposition of Sodium Alanate in the presence of MmNi4.5Al0.5 nanoparticles. Material Research Express. IOP Science
Zulkarnain Jalil and Adi Rahwanto (2018) The use of nano-silicon carbide and nickel as catalyst in magnesium hydrides (MgH2) for hydrogen storage material application. Materials Research Express. 5. IOP Science (2018)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Kalamkar, R., Yakkundi, V., Gangal, A. (2021). Behavior of Ammonia Borane as Solid-State Hydrogen Storage Material. In: Pawar, P.M., Balasubramaniam, R., Ronge, B.P., Salunkhe, S.B., Vibhute, A.S., Melinamath, B. (eds) Techno-Societal 2020. Springer, Cham. https://doi.org/10.1007/978-3-030-69925-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-69925-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69924-6
Online ISBN: 978-3-030-69925-3
eBook Packages: EngineeringEngineering (R0)