Abstract
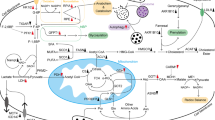
Currently, approximately 95% of pancreatic cancers are pancreatic ductal adenocarcinomas (PDAC), which are the most aggressive form and the fourth leading cause of cancer death with extremely poor prognosis [1]. Poor prognosis is primarily attributed to the late diagnosis of the disease when patients are no longer candidates for surgical resection [2]. Cancer cells are dependent on the oncogenes that allow them to proliferate limitlessly. Thus, targeting the expression of known oncogenes in pancreatic cancer has been shown to lead to more effective treatment [3]. This chapter discusses the complexity of metabolic features in pancreatic cancers. In order to comprehend the heterogeneous nature of cancer metabolism fully, we need to take into account the close relationship between cancer metabolism and genetics. Gene expression varies tremendously, not only among different types of cancers but also within the same type of cancer among different patients. Cancer metabolism heterogeneity is often prompted and perpetuated not only by mutations in oncogenes and tumor-suppressor genes but also by the innate diversity of the tumor microenvironment. Much effort has been focused on elucidating the genetic alterations that correlate with disease progression and treatment response [4, 5]. However, the precise mechanisms by which tumor metabolism contributes to cancer growth, survival, mobility, and aggressiveness represent a functional readout of tumor progression (Fig. 1).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Oncogenic KRAS regulates glucose and glutamine metabolism in pancreatic cancer cells.
-
MUC1 overexpression leads to increased glucose metabolism.
-
p53 functions predict the sensitivity of pancreatic cancer tumors to glycolytic inhibition.
-
Targeting alpha-ketoglutarate dehydrogenase function by CPI-613 slows mitochondrial metabolism.
-
The antidiabetic drug, metformin, targets pancreatic cancer stem cells.
-
Combined therapy is used to target pancreatic metabolism heterogeneity.
1 Introduction
Currently, approximately 95% of pancreatic cancers are pancreatic ductal adenocarcinomas (PDAC), which are the most aggressive form and the fourth leading cause of cancer death with extremely poor prognosis [1]. Poor prognosis is primarily attributed to the late diagnosis of the disease when patients are no longer candidates for surgical resection [2]. Cancer cells are dependent on the oncogenes that allow them to proliferate limitlessly. Thus, targeting the expression of known oncogenes in pancreatic cancer has been shown to lead to more effective treatment [3]. This chapter discusses the complexity of metabolic features in pancreatic cancers. In order to comprehend the heterogeneous nature of cancer metabolism fully, we need to take into account the close relationship between cancer metabolism and genetics. Gene expression varies tremendously, not only among different types of cancers but also within the same type of cancer among different patients. Cancer metabolism heterogeneity is often prompted and perpetuated not only by mutations in oncogenes and tumor-suppressor genes but also by the innate diversity of the tumor microenvironment. Much effort has been focused on elucidating the genetic alterations that correlate with disease progression and treatment response [4, 5]. However, the precise mechanisms by which tumor metabolism contributes to cancer growth, survival, mobility, and aggressiveness represent a functional readout of tumor progression (Fig. 1).
2 Oncogenic KRAS Regulates Metabolism in Pancreatic Cancer Cells (Fig. 2)
2.1 Oncogenic KRAS Regulates Glutamine Metabolism
Cancer’s specific metabolic adaptations in nutrient uptake and biosynthesis have been linked to a particular genetic mutation. The KRAS (Kirsten rat sarcoma) oncogene homolog is a known regulator of glutamine metabolism among other intermediary metabolic pathways that render cancer cells addicted to glutamine [6,7,8,9]. A range of mutations in the KRAS oncogene occur in over 90% of PDAC [10, 11]. Typically, glutamate feeds into the TCA cycle after being converted to alpha-ketoglutarate in the mitochondrion via glutamate dehydrogenase 1 (GLUD1) or aminotransferases. A study by Son et al. showed that KRAS regulated the reprogramming of glutamine metabolism through transcriptional regulation of key metabolic enzymes of transaminase reactions, which, in turn, determine PDAC tumor growth. Notably, they concluded that PDAC cells greatly depend on these reactions for redox homeostasis. Given that these pathways are nonessential in normal cells, the unique importance of these pathways in PDAC suggests novel approaches for therapy in treating PDAC [7]. KRAS mutation led to the reprogramming of glutamine metabolism, which was partially due to increased cytosolic aspartate aminotransferase or glutamic-oxaloacetic transaminase 1 (GOT1) expression and decreased GLUD1 expression, which led to increased aspartate production via the mitochondrial isozyme GOT2. The change in the ratio of expression of GOT1 and GLUD1 thus shunts glutamine flux through the aspartate aminotransferase pathway. According to Lyssiotis et al., the observation that the glutamine metabolism pathway is downstream of mutant KRAS serves as an explanation for the distinct glutamine dependency of pancreatic cancer. Not only do their results yield novel targets for pancreatic cancer therapy, but they also suggest that inhibiting glutamine metabolism in pancreatic cancer therapies may synergize with therapies that increase reactive oxygen species (ROS) [8].
Oncogenic KRAS affects multiple metabolic pathways that contribute to cancer cell growth. Of note are increased macropinocytosis, increased glutamine metabolism, and increased glucose metabolism. This leads to an increase in macromolecules that allow cancer cell growth. GOT1 glutamic-oxaloacetic transaminase s1, GLUD1 glutamate dehydrogenase 1, GLUT1 glucose transporter 1, MCT4 monocarboxylate transporter 4, LDHA lactate dehydrogenase A
It has been recently found that oncogenic KRAS also activates a nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent antioxidant program to suppress ROS levels within the cells [12]. The upregulated Nrf2 pathway in cancer cells also increases the shunt of both glucose and glutamine towards anabolic metabolism, specifically towards the pentose phosphate pathway (PPP), when triggered by proliferative signaling [13]. Finally, Nrf2 also promotes pancreatic tumorigenesis and proliferation [14], further suggesting the multifaceted role of KRAS in driving pancreatic cancers.
2.2 Oncogenic KRAS Regulates Glucose Metabolism
The KRAS oncogene is also known to contribute to the glucose metabolism in pancreatic cancer cells via the upregulation of glucose uptake and the diversion of glucose into the hexosamine biosynthesis pathways [15]. Oncogenic KRAS also controls the diversion of glycolytic intermediates into ribose biosynthesis pathways via the upregulation of the non-oxidative pentose phosphate pathway (PPP), a pathway that is fundamental to nucleic acid synthesis and thus cancer cell proliferation [15]. Expression of glucose transporter-1 (GLUT1), hexokinase-II (HK2), a kinase that phosphorylates glucose to drive intracellular glucose flux, and lactate dehydrogenase A (LDHA), an enzyme that catalyzes the reaction of pyruvate to lactate, are greatly enhanced by KRAS in pancreatic tumor cells [15]. Consequently, glycolytic flux, the production of lactate from glucose, was high in KRAS-mutant tumors. It is of note that these alterations are not nearly as pronounced in the stromal cells of these tumors, which can uptake the lactate generated by tumor cells and convert the lactate back to pyruvate in order to fuel the TCA cycle [16, 17]. Yun et al. found that cells with mutated KRAS underwent the Warburg effect and survived better in low-glucose environments compared to cells with wild-type KRAS due to the fact that KRAS upregulated GLUT1 [18, 19], suggesting that KRAS mutation is involved in the altering of a cancer cell’s bioenergetics that is seen in most PDAC tumor cells, which take advantage of the altered metabolic pathways to proliferate and grow successfully.
2.3 Oncogenic KRAS Upregulates Macropinocytosis and Lipid Scavenging
Fatty acids are required for cancer cells to replicate their cell membranes and undergo essential lipid-dependent processes for proliferation. Besides shunting glutamine carbon to de novo fatty acid synthesis [20, 21], oncogenic KRAS also upregulates macropinocytosis, the endocytic process of cellular internalization of extracellular fluid, and its contents, further demonstrating the impact of oncogenic KRAS on PDAC. Recently, it was found that Ras-transformed cells utilize macropinocytosis as a way to uptake amino acids, including glutamine. In Ras-transformed pancreatic tumor xenografts, inhibiting macropinocytosis significantly inhibited tumor growth [22]. Ras-transformed cells, along with hypoxic cells, were also shown to support growth by increased scavenging for serum fatty acids [23]. Inhibiting low-density lipoprotein receptor (LDLR), which facilitates cholesterol intake and is associated with increased risk of PDAC recurrence, sensitized PDACs to chemotherapy drugs [24]. The metabolic importance of macropinocytosis in oncogenic KRAS-transformed cancer cells provides yet another metabolic target worth considering for therapy. Contradictorily, multiple studies have demonstrated that lipids are decreased in both cancerous and noncancerous regions of PDACs [25, 26].
3 Other Alternative Metabolisms in Pancreatic Cancer
3.1 MUC1 Overexpression Leads to Increased Glucose Metabolism
A study by Chaika et al. revealed that the overexpression of transmembrane protein mucin 1 (MUC1) led to elevated glucose metabolism and related activities, such as increased glucose uptake and lactate production resulting from increases in GLUT1 expression and LDHA expression, respectively. These metabolic effects are particularly pronounced under hypoxic conditions, which are associated with the stabilization of hypoxia-inducible factor 1-alpha (HIF-1α), a transcription factor for many genes involved in regulating glucose uptake, through the overexpression of MUC1 [27]. Pancreatic cancer cells that do not overexpress MUC1 have a reduction in lactate and glycolytic intermediates. Overall, the overexpression of MUC1 is capable of influencing glucose metabolism, the elevation of amino acid metabolism, and the TCA cycle, all of which are important in the biosynthesis of cellular building blocks, and thus leading to tumorigenesis. The signaling pathway between MUC1 and HIF-1α plays an important role in the facilitation of tumor growth and metastasis, serving as a potential target for manipulation in the treatment of diseases reliant upon these proteins [27].
3.2 p53 Functions Predict the Sensitivity of Pancreatic Cancer Tumors to Glycolytic Inhibition
The heterogeneity of metabolic alterations within the same cancer type is best illustrated in a study of Rajeshkumar et al. They showed that PDAC’s sensitivity to the same metabolic inhibition could vary drastically from one tumor to another, depending on the specific tumor’s genetic status and unique metabolic phenotype [28]. More specifically, they found that responses to LDHA inhibition by the small-molecule FX11 [29, 30] were determined by the status of a tumor’s p53, a tumor-suppressor gene that is largely inactivated in many cancers [28, 31]. Within the same PDAC type, tumors with wild-type TP53 demonstrated resistance to FX11, while those with mutant TP53 exhibited sensitivity in the form of increased apoptosis, reduced proliferation, and attenuated tumor growth. Their data show that FX11 specifically decreased pyruvate-to-lactate conversion by LDHA only in the TP53-mutant tumor, suggesting LDHA inhibition as a possible therapeutic target to reduce TP53-mutant tumor growth. Resistance in TP53-WT tumors is thought to result from reduced dependence on glucose, as corroborated by their data showing higher levels of TIGAR, a p53-inducible protein that lowers glycolytic flux, in these tumors [28, 32]. This study supports growing evidence for variable metabolic phenotypes not only across cancer types but also within cancers of the same type. From a clinical perspective, this insight emphasizes the importance of metabolic phenotypes in pancreatic cancer sub-characterization in order to pair drug therapies according to the phenotypic sensitivity for more selective and personalized treatment.
3.3 Alternative Source of Glutamate in PDAC
3.3.1 Neurotransmitter N-Acetyl-Aspartyl-Glutamate (NAAG) as a Glutamate Reservoir in Cancer
In a recent study, Nguyen et al. found that in addition to the utilization of glutamate through traditional metabolic pathways, cancer cells actively convert glutamate into N-acetyl-aspartyl-glutamate (NAAG), a metabolite commonly known as a neurotransmitter, which can be hydrolyzed back to glutamate when needed by the oncogenic cells via glutamate carboxypeptidase II (GCP II) [33]. They further demonstrated that knocking down GCPII expression or inhibiting GCPII led to a reduction in PDAC growth, suggesting that GCPII is a viable target for cancer therapy, either alone or in combination with glutaminase inhibition.
3.3.2 Glutaminase II Pathway Is Another Source of Glutamate in Cancer
Pancreatic cancer cells can utilize the conversion of glutamine to glutamate via glutaminase 1 (GLS1) to fuel their energetic needs [34]. Although inhibition of GLS1 led to decreased tumor growth and is being explored in clinical trials, there is still room for improvement. A recent study uncovered that pancreatic cancer cells utilized the glutaminase II pathway, an alternative pathway where glutamine is converted to alpha-ketoglutaramate, then eventually glutamate when GLS1 (glutaminase I pathway) is inhibited [35]. Knocking down glutamine transaminase K (GTK) expression, a key enzyme in the glutaminase II pathway, was found to inhibit the growth of pancreatic cancer cells in vitro. When translating to in vivo models, genetic suppression of GTK was found to inhibit tumorigenesis. The uncovering of the role of the glutaminase II pathway as a source of the carbon backbone of glutamate upon single-therapy GLS1 inhibition opens up new strategic approaches. Specifically, the study suggested a combination therapy of GLS1 and GTK inhibition for pancreatic cancer therapy.
4 Pancreatic Tumor Microenvironment
Pancreatic tumors were found to be hypovascular and constantly deprived of nutrients [36], leading to these tumors relying on alternative sources of nutrients to continue proliferating. The tumor microenvironment (TME) [37] was also found to be under such intense physical and oxidative stress that the interstitial pressures in pancreatic tumors induced vascular collapse [38]. This resulted in tumor hypoperfusion, limiting oxygen and nutrients, and drug delivery to cancer cells [39], creating an intratumoral heterogeneity of metabolism [40]. Due to this hindrance in nutrient acquisition, pancreatic cancer cells must support themselves using alternative sources. Part of sustaining tumor viability involved the dense stromal components that are largely populated by fibroblasts and immune cells [41]. Although this dense fibrotic stroma metabolically supported pancreatic cancer cells, studies have demonstrated that it could actually restrain cancer progression, likely due to the stroma’s role in restraining angiogenesis [42, 43].
4.1 PDACs are Dependent on Autophagy
Yang et al. showed that pancreatic cancer cells are dependent on autophagy for tumor progression [44]. They found that suppression of autophagy via genetic depletion or pharmacological inhibition led to tumor regression in vivo and suppressed proliferation of multiple PDAC cell lines, along with an increase in ROS and a decrease in mitochondrial oxidative phosphorylation. However, in a recent study, Bryant and colleagues found that suppression of oncogenic KRAS or mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) actually increased autophagic flux [45]. These results were surprising since it was previously believed that oncogenic KRAS increased autophagy in PDAC [46]. Bryant et al. also found that ERK inhibition sensitizes PDAC to chloroquine (CQ), an autophagy inhibitor, and decreased tumor cell proliferation and tumor growth in vivo. Similar results were obtained in a study by Kinsey et al., who found antitumor activity when combining hydroxychloroquine (HCQ) with MEK inhibitors [47]. These two studies point towards a combined therapy of HCQ with downstream KRAS inhibitors, with a current clinical trial of HCQ and MEK inhibitor combined treatment (NCT03825289) in progress. It is also worth noting that autophagy plays a paradoxical role in PDAC progression, in that genetic ablation of autophagy in the pancreas resulted in increased tumor initiation but impaired the tumor’s ability to develop into invasive cancer [48]. Therefore, more studies into the role of autophagy in PDACs are required to better understand the clinical complexities of PDACs.
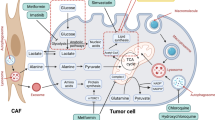
4.2 Stromal Interactions Create Complex PDAC Metabolic Networks
Metabolic networks within tumors can have profound effects on tumor progression and aggression. One example includes differences in regions of hypoxia and normoxia, where pancreatic cancer cells in hypoxic areas export lactate via monocarboxylate transporter 4 (MCT4) and PDAC cells in normoxic regions import this lactate via monocarboxylate 1 (MCT1) [49]. This increases the proliferation of PDAC cells in normoxic areas of the tumor. On the other hand, MCT4 expression has also been shown to promote an immunosuppressive environment and is associated with worse patient outcomes [50]. While PDAC cells can alter the tumor microenvironment to be immunosuppressive, PDAC cells can also stimulate stromal cells to release nutrients. Specifically, Sousa et al. found that stroma-associated pancreatic-stellate cells (PSC) secrete nonessential amino acids via autophagy and are critical for PDAC metabolism [51]. In fact, Sousa and colleagues found that alanine outperforms glucose and glutamine-derived carbon in PDAC to fuel TCA cycle intermediates by being converted to pyruvate. Other nutrients may also be taken up by PDAC cells via exosomes released by cancer-associated fibroblasts [52, 53]. Ultimately, there appears to be an incredibly complex metabolic network within PDAC tumors and the TME.
5 Suggested Therapy (Fig. 3)
Restricting fuel sources for homeostasis and proliferation in PDAC are the new therapeutic avenues for pancreatic cancer treatment [54]. KRAS appears to have a prominent role in the metabolic rewiring of PDAC tumors and PDAC pathogenesis [11]. Subsequently, it requires the cancer cell to become dependent on the oncogenic KRAS to continue proliferation [55]. This is known as oncogene addiction, in which the cancer cell becomes dependent on the activity of the oncogene for survival and proliferation [3]. Since KRAS mutations are found in a majority of PDAC cancer cells, and KRAS regulates cancer cell’s metabolism, targeting these regulations for cancer therapy is an approach that researchers are taking [55].
5.1 Targeting Alpha-Ketoglutarate Dehydrogenase Complex Function by CPI-613 to Slow Mitochondrial Metabolism
Drugs have been developed to target mitochondrial metabolism in cancers [55]. One of these drugs is CPI-613, an inhibitor of cancer-specific mitochondrial energy metabolism. The drug causes tumor cell apoptosis, necrosis, and autophagy by selectively targeting alterations in mitochondrial enzyme activities and redox status [56]. CPI-613 is a small molecule that attacks alpha-ketoglutarate dehydrogenase complex, an enzyme complex involved in the TCA cycle, in tumor cells through a redox process [57]. The drug is known to simultaneously attack multiple essential components of tumor cell regulation [57]. However, the exact mechanism is not well understood. Nevertheless, CPI-613 has been recognized to be effective against various types of cancers [58], including metastatic pancreatic cancer [56]. CPI-613 used in combination with modified FOLFIRINOX (mFOLFIRINOX) in patients with metastatic pancreatic cancer demonstrated better survival. However, since this phase I study was not designed to determine the efficacy of combining CPI-613 with mFOLFIRINOX, the results should be interpreted with caution. Nevertheless, Alistar et al. have obtained encouraging results from the phase I study and are currently performing a randomized phase III trial to compare FOLFIRINOX against mFOLFIRINOX with CPI-613 (NCT03504423). These results suggest that targeting mitochondrial metabolism holds enormous potential in combating pancreatic cancer.
5.2 Antidiabetic Drug, Metformin, Targets Pancreatic Cancer Stem Cells
Recent studies have shown that tumorigenic cancer stem cells (CSCs), a highly chemoresistant subclass of PDAC, are strongly dependent on oxidative metabolism [59, 60]. Retrospective analysis showed that oral administration of metformin in patients with type 2 diabetes was associated with a reduced risk of developing PDAC [61], along with a better outcome for patients that had established PDAC [62]. More recently, it has been discovered that metformin targets pancreatic CSCs but not the differentiated progenies (non-CSCs) [59]. KRAS targeting has resulted in tumor shrinkage but failed to kill all the CSCs [63]. Viale et al. established that dormant tumor cells that survived oncogene ablation have high sensitivity to oxidative phosphorylation inhibitors [63]. Lonardo et al. showed that metformin uniformly reduced ATP levels in CSCs, but not in non-CSCs [60]. Although the mechanism of action for metformin in CSCs is largely unknown, what is known is that metformin slowly accumulates in the mitochondria and directly inhibits complex 1 (NADH dehydrogenase) in the electron transport chain, affecting oxidative phosphorylation [60]. However, phase II clinical trials found no benefit of metformin treatment when it is administered to patients with advanced or metastatic pancreatic cancers [64, 65]. Although disappointing, there is still evidence to support the use of metformin as maintenance therapy in patients with stabilized metastatic PDAC [66], along with a recently finished clinical trial (NCT02048384). In addition, there may be more antitumor potential in metformin analogs with improved mitochondrial targeting ability [67]. Therefore, a potentially strong therapeutic strategy to manage pancreatic cancer is the combined targeting of the KRAS pathway and mitochondrial respiration [63].
5.3 Combined Therapy to Target Pancreatic Metabolism Heterogeneity
Combination therapy to target multiple metabolic pathways in pancreatic cancer has been demonstrated as a favorable therapeutic solution. Elgogary et al. found that targeting glutamine metabolism using the glutaminase inhibitor bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) encapsulated in nanoparticles effectively shrank pancreatic cancer tumor size and slowed proliferation [34]. They also found, using metabolomics technologies [68], that the tumor cells remaining after glutaminase inhibition were dependent on glycolysis and glycogen synthesis. Elgogary et al. continued the study by using both BPTES nanoparticles and metformin to target both glutamine and glucose metabolisms in pancreatic cancer cells. They discovered that the combined therapy provided enhanced efficacy that inhibited tumor growth significantly more compared to the single treatment of BPTES or metformin alone. This highlights the fact that there is great heterogeneity in pancreatic cell metabolism since targeting only glutamine metabolism did not kill all the pancreatic cancer cells, but targeting both glutamine and glucose metabolisms reduced tumor growth with considerably larger efficacy than targeting either glutamine or glucose metabolism alone. More clinical trials must be done in order to see whether combination therapy can assist in pancreatic cancer patient survival. BPTES analogs are being developed and investigated in glutamine-dependent tumors [34, 69,70,71,72,73].
5.4 Targeting PDACs Based on Metabolic Subtype within the PDAC Tumor Microenvironment
Increasing evidence within the past decade shows that PDAC heterogeneity can be characterized by the cell’s molecular biology and tumor microenvironment. Daemen et al. investigated metabolic profiles of PDACs and defined two subtypes: glycolytic and lipogenic [74]. The glycolytic subtype have elevated gene expression associated with glycolysis and PPP, while the lipogenic subtype have increased gene expression associated with lipogenesis. Daemen et al. found strong associations between the glycolytic subtype with a mesenchymal phenotype and the lipogenic subtype with an epithelial phenotype. These results were consistent with the results of previous PDAC classification by Collison et al. [75]. Daemen et al. proposed a model where glycolytic (mesenchymal) PDACs favor utilizing glucose for glycolysis and lactate production and glutamine for the TCA cycle while lipogenic (epithelial) PDACs favor utilizing glucose for the TCA cycle and de novo lipogenesis. Using xenograft models, Daemen et al. found that patient-derived PDACs characterized as glycolytic were sensitive to LDHA knockdown, while those characterized as lipogenic were mildly affected, demonstrating functionally distinct PDAC subtypes with varying metabolic inhibition sensitivity [74]. It is worth noting that the PDAC subtype classification varies depending on what system each group utilized [76,77,78]. However, developing personalized therapies based on patients’ PDAC subtypes appears to be a valid strategy as certain treatments appear to be better equipped in treating specific PDAC subtypes due to phenotypic differences.
5.5 Autophagy Inhibition via Hydroxychloroquine
As previously discussed, PDAC cells rely on autophagy. The Yang group furthered their study by taking the known autophagy inhibitor, hydroxychloroquine (HCQ) , and demonstrating its antitumor effects in a mouse preclinical model using patient-derived xenografts [48]. Although HCQ has not demonstrated much success as a monotherapy [79], surgical outcomes have improved with combination therapy of HCQ with gemcitabine and nab-paclitaxel as a preoperative treatment in PDAC patients (NCT01978184) [80]. There may be more promise with the combination therapy of HCQ and MEK inhibitors (NCT03825289).
6 Conclusion
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the United States and is expected to be the second largest by 2030 [81, 82]. The deadliness of this disease can be attributed to its metabolic heterogeneity, which develops through cancerous evolution. With that in mind, the investigation of PDAC within the past few years has been exponentially increasing with improved technologies and research methods that allow us to understand these intricate mechanisms better. It has also become clear that the heterogeneity in PDAC metabolism raises questions on how to approach new therapies that take into account a personalized approach to a patient’s specific PDAC metabolic characteristics. Exploration of more aspects of pancreatic cells enables scientists and clinicians to better target multiple facets of pancreatic cancer cells, resulting in more effective therapeutic and diagnostic methods.
Abbreviations
- Asp:
-
Aspartate
- EGFR:
-
Epidermal growth factor receptor
- GLS:
-
Glutaminase
- GLUD1:
-
Glutamate dehydrogenase 1
- GLUT:
-
Glucose transporter
- GOT1:
-
Glutamic-oxaloacetic transaminase 1
- HCQ:
-
Hydroxychloroquine
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- HK2:
-
Hexokinase 2
- KRAS:
-
Kirsten rat sarcoma viral oncogene homolog
- LDH:
-
Lactate dehydrogenase
- MAPK:
-
Mitogen-activated protein kinase
- MCT:
-
Monocarboxylate transporter
- MEK:
-
Mitogen-activated protein kinase kinase
- MUC:
-
Mucin
- NAA:
-
N-acetyl-aspartate
- NAAG:
-
N-acetyl-aspartyl-glutamate
- OAA:
-
Oxaloacetate
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PDH:
-
Pyruvate dehydrogenase
- PFK1:
-
Phosphofructokinase 1
- PPP:
-
Pentose phosphate pathway
- ROS:
-
Reactive oxygen species
- TCA:
-
Tricarboxylic acid
References
Hariharan, D., Saied, A., & Kocher, H. M. (2008). Analysis of mortality rates for gallbladder cancer across the world. HPB: The Official Journal of the International Hepato Pancreato Biliary Association, 10(5), 327–331.
Hidalgo, M. (2010). Pancreatic cancer. The New England Journal of Medicine, 362(17), 1605–1617.
Weinstein, I. B., & Joe, A. (2008). Oncogene addiction. Cancer Research, 68(9), 3077–3080; discussion 3080.
Verhaak, R. G., et al. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell, 17(1), 98–110.
Hirschey, M. D., et al. (2015). Dysregulated metabolism contributes to oncogenesis. Seminars in Cancer Biology, 35(Suppl), S129–S150.
Birnbaum, D. J., et al. (2011). Genome profiling of pancreatic adenocarcinoma. Genes, Chromosomes & Cancer, 50(6), 456–465.
Son, J., et al. (2013). Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature, 496(7443), 101–105.
Lyssiotis, C. A., et al. (2013). Pancreatic cancers rely on a novel glutamine metabolism pathway to maintain redox balance. Cell Cycle, 12(13), 1987–1988.
Li, T., Copeland, C., & Le, A. (2021). Glutamine metabolism in cancer. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_2
di Magliano, M. P., & Logsdon, C. D. (2013). Roles for KRAS in pancreatic tumor development and progression. Gastroenterology, 144(6), 1220–1229.
Sousa, C. M., & Kimmelman, A. C. (2014). The complex landscape of pancreatic cancer metabolism. Carcinogenesis, 35(7), 1441–1450.
DeNicola, G. M., et al. (2011). Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature, 475(7354), 106–109.
Mitsuishi, Y., et al. (2012). Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell, 22(1), 66–79.
Chio, I. I. C., et al. (2016). NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell, 166(4), 963–976.
Ying, H., et al. (2012). Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell, 149(3), 656–670.
Chaika, N. V., et al. (2012). Differential expression of metabolic genes in tumor and stromal components of primary and metastatic loci in pancreatic adenocarcinoma. PLoS One, 7(3), e32996.
Maher, J. C., et al. (2005). Differential sensitivity to 2-deoxy-D-glucose between two pancreatic cell lines correlates with GLUT-1 expression. Pancreas, 30(2), e34–e39.
Yun, J., et al. (2009). Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science, 325(5947), 1555–1559.
Bose, S., Zhang, C., & Le, A. (2021). Glucose metabolism in cancer: The Warburg effect and beyond. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_1
Bar-Sagi, D., & Feramisco, J. R. (1986). Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by Ras proteins. Science, 233(4768), 1061–1068.
Park, J. K., et al. (2021). The heterogeneity of lipid metabolism in cancer. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_3
Commisso, C., et al. (2013). Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature, 497(7451), 633–637.
Kamphorst, J. J., et al. (2013). Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proceedings of the National Academy of Sciences of the United States of America, 110(22), 8882–8887.
Guillaumond, F., et al. (2015). Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America, 112(8), 2473–2478.
Ma, X., et al. (2011). The metabolic features of normal pancreas and pancreatic adenocarcinoma: Preliminary result of in vivo proton magnetic resonance spectroscopy at 3.0 T. Journal of Computer Assisted Tomography, 35(5), 539–543.
Yabushita, S., et al. (2013). Metabolomic and transcriptomic profiling of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Carcinogenesis, 34(6), 1251–1259.
Chaika, N. V., et al. (2012). MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America, 109(34), 13787–13792.
Rajeshkumar, N. V., et al. (2015). Therapeutic targeting of the Warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Research, 75(16), 3355–3364.
Dutta, P., et al. (2013). Evaluation of LDH-A and glutaminase inhibition in vivo by hyperpolarized 13C-pyruvate magnetic resonance spectroscopy of tumors. Cancer Research, 73(14), 4190–4195.
Le, A., et al. (2010). Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proceedings of the National Academy of Sciences of the United States of America, 107(5), 2037–2042.
Surget, S., Khoury, M. P., & Bourdon, J. C. (2013). Uncovering the role of p53 splice variants in human malignancy: A clinical perspective. OncoTargets and Therapy, 7, 57–68.
Bensaad, K., et al. (2006). TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell, 126(1), 107–120.
Nguyen, T., et al. (2019). Uncovering the role of N-acetyl-aspartyl-glutamate as a glutamate reservoir in cancer. Cell Reports, 27(2), 491–501. e6.
Elgogary, A., et al. (2016). Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America, 113(36), E5328–E5336.
Udupa, S., et al. (2019). Upregulation of the glutaminase II pathway contributes to glutamate production upon glutaminase 1 inhibition in pancreatic cancer. Proteomics, 19(21–22), e1800451.
Kamphorst, J. J., et al. (2015). Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Research, 75(3), 544–553.
Antonio, M. J., Zhang, C., & Le, A. (2021). Different tumor microenvironments lead to different metabolic phenotypes. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_10
Provenzano, P. P., et al. (2012). Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 21(3), 418–429.
Olive, K. P., et al. (2009). Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science, 324(5933), 1457–1461.
Nabi, K., & Le, A. (2021). The intratumoral heterogeneity of cancer metabolism. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_11
Chu, G. C., et al. (2007). Stromal biology of pancreatic cancer. Journal of Cellular Biochemistry, 101(4), 887–907.
Ozdemir, B. C., et al. (2014). Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell, 25(6), 719–734.
Rhim, A. D., et al. (2014). Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell, 25(6), 735–747.
Yang, S., et al. (2011). Pancreatic cancers require autophagy for tumor growth. Genes & Development, 25(7), 717–729.
Bryant, K. L., et al. (2019). Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nature Medicine, 25(4), 628–640.
Guo, J. Y., et al. (2011). Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes & Development, 25(5), 460–470.
Kinsey, C. G., et al. (2019). Protective autophagy elicited by RAF-->MEK-->ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nature Medicine, 25(4), 620–627.
Yang, A., et al. (2014). Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discovery, 4(8), 905–913.
Guillaumond, F., et al. (2013). Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America, 110(10), 3919–3924.
Hutcheson, J., et al. (2016). Immunologic and metabolic features of pancreatic ductal adenocarcinoma define prognostic subtypes of disease. Clinical Cancer Research, 22(14), 3606–3617.
Sousa, C. M., et al. (2016). Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature, 536(7617), 479–483.
Zhao, H., et al. (2016). Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife, 5, e10250.
Sazeides, C., & Le, A. (2021). Metabolic relationship between cancerassociated fibroblasts and cancer cells. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_14
Le, A., et al. (2012). Conceptual framework for cutting the pancreatic cancer fuel supply. Clinical Cancer Research, 18(16), 4285–4290.
Weinberg, S. E., & Chandel, N. S. (2015). Targeting mitochondria metabolism for cancer therapy. Nature Chemical Biology, 11(1), 9–15.
Alistar, A., et al. (2017). Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: A single-centre, open-label, dose-escalation, phase 1 trial. The Lancet Oncology, 18(6), 770–778.
Stuart, S. D., et al. (2014). A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer & Metabolism, 2(1), 4.
Pardee, T. S., et al. (2014). A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clinical Cancer Research, 20(20), 5255–5264.
Sancho, P., et al. (2015). MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metabolism, 22(4), 590–605.
Lonardo, E., et al. (2013). Metformin targets the metabolic Achilles heel of human pancreatic cancer stem cells. PLoS One, 8(10), e76518.
Evans, J. M., et al. (2005). Metformin and reduced risk of cancer in diabetic patients. BMJ, 330(7503), 1304–1305.
Sadeghi, N., et al. (2012). Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clinical Cancer Research, 18(10), 2905–2912.
Viale, A., et al. (2014). Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature, 514(7524), 628–632.
Kordes, S., et al. (2015). Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. The Lancet Oncology, 16(7), 839–847.
Reni, M., et al. (2016). (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: An open-label, randomized phase II trial. Clinical Cancer Research, 22(5), 1076–1085.
Yang, Y. X., & Rustgi, A. K. (2016). Impact of metformin on advanced pancreatic cancer survival: Too little, too late? Clinical Cancer Research, 22(5), 1031–1033.
Cheng, G., et al. (2016). Mitochondria-targeted analogues of metformin exhibit enhanced antiproliferative and radiosensitizing effects in pancreatic cancer cells. Cancer Research, 76(13), 3904–3915.
Hoang, G., Udupa, S., & Le, A. (2019). Application of metabolomics technologies toward cancer prognosis and therapy. International Review of Cell and Molecular Biology, 347, 191–223.
Gross, M. I., et al. (2014). Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Molecular Cancer Therapeutics, 13(4), 890–901.
Robinson, M. M., et al. (2007). Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). The Biochemical Journal, 406(3), 407–414.
Wang, J. B., et al. (2010). Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell, 18(3), 207–219.
Willis, R. C., & Seegmiller, J. E. (1977). The inhibition by 6-diazo-5-oxo-l-norleucine of glutamine catabolism of the cultured human lymphoblast. Journal of Cellular Physiology, 93(3), 375–382.
Zimmermann, S. C., et al. (2016). Allosteric glutaminase inhibitors based on a 1,4-di(5-amino-1,3,4-thiadiazol-2-yl)butane scaffold. ACS Medicinal Chemistry Letters, 7(5), 520–524.
Daemen, A., et al. (2015). Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 112(32), E4410–E4417.
Collisson, E. A., et al. (2011). Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature Medicine, 17(4), 500–503.
Bailey, P., et al. (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature, 531(7592), 47–52.
Moffitt, R. A., et al. (2015). Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nature Genetics, 47(10), 1168–1178.
Puleo, F., et al. (2018). Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology, 155(6), 1999–2013. e3.
Wolpin, B. M., et al. (2014). Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. The Oncologist, 19(6), 637–638.
Bryant, K. L., & Der, C. J. (2019). Blocking autophagy to starve pancreatic cancer. Nature Reviews. Molecular Cell Biology, 20(5), 265.
Rahib, L., et al. (2014). Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Research, 74(11), 2913–2921.
Rossi, M. L., Rehman, A. A., & Gondi, C. S. (2014). Therapeutic options for the management of pancreatic cancer. World Journal of Gastroenterology, 20(32), 11142–11159.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Camelo, F., Le, A. (2021). The Intricate Metabolism of Pancreatic Cancers. In: Le, A. (eds) The Heterogeneity of Cancer Metabolism. Advances in Experimental Medicine and Biology, vol 1311. Springer, Cham. https://doi.org/10.1007/978-3-030-65768-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-65768-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65767-3
Online ISBN: 978-3-030-65768-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)