Abstract
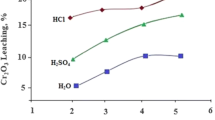
Tailings from inactive gold mines, that are not yet successfully restored (generation of As- and Co-contaminated neutral mine drainage), represent a promising secondary source of strategic metals including Co and Ni. Three different mine tailings (sites A, B and C) from Cobalt Mining Camp were collected and characterized. Preliminary chemical leaching tests were conducted with inorganic acids (HCl, H2SO4 and HNO3) to solubilize Co and Ni at different concentrations (0.01–0.5 N). The influence of the number of the leaching steps on the recovery of Co and Ni was also evaluated. Promising concentrations of Co (0.7%) and Ni (0.3%) were reported in tailings from site A, while lower concentrations were measured in tailings from sites B and C (0.02–0.1%), requiring pre-concentration steps (not tested in this preliminary study) before leaching to reduce operating costs. More than 85% of both Co and Ni were solubilized from tailings from site A after only 30 min using H2SO4 (0.25 N) at room temperature. Lower efficiencies (36–62%) were observed for tailings from sites B and C, which can be partially explained by the higher amounts of acid-consuming minerals present in the gangue. Additional experiments are required to better understand the mechanisms involved in Co and Ni solubilization and to optimize operating conditions in terms of Co and Ni recovery.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Pazik PM, Chielewski T, Glass HL, Kowalczuk PB (2016) World production and possible recovery of from the Kupferschiefer stratiform copper ore. In: E3S web of conferences–mineral engineering conference MEC2016, vol 8, pp 01063, 1–9. https://doi.org/10.1051/e3sconf/20160801063
Commission Européenne (2017) Communication de la commission au parlement européen, au conseil, au comité économique et social européen et au comité des régions relative à la liste 2017 des matières premières critiques pour l’UE, p 8
MERN (2019) Réflexion sur la place du Québec dans la mise en valeur des minéraux critiques et stratégiques, p 22
Falagán C, Grail BM, Johnson DB (2017) New approaches for extracting and recovering metals from mine tailings. Miner Eng 106:71–78. https://doi.org/10.1016/j.mineng.2016.10.008
Landenberger A, Arvanitidis N, Jonsson E, Arvidsson R, Casanovas S, Lauri L (2016). Identification and quantification of secondary CRM resources in Europe. SCRREEN, p 129
Petavratzi E, Gunn G, Kresse C (2019) Cobalt–British geological survey commodity review, p 72
Crundwell F, Moats M, Ramachandran V, Robinson T, Davenport WG (2011) Extractive metallurgy of nickel, cobalt and platinum group metals. Elsevier, p 622
Bellenfant G, Guezennec AG, Bodéman F, d’Hugues P, Cassard D (2013) Re-processing of mining waste: Combining environmental management and metal recovery? In: Proceedings of the eighth international seminar on mine closure. Australian Centre for Geomechanics, Cornwall, pp 571–582. https://doi.org/10.36487/ACG_rep/1352_48_Bellenfant
Shaw RA, Petavratzi E, Bloodworth AJ (2013) Resource recovery from mine waste–Chapter 2. In: Hester RE, Harrison RM (ed.) Waste as a resource, Royal Society of Chemistry, pp 44–65
Ahmadi A, Khezri M, Abdollahzadeh AA, Askari M (2015) Bioleaching of copper, nickel and cobalt from the low grade sulfidic tailing of Golgohar Iron Mine. Iran Hydromet 154:1–8. https://doi.org/10.1016/j.hydromet.2015.03.006
Chen G, Yang H, Li H, Tong L (2016) Recovery of cobalt as cobalt oxalate from cobalt tailings using moderately thermophilic bioleaching technology and selective sequential extraction. Miner 6(3):1–11. https://doi.org/10.3390/min6030067
Mondal S, Kumar BP, Singh DK, Chakravartty JK (2015) Parametric optimization for leaching of cobalt from Sukinda ore of lateritic origin–a Taguchi approach. Sep Purif Technol 156(2):827–834. https://doi.org/10.1016/j.seppur.2015.11.007
Xie Y, Xu Y, Yan L, Yang R (2005) Recovery of nickel, copper and cobalt from low-grade Ni-Cu sulfide tailings. Hydrometallurgy 80:54–58. https://doi.org/10.1016/j.hydromet.2005.07.005
Wang Y, Zhou C (2002) Hydrometallurgical process for recovery of cobalt from zinc plant residue. Hydrometallurgy 63:225–234. https://doi.org/10.1016/S0304-386X(01)00213-4
Blengini GA, Mathieux F, Mancini L, Viegas HM (2019) Recovery of critical and other raw materials from mining waste and landfills. JRC Science for policy report, European Union, Luxembourg, p 130
Cs Gahan, Srichandan H, Kim DJ, Akcil A (2012) Biohydrometallurgy and biomineral processing technology: a review on its past, present and future. Res J Rec Sci 1(10):85–99
Mäkinen J, Salo M, Khoshkhoo M, Sundkvist JE, Kinnunen P (2020) Bioleaching of cobalt from sulfide mining tailings: a mini-pilot study. Hydrometallurgy 196:1054181–6. https://doi.org/10.1016/j.hydromet.2020.105418
Zhen S, Yan Z, Zhang Y, Wang J, Campbell M, Qin W (2009) Column bioleaching of a low grade nickel-bearing ore containing high magnesium as olivine, chlorite and antigorite. Hydrometallurgy 96:337–341. https://doi.org/10.1016/j.hydromet.2008.11.007
Coto O, Galizia F, Hernández I, Marrero J, Donati E (2008) Cobalt and nickel recoveries from laterite tailings by organic and inorganic bio-acids. Hydrometallurgy 94:18–22. https://doi.org/10.1016/j.hydromet.2008.05.017
Ozer M (2019) Cobalt and copper recovery from the ancient flotation tailings by selective sulfation roast-leaching process. J Min Metall Sect B-Metall 55(3):315–324. https://doi.org/10.2298/JMMB190304043O
Shengo ML, Kime MB, Mambwe MP, Nyembo TK (2019) A review of the beneficiation of copper-cobalt-bearing minerals in the Democratic Republic of Congo. J Sustain Mining 18:226–246. https://doi.org/10.1016/j.jsm.2019.08.001
Dumaresq CG (1993) The occurrence of arsenic and heavy metal contamination from natural and anthropogenic sources in the Cobalt area of Ontario. MSc thesis, Carleton University, Ottawa, Canada, p 355
Kwong YTJ, Beauchemin S, Hossain MF, Gould WD (2007) Transformation and mobilization of arsenic in the historic Cobalt mining camp, Ontario, Canada. J Geochem Explor 92:133–150. https://doi.org/10.1016/j.gexplo.2006.08.002
Percival JB, Kwong YJT, Dumaresq CG, Michel FA (2007) Distribution of As, Ni and Co in tailings and surface waters in the Cobalt area, Ontario. Mining and the Environment IV Conference, Sudbury, Ontario, Canada, October 19–27, p 10
CEAEQ (2013) Détermination du carbone et du soufre: méthode de combustion et dosage par spectrophotométrie infrarouge, MA 310-CS 1.0 Rév 3, Ministère du Développement Durable, de l’Environnement, de la Faune et des Parcs du Québec, Québec, Canada, p 8
Lawrence RW, Scheske M (1997) A method to calculate the neutralization potential of mining wastes. Environ Geol 32:100–106
Ure A, Davidson C (2002) Chemical speciation in soils and related materials by selective chemical extraction. Chem Spec Environ 265–300
Investmine (2020) Copper prices and copper price charts. http://www.infomine.com/investment/metal-prices/copper/. Accessed 25 Feb 2020
Drahota P, Filippi M (2009) Secondary arsenic minerals in the environment: a review. Environ Int 35(8):1243–1255. https://doi.org/10.1016/j.envint.2009.07.004
Jambor JL, Dutrizac JE (1995) Solid solutions in the annabergite–erythrite–hörnesite synthetic system. Can Mineral 33:1063–1071
Battaglia F, Morin D, Ollivier P (1994) Dissolution of cobaltiferous pyrite by Thiobacillus ferrooxidans and Thiobacillus thiooxidans: factors influencing bacterial leaching efficiency. J Biotechnol 32(1):11–16. https://doi.org/10.1016/0168-1656(94)90115-5
D’Hugues P, Cezac P, Cabral T, Battaglia F, Truong-Meyer XM, Morin D (1997) Bioleaching of a cobaltiferous pyrite: a continuous laboratory-scale study at high solids concentration. Miner Eng 10:507–527. https://doi.org/10.1016/S0892-6875(97)00029-0
Tanong K, Coudert L, Mercier G, Blais JF (2016) Recovery of metals from a mixture of various spent batteries by a hydrometallurgical process. J Environ Manage 181:95–107. https://doi.org/10.1016/j.jenvman.2016.05.084
Altinkaya P, Liipo J, Kolehmainen E, Haapalainen M, Leikola M, Lundström M (2019) Leaching of trace amounts of metals from flotation tailings in cupric chloride solutions. Mining Metall Explor 36:335–342. https://doi.org/10.1007/s42461-018-0015-9
Yaylali B, Yazici EY, Celep O, Deveci H (2016) Extraction of cobalt from a flotation tailngs in different mineral acids under oxidative conditions. In: Proceedings of the 15th international mineral processing symposium, Istanbul, Turkey, October 19–21, p 13
Innocenzi V, Veglio F (2012) Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations. J Power Sources 211:184–191. https://doi.org/10.1016/j.jpowsour.2012.03.064
Jouini M, Rakotonimaro TV, Neculita CM, Genty T, Benzaazoua M (2019) Stability of metal-rich residues from laboratory multi-step treatment system for ferriferous acid mine drainage. Environ Sci Pollut Res 26:35588–35601. https://doi.org/10.1007/s11356-019-04608-1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
Rights and permissions
Copyright information
© 2021 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Jouini, M., Perrin, M., Coudert, L. (2021). Chemical Leaching of Inactive Gold Mine Tailings as a Secondary Source of Cobalt and Nickel—A Preliminary Case Study. In: Anderson, C., et al. Ni-Co 2021: The 5th International Symposium on Nickel and Cobalt. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-65647-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-65647-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65646-1
Online ISBN: 978-3-030-65647-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)