Abstract
Otto Stern became famous for molecular beam physics, matter-wave research and the discovery of the electron spin, with his work guiding several generations of physicists and chemists. Here we discuss how his legacy has inspired the realization of universal interferometers, which prepare matter waves from atomic, molecular, cluster or eventually nanoparticle beams. Such universal interferometers have proven to be sensitive tools for quantum-assisted force measurements, building on Stern’s pioneering work on electric and magnetic deflectometry. The controlled shift and dephasing of interference fringes by external electric, magnetic or optical fields have been used to determine internal properties of a vast class of particles in a unified experimental framework.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 From Otto Stern to Universal Molecule Interferometry
Our contribution honors the legacy of Otto Stern, who paved the path for 100 years of exciting research into atomic and molecular beams, spin physics and matter-wave interferometry. Many of his ideas and original methods are still implemented in our present-day experiments. On the one hand it is impressive how much progress these fields have made since Stern’s time, but it is also humbling to realize how many of Stern’s experimental challenges remain even in the most advanced experiments today.
1.1 Stern’s Legacy in Molecular Beam Deflection
It is enlightening to look at one of Stern’s early papers, Zur Methode der Molekularstrahlen [1], in which he describes the first applications of atomic and molecular beam deflectometry and formulates criteria for achieving the highest possible metrological sensitivity. His idea was straightforward and is sketched in Fig. 1a: a beam of atoms or molecules is launched into high vacuum, collimated, deflected by external fields and detected downstream with position resolution. Following Stern’s notation, the deflection s of a particle of mass m after traveling a distance l with a velocity v in a uniform force field K is
Knowing the beam velocity, geometry and fields involved, it is then straightforward to extract electronic or magnetic properties of the atoms or molecules, since they are contained within K. Stern formulated three criteria to achieve high sensitivity for deflectometryFootnote 1:
-
1.
Narrow beam width: “make the beam as narrow as possible, because the narrower it is, the smaller the deflection s we can measure”.
-
2.
Large deflection region: “make the path l through the field as long as possible, because \(\mathrm {s \sim l^2}\)”.
-
3.
Strong fields: “make the force K as big as possible, because \(\mathrm {s \sim K}\)”.
Since the first two criteria reduce the flux of detected particles, Stern proposed to “...increase the intensity to the required amount by placing 100 identical furrows next to each other on the pole shoe, all of them pointing to the same detector area, such that their images fall on top of each other.”
Stern envisioned far-reaching applications of his beam deflection apparatus, such as the measurement of nuclear magnetic moments,Footnote 2 induced moments, electric dipole moments, and higher-order moments. Many of our experiments with atoms, molecules and clusters are rooted in these ideas.
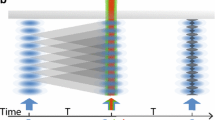
a A molecular beam deflection experiment in the spirit of Otto Stern consists of an intense beam source, narrow collimators, an inhomogeneous deflection field and a position resolving detector. b Deflectometry in a near-field Talbot-Lau interferometer (TLI): the first grating, G\(_1\), prepares transverse coherence from an initially incoherent beam, while the second grating imparts a superposition of momenta to the delocalized matter wave. Interference manifests itself as a particle density pattern with the same periodicity of as the gratings which can be detected by scanning the third grating and counting the transmitted particles. c Near-field interferometry requires either coherent sources or an absorptive first grating G\(_1\). This can be realized by a material mask or photo-induced depletion of the molecular beam in a standing light wave grating [2]. In Sec. 2, different variants of Talbot-Lau interferometers are discussed, including the all-optical OTIMA experiment and the Kapitza-Dirac Talbot-Lau scheme, which constitutes a hybrid of (b) and (c) with G\(_1\) and G\(_3\) as material gratings, but an optical phase grating serving as G\(_2\)
1.2 Stern’s Legacy in Matter-Wave Research
Our experiments are also based on a second series of pioneering studies by Otto Stern: while textbooks correctly ascribe the first demonstration of matter-wave diffraction to the electron experiments by Davisson and Germer [3], it is noteworthy that Estermann and Stern were already working toward matter-wave experiments in 1926. In 1930 they succeeded in demonstrating the first diffraction of atoms (He) and molecules (H\(_2\)) from a crystal surface [4].
Since then, huge progress has been made in atom interferometry based on improved beam sources, nanomechanical gratings, laser physics and optical beam-splitting techniques. Important milestones in this field are the first atom diffraction at optical [5, 6] and nanomechanical gratings [7] in the group of David Pritchard, who also realized the first atom interferometer using free-standing nanomechanical gratings [8]. Christian Bordé realized that single-photon absorption can act as a coherent beam splitter for atoms [9] and reinterpreted earlier spectroscopy experiments on SF\(_6\) [10] as Ramsey-Bordé interferometry. A time-domain atom interferometer based on Raman transitions was built by Mark Kasevich and Steven Chu [11] and became a model for many atom interferometer realizations around the world.
Atom interferometry is now a thriving field of physics with applications ranging from precision tests of fundamental physics to quantum metrology, geodesy and inertial navigation. Some noteworthy applications include the measurement of the Earth’s gravity [12], the gravitational constant G [13], tests of the weak equivalence principle and the universality of free-fall [14], measurements of the fine structure constant [15] and rotation sensing [16, 17]. Interferometry experiments have also been proposed for gravitational wave detection [18] and for dark matter [19] and dark energy [20, 21] searches. For reviews covering these topics see e.g. Refs. [22,23,24]. With the advent of ultra-cold quantum degenerate gases and Bose-Einstein condensates [25, 26], a wide range of mesoscopic matter-wave experiments have also become possible, with too many examples to be listed here; the same holds for molecular quantum gases [27, 28].
Significant progress has also been made in molecule interferometry since Stern’s early experiments with H\(_2\). Diffraction at a nanomechanical mask was key to the discovery of the extremely weakly bound helium dimer He\(_2\) [29] and the basis for Mach-Zehnder interferometry with Na\(_2\) [30]. The combination of four \(\pi /2\)-pulse beam splitters in a Ramsey-Bordé interferometer was demonstrated with I\(_2\) [31]. We refer to contributions by Jan-Peter Toennies, Wieland Schoellkopf and David Pritchard in this book for more on these topics.
Stern’s original idea was to exploit beam deflectometry as a tool to learn about the inner structure and physics of atoms and molecules, and the techniques of atom interferometry have enabled a number of such measurements. The nature of the bonding of He\(_2\) [32] was measured via quantum reflection from a grating, since other techniques would have been too invasive to probe the extremely fragile bond. The static polarizability of sodium [33], lithium [34] and other alkali atoms [35] was measured using atom interferometry, and long-range potential properties [36], van der Waals coefficients [37], atomic tune-out wavelengths [38] and transition matrix elements [39, 40] as well as surface excitations [41] have all been studied using matter-wave diffraction.
2 Interferometer Concepts for Studying the ‘Wave-Nature of Everything’
Building on the work with atoms and dimers and fueled by advances in lasers and nanotechnology, the investigation of the quantum nature of more massive objects became possible by the end of the 20th century.
The fullerene C\(_{60}\) was the first complex molecule for which de Broglie interference was demonstrated in far-field diffraction [42]. Fullerenes are particularly well-suited for beam experiments since they are thermally stable and can be evaporated in a simple furnace. Since a thermal source lacks both transverse and longitudinal coherence, the particles were sent through a pair of collimation slits to generate the required transverse coherence before being diffracted at a nanofabricated grating with a period of \(d=100\) nm. The far-field diffraction pattern is a convolution of the single-slit and multi-slit pattern as familiar from optics textbooks, with the relevant wavelength in this case \(\lambda _\mathrm {dB}=h/mv\). The molecular density pattern was detected by scanning a tightly focused green laser over the molecular beam to cause thermal ionization and create countable ions [43]. The experiment is illustrated in Fig. 2.
Fullerene diffraction at a 100 nm period nanomechanical grating as realized in Vienna [42, 44]. For a central velocity of 136 m/s, the de Broglie wavelength is 4 pm and thus less than 200 times the molecular diameter. Two slits of \(10\,\upmu \)m width separated by 104 cm prepare the transverse coherence required to illuminate several slits of the grating
At first glance it is intriguing that high-contrast interference could be observed despite the fact that the molecules were heated to about 900 K, thus exciting many rotational and vibrational levels. While each individual molecule is distinguishable by virtue of its unique internal state, interference is still observed because each particle interferes with itself, and the evolution of its vibrational and rotational modes occurs simultaneously along each arm of the interferometer. The center-of-mass motion is the only relevant degree of freedom—as long as coupling to the environment can be suppressed.
A more visual way of revealing the molecular wave-particle duality is via fluorescence imaging. Starting from a micron sized laser emission source, molecules were diffracted at a nanomechanical mask and deposited on a quartz slide, where they were detected in real time by fluorescence microscopy with a spatial resolution of about 10 nm [45].
The de Broglie wavelength of a particle with \(m\simeq 1000\,\)Da travelling at 100–300 m/s is of order \(\lambda _\mathrm {dB}\simeq 10^{-12}\) m. Typical gratings – both nanomechanical masks and standing light waves – have periods of \(d\simeq 100\) nm or larger, which yield diffraction angles of 10 \(\mu \)rad. Resolving this small angle requires collimation and an angular resolution of the detector of a few \(\mu \)rad. Increasing the mass by a factor of ten thus requires improving the collimation and detector resolution by the same factor.
Textbook-style far-field diffraction thus becomes quickly impractical for beams of high-mass particles. Near-field optics, however, provides a viable solution. The phenomenon of lens-less grating self-imaging was first observed with light by Henry Fox Talbot in 1836 [46] and later extended by Ernst Lau to incoherent sources [47]. A Talbot-Lau interferometer (TLI) relies on this self-imaging phenomenon and can be formed with two gratings, as illustrated in Fig. 1b. In the symmetric configuration, two identical gratings are spaced apart by a multiple of the Talbot length, \(L_T = d^2/\lambda _\mathrm {dB}\), with d the grating period. In general, near field diffraction causes a complicated pattern, known as a Talbot carpet, to be imprinted into the light or matter-wave beam behind the second grating. At certain distances (in the symmetric case simply the same distance as the \(G_1\)–\(G_2\) separation), the pattern is an exact self-image of the second grating, i.e., fringes with period d. A third grating with the same period can be employed to detect this self image: scanning it transversely to the beam will yield a sinusoidal modulation in the flux as detected by a spatially integrating detector.
The TLI scheme has several advantages for interferometry of massive particles. Compared to far-field schemes like the Mach-Zehnder interferometer, a TLI has relaxed requirements on transverse coherence, permitting the use of relatively uncollimated molecular beams. This is because each opening of the first grating acts as a coherent source for the second grating, with the symmetry of the setup ensuring that the many interferometer trajectories emanating from the first grating recombine in phase at the position of the third grating. In other words, the Talbot-Lau scheme benefits largely from multiplexing, a strategy already envisioned by Stern for deflectometry. The large gain in throughput is an important asset especially when dealing with large (organic) molecules, which are typically very fragile and difficult to volatilize intact, resulting in low beam intensities.
Another reason for working with near-field interferometers is due to their favorable scaling with particle mass, as pointed out by Clauser [48]. For a given length of the setup, the minimum resolvable de Broglie wavelength scales with \(d^2\), in contrast to d in far-field diffraction. Increasing m by a factor of 100 – thereby reducing \(\lambda _\mathrm {dB}\) by the same factor – would require gratings with a 10 times smaller period d in a near-field scheme, whereas they would have to be 100 times smaller to observe the diffraction of the same mass in the far-field. Talbot-Lau interferometry thus allows us to access a high mass range even with moderate interferometer baselines and grating periods.
There are a variety of ways to realize gratings in the lab, with the restriction that \(G_1\) and \(G_3\) must be transmission masks to fulfill their roles of spatially confining the beam and spatially filtering the beam, respectively.
Nanofabricated masks can be used for a large variety of particles because their action does not depend on any specific internal particle property or transition. Fullerenes once again served as the first species to be studied in a three-grating TLIFootnote 3 at the University of Vienna [49]. A TLI setup was also used to observe the wave nature of tetraphenylporphyrins (TPP) and the fluorofullerenes C\(_{60}\)F\(_{48}\) [50] as well as to demonstrate the prospects of molecule lithography [51].
Material gratings, however, also induce strongly velocity-dependent (dispersive) Casimir-Polder phase shifts on matter waves, which limits the maximal fringe contrast particularly for highly polarizible, slow molecules [52]. This particle-wall interaction may also be enhanced by local charges deposited in the fabrication process. Surface effects can be partially mitigated by reducing the grating thickness, but even at the ultimate limit of an atomically thin diffraction grating made from single-layer graphene [53], a sizeable phase shift remains.
Optical beam splitters are particularly appealing for molecule interferometry, since they are free from effects of surface geometry and quality, contamination and charges, and they can be precisely defined both spatially and temporally. Diffraction at a standing light wave was originally proposed by Kapitza and Dirac for electrons in the Bragg regime [54]. It was first realized with atoms using off-resonant optical dipole force phase gratings in the Raman-Nath regime [5].Footnote 4 Kapitza-Dirac diffraction was demonstrated both for electrons [55] and fullerenes [56] in 2001.
While the polarizability of atoms varies by several orders of magnitude around an optical resonance, it does not typically vary by more than 50% across a large part of the optical spectrum for complex, warm molecules or nanoparticles. An optical phase grating of a fixed wavelength may therefore serve as a universal coherent beam splitter for a large variety of particles. This universality, however, comes at a price: without resonant enhancement, the polarizability remains moderate and the process requires high laser intensities. The wavelength should also be chosen to avoid photon absorption and re-emission processes, which tend to reduce interference contrast.
The benefits of both optical gratings and of near-field interferometry led to the proposal of a Talbot-Lau interferometer whose central element \(G_2\) is an optical phase grating (see Fig. 1) [57]. In this scheme, the Kapitza-Dirac-Talbot-Lau Interferometer (KDTLI), the first and last grating remain material masks while the central grating is formed by a thin standing light wave with a period matching that of the outer two gratings. Such an interferometer was built and successfully employed with a variety of complex molecules with masses up to \(10^4\) Da [52, 58, 59].
The use of nanomechanical gratings for G\(_1\) and G\(_3\) will eventually be limited when the Casimir Polder potential becomes sufficiently strong (for slow, highly-polarizable molecules) that the particles are completely deflected to the grating walls and cannot pass. It was therefore proposed to form transmission gratings using a standing light wave. This can work via photo-ionization, where particles passing the anti-nodes of a standing light wave are ionized and removed from the beam, leaving every node as an effective grating slit [2]. This process works best if the photon energy exceeds the ionization energy and the absorption cross section is sufficiently high to allow absorption of more than one photon in every anti-node, conditions which can be met, for example, by tryptophan-rich peptides or low work function metal clusters.
The Optical TIme-domain MAtter-wave interferometer (OTIMA) is a time-domain interferometer that employs pulsed absorptive optical gratings [60, 61]. Since all particles from the pulsed source interact with the same grating pulse at the same time, independent of their position, various dispersive phase shifts are eliminated, most prominently the shift related to Earth’s gravitational acceleration: \(\varDelta \varphi \propto kgT^2\), where \(k=4\pi / \lambda _\mathrm {L}\) is the wave number of the optical grating and T the pulse separation time. However, phase shifts with explicit velocity dependence remain, such as the Coriolis shift induced by the rotation of the Earth, \(\varDelta \varphi \propto k (2\vec {v} \times \vec {\varOmega _\mathrm {E}}) T^2\).
Photo-depletion gratings have been extensively studied in atom interferometry [62, 63]. For molecular quantum optics, we are developing mechanisms that can act on the widest class of particles possible. The fluorine (F\(_2\)) grating lasers in the OTIMA experiment have a wavelength of \(\lambda _\mathrm {L}=157.6\) nm (7.9 eV). This suffices to ionize van der Waals clusters of aromatic anthracene and caffeine [61, 64] as well as tryptophan-rich polypeptides [65]. Combined with ultrafast desorption techniques [65], the first interference of a natural antibiotic polypeptide, Gramicidin A1, was recently demonstrated in this experiment [66] (see Fig. 3a).
Photo-fragmentation is another mechanism which can be used to achieve an optical transmission grating, as demonstrated with van der Waals clusters of hexafluorobenzene [64]. A major goal is to develop single-photon cleavage of tagged biopolymers for future interference experiments with proteins and DNA. The cleavage mechanism itself has recently been successfully demonstrated with functionalized insulin [68].
Since the grating periods of KDTLI and OTIMA are already at the lower limit of commercially available high power lasers, pushing high mass quantum experiments even further requires increasing the flight time between the gratings. This may be achieved by advanced particle cooling schemes or by increasing the interferometer length. The Long-baseline Universal Matter-wave Interferometer (LUMI) is a ten-fold stretched realization of the KDTLI experiment. This instrument accepts de Broglie wavelengths as small as 35 fm and has already demonstrated interference with a molecular library centered at 27,000 Da, with a typical molecule in the library consisting of nearly 2000 atoms and travelling at 260 m/s [67] (see Fig. 3b). These molecules were synthesized at the University of Basel for the purpose of these interference experiments, as described in Sect. 3.2. To date, they are the most massive and complex objects for which quantum interference has been demonstrated.
The LUMI design is modular: the central grating can be interchanged between a nanomechanical grating and an optical phase grating. Generalized versions of the scheme, including optical transmission gratings and surface detection have also been theoretically investigated [69]. The present LUMI experiment is compatible with both atoms and complex molecules, and an upgrade that is currently being implemented will allow it to also work with massive metal clusters. In this sense LUMI is a truly ‘universal’ interferometer as well as a powerful instrument for metrological studies.
3 Quantum-Assisted Deflectometry of Atoms and Complex Molecules
Otto Stern demonstrated that molecular beam methods can boost the precision in the measurement of atomic and molecular properties, external forces or fundamental constants. The Stern-Gerlach experiment has become standard textbook material, and his electric deflection experiments paved the way for a wide range of studies in physics and chemistry in the decades that followed.
Classical beam deflectometry measures the shift of a tightly collimated molecular beam as a result of its interaction with an external field (see Fig. 1 and Eq. (1)) [70,71,72,73,74]. The magnitude of the shift encodes information about the field and the molecular coupling to it. Beam deflectometry can also be used to separate isomers [72, 75, 76] or sort molecules by their quantum state [77]. The sensitivity of classical measurements is determined by the width of the beam and the detector resolution. Beam flux requirements typically constrain the beam width to \(>\!10\,\upmu \)m while position-sensitive time-of-flight mass spectrometers can typically resolve shifts \(>\!50\,\upmu \)m [78].
Here we review a method for improving the spatial resolution and flux in beam deflectometry: Quantum-assisted deflectometry. The technique is particularly fitting in a tribute to Otto Stern since it combines beam deflectometry and matter-wave diffraction, two fields he helped pioneer. Combining these techniques gains orders of magnitude in spatial resolution, allowing us to resolve nanometer, rather than micrometer, deflections.
As described in Sect. 2, the coherent evolution of molecules in a generalized Talbot-Lau interferometer (e.g. TLI, KDTLI, OTIMA, LUMI) manifests as density fringes imprinted into the molecular beam. In a symmetric setup these fringes have the same periodicity as the interferometer gratings, with \(d=79\) nm in OTIMA and \(d=266\) nm in KDTLI and LUMI. The capability to track fringe shifts on the nanometer level yields the high spatial resolution of this technique, enabling the measurement of tiny forces which would be nearly impossible to resolve with classical beam deflection methods. In analogy to Eq. (1), the fringe shift due to a uniform force acting transversely along the entire interferometer is
where \(k = 2\pi /d\), T the time between gratings (for pulsed experiments like OTIMA) and L the inter-grating separation.
3.1 Quantum versus Classical Deflection
In the matter-wave deflectometry experiments described here, a force is applied to the molecules and the resulting phase shift of the interference fringes is detected. Consider a particle beam in a uniform electric field gradient. It will be broadened and/or shifted depending on whether the particles have a permanent electric dipole moment or are only polarizible. In quantum-assisted deflectometry this corresponds to contrast reduction and the deflection of the fringe pattern, where the fringes provide a ruler with high spatial resolution.
The Talbot-Lau deflectometry scheme can be compared to similar experiments using Mach-Zehnder interferometry [33] and classical Moiré deflectometry [79]. The three schemes are limiting cases of the same physical setup: a molecular beam traversing three gratings of period d each separated by a distance L. The relevant length scales are the Talbot length \(L_T=d^2/\lambda _\mathrm {dB}\) and the “aperture Talbot Length”, \(L_a = a^2/\lambda _\mathrm {dB}\), where a is the width of the beam at the first grating.
-
1.
The far-field (Mach-Zehnder) regime is reached when the grating separation satisfies \(L \gg L_a\). In this limit, the diffraction orders emerge from \(G_1\) as distinct partial beams which are diffracted back by \(G_2\) and recombined by \(G_3\). The isolated partial beams can be made to locally interact with potentials, which induce a measurable phase shift in the interference pattern. This setting was realized in the first atom interference [8] and metrology [33] experiments.
-
2.
If the grating separation satisfies \(L_a/N> L > L_T\), with N the number of illuminated grating slits, the apparatus realizes a Talbot-Lau interferometer [80, 81], which is the regime of our molecule interference experiments. Each individual molecule is still spatially delocalized across several, or in some cases up to 100, grating periods [82]. However, the molecular beam is so wide and the diffraction angles so small that all of these partial interferometers overlap. While this makes it impossible to address individual partial beams, the symmetry of the interferometer ensures that all partial waves converge at the position of the third grating, giving rise to high-contrast interference fringes. A potential gradient can be applied within the interferometer to induce an envelope phase shift of the interference fringes.
-
3.
The third limit is that of classical Moiré deflectometry, in which \(L_a,L_T \gg L\). In this setting, the wavelets originating in \(G_1\) do not evolve fast enough to cover two slits in \(G_2\) coherently. Even in this classical regime, sensitive force sensing is still possible [79]. This is the closest realization to the classical beam multiplexing proposed by Stern.
Among the three different regimes, only the far-field Mach-Zehnder features well-separated interferometer arms, and it still holds the record for the most sensitive polarizability measurements [33,34,35]. In this regime one can also explore topological and geometric phases, such as the Aharanov-Bohm [83], Aharanov-Casher [84], Berry [85] and He-McKellar-Wilkens phases [86].
The TLI regime, on the other hand, has the best mass scalability of the three limits and it is therefore currently the only setting compatible with quantum-enhanced measurements of large molecules. In both the far- and near-field limits (1 and 2 above), the sensitivity to external forces depends on the enclosed interferometer area and the detected signal-to-noise ratio. Compared to a classical Moiré deflectometer, the TLI employs smaller grating periods and/or longer machine length and therefore has intrinsically better sensitivity to small fringe displacements.
3.2 Molecules for Interferometry: Choice, Synthesis and Sources
3.2.1 General Strategies
Among all nanoscale particles, molecules are ideally suited for matter-wave experiments due to their monodisperse nature. Being virtually identical, they also exhibit a very narrow isotopomeric mass distribution, typically within a few Daltons. Over the years, we have explored a large variety of structures, from commercially available molecules to tailor-made model compounds with properties optimized for the particular experiment. The collaboration between experimental physicists and synthetic chemists has enabled access to higher mass regimes and the development of new diffraction mechanisms.
Three challenges need to be considered when selecting molecules for interference experiments: the preparation of neutral particle beams, novel diffraction mechanisms and detection schemes with high sensitivity and resolution. For different molecules different techniques may apply and one research goal is to find the most generic combinations that allow treating the largest class of particles.
While thermal evaporation from a Knudsen cell is a simple experimental technique, it requires molecules with sublimation temperatures below their degradation temperature to guarantee the launch of individual and intact molecules of known composition and mass. This calls for a molecular design of thermally stable molecules with minimal intermolecular attraction. An equally challenging criterion is to provide these substances in sufficient quantities (a few grams) to realize constant beams for a sufficient period of time to enable both alignment and interference experiments. While the requirement of ’large scale availability’ constrains the variety of suitable structures, clever design and synthesis enabled us to push the mass limit to beyond 10 kDa [59]. Pulsed laser desorption of functionalized molecules from thin surfaces [87] has been shown to be more economical and applicable to an even larger variety of potential structures.
To minimize intermolecular attraction and to improve the volatility of molecules, their peripheral decoration with highly fluorinated alkyl chains is a successful strategy. The strong electron-withdrawing character of the fluorine atoms localizes the electron densities and decreases the electron mobility. The decoration with perfluorinated alkyl chains thus increases the mass of the target structure, while keeping the polarizability and induced dipole interaction low, a particularly appealing feature when ‘heavy’ particles are of interest. Furthermore, the stability of a C-F bond compares favorably with a C-H bond and the mass spectrum is kept clean, since fluorine is a monoisotopic element. The strategy has been successfully applied to a variety of model compounds ranging from simple dyes like azobenzenes [88], porphyrins [58, 89, 90], and phthalocyanines [45], to interlinked benzene subunits [91], and advanced molecular libraries pushing the limits of diffraction experiments [59, 67].
The feasibility of thermal peptide beams was studied using derivatives of a tryptophan-containing tripeptide. While an unprotected alanine-tryptophan-alanine (Ala-Trp-Ala) showed only fragments in the VUV-post-ionization mass spectrum (\(\lambda = 157\) nm), the intact molecular ion could be observed after removing internal charges and hydrogen bond donors by acetylation and amidation of the termini and methylation of the peptidic amide protons [92, 93]. The introduction of fluoroalkyl chains at the N-terminus or both termini improved the relative intensity of the molecular ion substantially despite the increase in molecular mass. The best results with the least fragmentation were obtained when fluoroalkyl chains were introduced, and the N-methylation was omitted. Considerably more massive peptidic constructs could be launched and VUV-ionized under femtosecond laser desorption even reaching beyond 20 kDa for a 50 amino acid Trp-Lys construct which was extensively decorated with fluoroalkyl chains [65].
The second crucial factor that must be considered in the molecular design is the detection method. The observation of neutral molecules is challenging at low beam densities, and fragmentation-free post-ionization becomes generally more challenging with higher molecular mass [94]. The detectability, however, can be improved substantially by molecular design. The presence of a suitable chromophore enables the observation of individual molecules by fluorescence, which allowed real-time single-molecule imaging in far-field diffraction experiments [45]. Large, electron-rich \(\pi \)-systems are also attractive for photo-induced post-ionization [87]. Oligopeptides with tryptophan units turned out to be particularly suited for photoionization mass spectrometry because of the high absorption cross section of the indole subunit, the only group in any natural amino acid that is susceptible to single-photon ionization at 157 nm [66, 93]. A high tryptophan density even permitted the VUV-ionization of a peptidic construct of more than 20 kDa, thereby exceeding the mass limit for VUV-ionization by one order of magnitude over the previous standard for biomolecules [65]. Detection by mass spectrometry is appealing, as it eases the requirement of monodispersivity and thereby allows a new approach based on molecular libraries. These ensembles of molecules have different numbers of identical subunits, have a broader mass range, but with well-defined and well-separated masses.
3.2.2 Specific Examples
Fullerenes were used in the first diffraction experiments with organic molecules [42] and in many calibration experiments ever since. Their high thermal stability facilitates the creation of intense thermal beams from simple Knudsen cells, and they can be detected by electron impact ionization or by thermal ionization in an intense laser field [43, 95] followed by ion counting. Pure fullerene powder is also readily available in bulk quantities. Vapor pressures of about 0.1 hPa can be reached by heating the powder to 900 K, which generates an intense molecular beam with velocities in the range of 100–200 m/s.
Vitamins and provitamins such as \(\alpha \)-tocopherol (vitamin E), \(\beta \)-carotene (provitamin A), 7-dehydrocholesterol (converted to provitamin D3 upon absorption of UV light) and phylloquinone (vitamin K1) have been interfered and deflected in the KDTLI experiment. Thermal sublimation of such fragile biomolecules always competes with fragmentation. At 500 K, the beam would typically last for only about 30 minutes.
Natural peptides do not evaporate or sublimate intact in a continuous thermal source. However, they can be launched by nanosecond [96] or femtosecond pulsed laser desorption sources, if they are immediately entrained into an adiabatically expanding seed gas. This recently enabled interference of a polypeptide with 15 amino acids, Gramicidin A, in the OTIMA experiment. The fragility of large peptides, limited photoionization cross sections (needed for optical gratings and post-ionization mass spectrometry) of complex peptides, and carrier gas velocity, are the reasons why Gramicidin A is the most complex natural peptide in matter-wave experiments to date [66] (Fig.3a).
Thermal beams of functionalized tripeptides: Peptides are very fragile compounds—even simple dipeptides hardly survive the temperature needed to build up the vapor pressure required for molecular beam experiments. Interestingly, perfluoroalkly functionalization can facilitate the formation of thermal beams of even tripeptides [97] to a degree that matter wave interference became possible [93] (see Fig.6e).
Laser desorbed beams of large tailored polypeptides: The combination of fluoroalkyl decoration and ultrafast (femtosecond) laser desorption into a cold seed gas enabled launching even complex neutral peptides composed of up to 50 amino acids [65]. Their successful intact detection using single-photon ionization at 157 nm required optimizing the peptides for a tryptophan content as high as 50%.
Electrosprays of modified biopolymers: We have recently started investigating a novel approach to generating neutral biomolecular beams using bioconjugation techniques. Peptides with a photocleavable tag, introduced by amidation of surface amino groups with N-hydroxysuccinimide esters (NHS-esters) can be readily volatilized and ionized in an electrospray source. The emerging ions can be guided and manipulated using electric fields and they can even be cooled in a buffer gas. Subsequent neutralization can be achieved by selective photocleavage of the tag in an intense pulsed laser field. This mechanism has been demonstrated for various peptides [98] and even for human insulin [68].
Molecular libraries have been developed and optimized for high-mass interferometry. The concept is displayed in Fig.6f. A readily ionizable porphyrin architecture exposing numerous pentafluorophenyl groups was synthesized as a pure compound. In a subsequent aromatic nucleophilic substitution reaction fluorine atoms were substituted by highly fluorinated alkyl thiol chains. Since each reaction replaces exactly one fluorine atom by one fluorinated alkyl thiol chain, an entire molecular library emerges with precisely known masses, differing by the value of (M(fluorinated alkyl thiol chain)-M(FH)). Electron impact ionization mass spectrometry (EI-QMS) allowed the selective detection of a particular mass range.
Near-field interferometry tolerates a mass distribution even in excess of \(\varDelta m/m\simeq 10\,\%\) and neither isomers nor isotopes impair the experiment. Each molecule constitutes its own de Broglie wave and as long as the electromagnetic properties are similar the interference fringes will appear at the same position. Such a library was first built around a single tetrakispentafluorophenylporphyrin with 20 substitutable fluorine atoms [89] (see Fig. 6f), and successfully used in KDTL interferometry [59]. A dendritic porphyrin architecture with 60 substitutable fluorine atoms gave access to an even larger library that allowed us pushing the mass record in LUMI to beyond 25 kDa [67] (see Fig. 3b).
3.3 Molecule Interference Experiments
Numerous molecular properties have already been probed in quantum-assisted measurements. Here we restrict ourselves to experiments performed at the University of Vienna. They all rely on measurements of interference contrast and fringe deflection in generalized Talbot-Lau interferometers. Experimental results are divided into four categories; electronic, magnetic, and optical properties, as well as the measurement of inertial forces, as summarized in Table 1.
3.3.1 Electronic Properties
Electric deflection experiments require an electrode that provides a uniform force field. The transverse force on a polarizable particle (without permanent dipole moment) is then given by
Here we include the possibility of a thermally induced dipole moment \(d_{ind}=\alpha _0 E\). The factor of 1/2 is due to the work done by the field inducing the dipole moment, and x is the direction transverse to both the molecular beam and to the grating bars.
Equation (3) shows that a field satisfying \((\vec {E}\cdot \nabla ) E_x = \mathrm {const}\) gives a constant transverse force proportional to the particles’ static polarizability \(\alpha _0\). Electrodes have been designed and built with a tailored geometry to provide such a force, as described in Ref. [99] (see Fig. 4a).
Static polarizability: The first polarizability measurements in Talbot-Lau interferometry were made with fullerenes [101]. These measurements were repeated with improved precison and accuracy in the LUMI experiment [102]. Here, we took advantage of the ability to calibrate the setup in-situ with atomic cesium, the polarizability of which has been precisely measured with Mach-Zehnder interferomtery [35, 115] (see Fig. 5). Improving the precision even further, to better much than 1%, is only sensible for cold molecules, with improved control over the internal state.
Structural isomers have identical chemical composition and mass but different geometries and electronic properties (see Fig.6c). We consider two specially synthesized isomers which differ in their susceptibility by more than 20% because the molecule on the left of Fig.6c has a widely delocalized electron system, while electron delocalization is constrained to the phenyl rings in the molecule on the right. This can be easily distinguished in KDTLI deflectometry [91].
Deflectors in our TLI, KDTLI and LUMI experiments. a A uniform \((\vec {E}\cdot \nabla ) E_x\) field to measure polarizabilities and induced dipole moments [99]. b A modified Halbach array with a uniform \((\vec {B}\cdot \nabla ) B_x\) to measure magnetic susceptibilities in LUMI [100]. c Anti-Helmholtz coils with a uniform \(\nabla B_x\) for probing permanent magnetic moments in LUMI (image: S. Pedalino)
Dynamic dipole moment: While fullerenes are rigid, isotropic bodies, well-characterized by the scalar static polarizability \(\alpha _0\), this is not the case for floppy molecules such as the perfluoroalkyl-functionalized diazobenzenes [88] (see Fig. 6a). At a source temperature of 500 K these molecules undergo rapid conformational changes on the picosecond time scale, which leave the static and optical polarizability nearly constant, but may change the instantaneous electric dipole moment \(d_e\) by as much as 300%. This contributes to the net electronic susceptibility according to
where the second term is due to the thermally averaged value of the dipole moment [116]. In the electric deflectometry experiments described here the total susceptibility is measured, and with the aid of ab initio molecular dynamics simulations the relative contributions of the static polarizability and the averaged thermally induced dipole moments can be extracted [88, 103]. It is interesting that de Broglie interferometry, which is primarily concerned with center-of-mass motion, can still reveal the influence of fast conformational changes through their influence on the molecules’ response in an electric field. It is expected that molecular sequence isomers will exhibit different dynamic dipole moments and be separable in experiments with good signal-to-noise ratio [117].
Interference-assisted deflectometry was used to elucidate the dynamic dipole moments of functionalized azobenzenes [88], b the fragmentation of a palladium catalyst precursor [105], c electron delocalization in constitutional isomers [91], and d permanent electric dipole moments in porpyhrin derivatives [104]. e Tripeptides optimized for both molecular beam formation and detection [93]. f Molecular libraries of members with well-defined molecular weight by random substitution of fluorine atoms with highly fluorinated alkyl chains [89]
Permanent dipole moment: Molecules with a permanent electric dipole moment experience a shift of their fringe pattern that depends upon the orientation of the molecule. Since most beam sources emit molecules with random initial orientation and in a mixture of thermally excited rotational states, each molecule experiences a different shift according to its orientation as it tumbles through the electric field.
Molecules with a permanent electric dipole moment thus exhibit a reduced interference contrast, which can be used to distinguish them from polarizable particles with no permanent moment. This has been demonstrated with the porphyrin derivatives Fe-TPP and Fe-TPP-Cl (see Fig. 6d), which differ only by a single chlorine atom and a dipole moment of 2.7 D. Measuring the fringe deflection as a function of electrode voltage showed that both compounds have similar polarizabilities, while measuring the interference contrast revealed a much faster decay in contrast for the polar compound [104].
3.3.2 Magnetic Properties
Magnetic deflection, even more so than electric deflection, represents the huge impact of Otto Stern on the landscape of experimental physics, and has triggered a number of Stern-Gerlach type beam experiments [118,119,120]. However, only recently have similar experiments been performed in molecule interferometry.
The conceptual design of quantum-assisted magnetic deflectometry is identical to that of electric deflection. Here we aim to measure the magnetic susceptibility of particles subject to a uniform force which is introduced via a specially designed Halbach array of permanent magnets [100], as illustrated in Fig. 4b. The magnet can be translated in and out of the molecular beam, allowing us to take differential phase measurements referenced to a no-field situation.
In analogy to electric deflection, we require a region with
such that magnetically susceptible particles with no permanent magnetic moment experience a uniform transverse force. Species with permanent magnetic moments, i.e. paramagnetic particles, will exhibit a reduced interference contrast, which is why this technique is best suited for measuring diamagnetic deflections or second order paramagnetic contributions (temperature-independent paramagnetism [116]).
The first measurements with the magnetic deflector described in Ref. [100] were performed in the LUMI experiment, taking advantage of the long interferometer baseline to observe the small diamagnetic deflection of thermal beams of the alkaline earth atoms barium and strontium [82]. The measured susceptibilities agreed well with the calculated values, and represent the first direct measurement of the ground-state diamagnetism of isolated particles. The sensitivity of the method was further illustrated by demonstrating the complete loss of interference contrast for the odd isotopes of barium and strontium which contain an unpaired nuclear spin, showing that even permanent moments on the order of a nuclear magneton are sufficient to completely dephase the interference fringes.
This work was recently extended to molecules [106], for which the situation is more complex than for atoms due to coupling with rotational states as well as alignment effects in the molecular beam. In this work, we measured the diamagnetic deflection of anthracene, a planar aromatic molecule, and adamantane, a tetrahedrally symmetric molecule. We observed the predicted isotropically averaged susceptibility for adamantane but a surprisingly large value for anthracene, which would be consistent with edge-on alignment of the planar molecules in the supersonic expansion. This alignment leads to the broadside orientation of anthracene being over-represented during its transit through the deflection region. Due to anthracene’s aromaticity, the molecular plane has a significantly larger susceptibility tensor component than the other orientations, leading to the larger-than-isotropic observed deflection.
In the LUMI experiment, we conducted the first beam deflection experiments to measure diamagnetic susceptibilities of atoms and molecules. In the first demonstration [82], the alkaline earth atoms barium and strontium were used in a TLI scheme. Here one can see both the diamagnetic deflection of the even isotopes of strontium (86Sr and 88Sr) and the complete washing out of the interference fringes of 87Sr, which contains a small permanent magnetic moment due to an unpaired nuclear spin. Reference data is shown in blue and deflection data in red
The difference to electric deflection experiments is apparent in the presence of a non-zero magnetic moment, as when there are unpaired nuclear or electron spins. In this case, as in the seminal Stern-Gerlach experiment, the quantization of the magnetic moment plays a role in the behavior of the particles in the magnetic field. When exposed to a \((\vec {B}\cdot \nabla ) B_x\) field, the various projections will be deflected in different directions, leading to a reduction in interference contrast unless a spin state is selected and maintained in the interferometer (see Fig. 7). However, a constant \(\nabla B_x\) field (such that the force on a permanent moment is uniform across the beam) can lead to revivals in interference visibility when the magnetic sub-levels are shifted by integer multiples of the grating period. This has been demonstrated in the LUMI experiment using anti-Helmholtz coils (illustrated in Fig. 4c) to observe the effect in atomic cesium [121], and experiments to observe the effect in triplet-excited fullerenes are in progress.
3.3.3 Optical Properties
There are several optical properties of molecules which can be extracted using quantum-assisted measurements. This can be accomplished by introducing an additional laser to the interferometer in analogy to the previously described deflectometry experiments and performing recoil spectroscopy, as proposed in Ref. [122]. The extraction of absolute absorption cross sections of dilute beams of C\(_{70}\) fullerenes was demonstrated using this technique in Ref. [110] in the KDTLI experiment.
Another approach to probe optical properties is to take advantage of the matter-light interactions which always occur in interferometer schemes with optical gratings. Since the contrast obtained in a KDTLI experiment depends on the AC polarizability of the molecule at the grating wavelength, this can be used as a measurement for optical polarizability, as done in Refs. [107, 108].
The sensitivity of the KDTLI scheme to optical polarizability can also be used to study molecular fragmentation [105]. The optical polarizability of a fragment of the palladium catalyst C\(_{96}\)H\(_{48}\)C\(_{12}\)F\(_{102}\)P\(_2\)Pd was extracted by measuring the interference contrast as a function of the optical grating power. By comparing the measured polarizability to that of the intact particle versus the fragment, it could be determined whether the molecule fragmented already in the source, or only during the detection, after traversing the interferometer. Classical beam deflectometry could not have distinguished the origin of fragmentation, since the deflection depends only on the polarizability-to-mass ratio, which is nearly the same for the parent molecule and its fragments. Here, since the phase imprinted by the second grating depends only on the optical polarizability, it could be determined that the molecule fragmented in the source rather than the detector.
Measuring the optical polarizability is also useful for estimating the static polarizability of molecules, since for fullerenes and many other large organic molecules, we find that the static polarizability approximates the optical polarizability to within a few 10%. This is in agreement with the observation that most optical transitions for molecules in this complexity class are 30–50 nm wide.
There have been several proposals for other ways to utilize the sensitive dependence of molecule diffraction on optical interactions. Several spectroscopy setups have been proposed in the context of the OTIMA experiment, including multi-photon recoil and polarizability spectroscopy [112]. In the far-field diffraction experiment, a near-resonant ultraviolet optical grating could potentially be used for efficient sorting of conformers [111]. It has also been proposed to employ optical helicity fringes to create a diffraction grating that discriminates chiral enantiomers [123].
3.3.4 Inertial Forces
Interferometers have long been used as inertial sensors, from Sagnac loop interferometers with light to sensitive gravity and gravity gradient sensors made with atom interferometers. Molecule interferometers do not compete in sensitivity due to the comparatively poor signal-to-noise ratios and smaller enclosed areas, but they are still sensitive to such effects. In Ref. [114], the competing phase shifts of fullerenes due to the Coriolis effect and gravity were mapped as a function of velocity for different roll angles of the interferometer setup (see Fig. 8). Molecule interferometry also enables weak equivalence principle measurements of a wider variety of species and internal energies and properties than atom interferometry experiments. This has been demonstrated in the OTIMA experiment by comparing the gravitational phase shift of various isotopomeres of tetraphenylporphyrin [113].
Time-resolved interference scans showing the competing effects of gravitational and Coriolis phase shifts as a function of the interferometer roll angle. On the far left gravity is responsible for the large shearing, while on the far right the gratings are nearly aligned with gravity and the shearing is due to the Coriolis force. In Ref. [114] these two phase shifts were used to passively compensate one another, but a similar technique could be used for the purpose of measuring gravitational or Sagnac phases directly
4 Outlook
Molecule interferometry and deflectometry have been inspired by work that was started by Otto Stern 100 years ago. Much of the research in the field since then can be seen as a very extended footnote to the ideas of Otto Stern. And yet we foresee years of exciting research in the attempt to push matter-wave interferometry to ever higher mass and complexity, and to further explore the interface to chemistry, biology and the classical world.
Otto Stern remarked in several of his writings on the particular challenge of preparing molecular beams. This is where quantum optics and chemistry have found a very fruitful overlap and where we still expect thrilling developments: the tailoring of molecules to the needs of quantum optics as well as the use of quantum optics to retrieve information about molecules is a new field of research that opens promising perspectives.
Notes
- 1.
Since Otto Stern’s early papers were written in German, we use our own translation where a verbatim citation is indicated.
- 2.
Stern’s Nobel Prize in 1943 was awarded for his measurement of the proton’s magnetic moment.
- 3.
From here on, TLI will refer to Talbot-Lau interferometers implemented with three nanomechanical gratings.
- 4.
Modern matter-wave literature often associates the names of Kapitza and Dirac with diffraction at a thin dipole force phase grating, even though their original idea applied to non-polarizable electron diffracted at the ponderomotive potential created by interaction with the light field.
References
O. Stern, Zeitschrift für Physik 39, 751–763 (1926)
E. Reiger, L. Hackermüller, M. Berninger, M. Arndt, Opt. Commun. 264(2), 326 (2006)
C. Davisson, L.H. Germer, Phys. Rev. 30, 705 (1927)
I. Estermann, O. Stern, Z. Phys. 61, 95 (1930)
P.E. Moskowitz, P.L. Gould, S.R. Atlas, D.E. Pritchard, Phys. Rev. Lett. 51, 370 (1983)
P.L. Gould, G.A. Ruff, D.E. Pritchard, Phys. Rev. Lett. 56, 827 (1986)
D.W. Keith, M.L. Schattenburg, H.I. Smith, D.E. Pritchard, Phys. Rev. Lett. 61, 1580 (1988)
D.W. Keith, C.R. Ekstrom, Q.A. Turchette, D.E. Pritchard, Phys. Rev. Lett. 66(21), 2693 (1991)
C.J. Bordé, Phys. Lett. A 140, 10 (1989)
C.J. Bordé, S. Avrillier, A. Van Lerberghe, C. Salomon, D. Bassi, G. Scoles, J. Phys. Coll. 42(C8), 15 (1981)
M. Kasevich, D.S. Weiss, E. Riis, K. Moler, S. Kasapi, S. Chu, Phys. Rev. Lett. 66, 2297 (1991)
A. Peters, K. Yeow-Chung, S. Chu, Nature 400, 849 (1999)
G. Lamporesi, A. Bertoldi, L. Cacciapuoti, M. Prevedelli, G. Tino, Phys. Rev. Lett. 100, 5 (2008)
P. Asenbaum, C. Overstreet, M. Kim, J. Curti, M.A. Kasevich, arXiv:2005.11624v1 (2020)
R.H. Parker, C. Yu, W. Zhong, B. Estey, H. Müller, Science 360(6385), 191 (2018)
I. Dutta, D. Savoie, B. Fang, B. Venon, C.L. Garrido Alzar, R. Geiger, A. Landragin, Phys. Rev. Lett. 116(18), 183003 (2016)
D. Savoie, M. Altorio, B. Fang, L.A. Sidorenkov, R. Geiger, A. Landragin, Sci. Adv. 4, 7948 (2018)
W. Chaibi, R. Geiger, B. Canuel, A. Bertoldi, A. Landragin, P. Bouyer, Phys. Rev. D 93(2), 021101 (2016)
Y.A. El-Neaj, C. Alpigiani, S. Amairi-Pyka, H. Araújo, A. Balaž, A. Bassi, L. Bathe-Peters, B. Battelier, A. Belić, E. Bentine, J. Bernabeu, A. Bertoldi, R. Bingham, D. Blas, V. Bolpasi, K. Bongs, S. Bose, P. Bouyer, T. Bowcock, W. Bowden, O. Buchmueller, C. Burrage, X. Calmet, B. Canuel, L.I. Caramete, A. Carroll, G. Cella, V. Charmandaris, S. Chattopadhyay, X. Chen, M.L. Chiofalo, J. Coleman, J. Cotter, Y. Cui, A. Derevianko, A. De Roeck, G.S. Djordjevic, P. Dornan, M. Doser, I. Drougkakis, J. Dunningham, I. Dutan, S. Easo, G. Elertas, J. Ellis, M. El Sawy, F. Fassi, D. Felea, C.H. Feng, R. Flack, C. Foot, I. Fuentes, N. Gaaloul, A. Gauguet, R. Geiger, V. Gibson, G. Giudice, J. Goldwin, O. Grachov, P.W. Graham, D. Grasso, M. van der Grinten, M. Gündogan, M.G. Haehnelt, T. Harte, A. Hees, R. Hobson, J. Hogan, B. Holst, M. Holynski, M. Kasevich, B.J. Kavanagh, W. von Klitzing, T. Kovachy, B. Krikler, M. Krutzik, M. Lewicki, Y.H. Lien, M. Liu, G.G. Luciano, A. Magnon, M.A. Mahmoud, S. Malik, C. McCabe, J. Mitchell, J. Pahl, D. Pal, S. Pandey, D. Papazoglou, M. Paternostro, B. Penning, A. Peters, M. Prevedelli, V. Puthiya-Veettil, J. Quenby, E. Rasel, S. Ravenhall, J. Ringwood, A. Roura, D. Sabulsky et al., EPJ Quant. Technol. 7, 1 (2020)
C. Burrage, E.J. Copeland, E.A. Hinds, J. Cosmol. Astroparticle Phys. 2015(03), 042 (2015)
P. Hamilton, M. Jaffe, P. Haslinger, Q. Simmons, H. Müller, J.T. Khoury, Science 349, 849 (2015)
P.R. Berman, B. Dubetsky, Phys. Rev. A 59, 2269 (1999)
A.D. Cronin, J. Schmiedmayer, D.E. Pritchard, Rev. Mod. Phys. 81(3), 1051 (2009)
G. Tino, M. Kasevich, Atom interferometry, in Proceedings of the International School of Physics “Enrico Fermi”, vol. 188 (IOS, Varenna, 2014)
M.H. Anderson, J.R. Ensher, M.R. Matthews, C.E. Wieman, E.A. Cornell, Science 269, 198 (1995)
K.B. Davis, M.O. Mewes, M.R. Andrews, N.J. van Druten, D.S. Durfee, D.M. Kurn, W. Ketterle, Phys. Rev. Lett. 75, 3969 (1995)
J. Herbig, T. Kraemer, M. Mark, T. Weber, C. Chin, H.C. Nagerl, R. Grimm, Science 301, 1510 (2003)
C. Kohstall, S. Riedl, E.R. Sánchez Guajardo, L.A. Sidorenkov, J. Hecker Denschlag, R. Grimm, New. J. Phys. 13(6), 065027 (2011)
W. Schöllkopf, J.P. Toennies, Science 266, 1345 (1994)
M.S. Chapman, T.D. Hammond, A. Lenef, J. Schmiedmayer, R.A. Rubenstein, E. Smith, D.E. Pritchard, Phys. Rev. Lett. 75, 3783 (1995)
C. Bordé, N. Courtier, F.D. Burck, A. Goncharov, M. Gorlicki, Phys. Lett. A 188, 187 (1994)
B.S. Zhao, G. Meijer, W. Schoellkopf, Science 331(6019), 892 (2011)
C. Ekstrom, J. Schmiedmayer, M. Chapman, T. Hammond, D. Pritchard, Phys. Rev. A 51(5), 3883 (1995)
A. Miffre, M. Jacquey, M. Büchner, G. Trenec, J. Vigue, Phys. Rev. A 73, 011603(R) (2006)
M.D. Gregoire, I. Hromada, W.F. Holmgren, R. Trubko, A.D. Cronin, Phys. Rev. A 92, 5 (2015)
J. Schmiedmayer, M. Chapman, C. Ekstrom, T. Hammond, S. Wehinger, D. Pritchard, Phys. Rev. Lett. 74(7), 1043 (1995)
V.P.A. Lonij, Atom optics, core electrons, and the van der Waals potential. Thesis (2011)
R. Trubko, J. Greenberg, M.T.S. Germaine, M.D. Gregoire, W.F. Holmgren, I. Hromada, A.D. Cronin, Phys. Rev. Lett. 114, 14 (2015)
A. Fallon, C. Sackett, Atoms 4, 2 (2016)
C. Lisdat, M. Frank, H. Knöckel, M.L. Almazor, E. Tiemann, Eur. Phys. J. D 12, 235 (2000)
P. Rousseau, H. Khemliche, A.G. Borisov, P. Roncin, Phys. Rev. Lett. 98, 1 (2007)
M. Arndt, O. Nairz, J. Voss-Andreae, C. Keller, G. van der Zouw, A. Zeilinger, Nature 401, 680 (1999)
D. Ding, J. Huang, R. Compton, C. Klots, R. Haufler, Phys. Rev. Lett. 73(8), 1084 (1994)
O. Nairz, M. Arndt, A. Zeilinger, Am. J. Phys. 71(4), 319 (2003)
T. Juffmann, A. Milic, M. Müllneritsch, P. Asenbaum, A. Tsukernik, J. Tüxen, M. Mayor, O. Cheshnovsky, M. Arndt, Nature Nanotech. 7, 297 (2012)
W.H.F. Talbot, Philos. Mag. 9, 401 (1836)
E. Lau, Ann. Phys. 6, 417 (1948)
J. Clauser, De Broglie-Wave Interference of Small Rocks and Live Viruses (Kluwer Academic, 1997), pp. 1–11
B. Brezger, L. Hackermüller, S. Uttenthaler, J. Petschinka, M. Arndt, A. Zeilinger, Phys. Rev. Lett. 88, 100404 (2002)
L. Hackermüller, S. Uttenthaler, K. Hornberger, E. Reiger, B. Brezger, A. Zeilinger, M. Arndt, Phys. Rev. Lett. 91(9), 090408 (2003)
T. Juffmann, S. Truppe, P. Geyer, A.G. Major, S. Deachapunya, H. Ulbricht, M. Arndt, Phys. Rev. Lett. 103, 26 (2009)
S. Gerlich, L. Hackermüller, K. Hornberger, A. Stibor, H. Ulbricht, M. Gring, F. Goldfarb, T. Savas, M. Müri, M. Mayor, M. Arndt, Nat. Phys. 3(10), 711 (2007)
C. Brand, M. Sclafani, C. Knobloch, Y. Lilach, T. Juffmann, J. Kotakoski, C. Mangler, A. Winter, A. Turchanin, J. Meyer, O. Cheshnovsky, M. Arndt, Nat. Nanotechnol. 10, 845 (2015)
P.L. Kapitza, P.A.M. Dirac, Proc. Camb. Philos. Soc. 29, 297 (1933)
D.L. Freimund, K. Aflatooni, H. Batelaan, Nature 413, 142 (2001)
O. Nairz, B. Brezger, M. Arndt, A. Zeilinger, Phys. Rev. Lett. 87, 160401 (2001)
B. Brezger, M. Arndt, A. Zeilinger, J. Opt. B. 5, 82 (2003)
S. Gerlich, S. Eibenberger, M. Tomandl, S. Nimmrichter, K. Hornberger, P. Fagan, J. Tüxen, M. Mayor, M. Arndt, Nat. Commun. 2, 263 (2011)
S. Eibenberger, S. Gerlich, M. Arndt, M. Mayor, J. Tüxen, Phys. Chem. Chem. Phys. 15, 14696 (2013)
S. Nimmrichter, K. Hornberger, P. Haslinger, M. Arndt, Phys. Rev. A 83, 043621 (2011)
P. Haslinger, N. Dörre, P. Geyer, J. Rodewald, S. Nimmrichter, M. Arndt, Nat. Phys. 9, 144–148 (2013)
R. Abfalterer, S. Bernet, C. Keller, M. Oberthaler, J. Schmiedmayer, A. Zeilinger, Act. Phys. Slov. 47(3/4), 165 (1997)
S. Fray, C.A. Diez, T.W. Hänsch, M. Weitz, Phys. Rev. Lett. 93, 24 (2004)
N. Dörre, J. Rodewald, P. Geyer, B. von Issendorff, P. Haslinger, M. Arndt, Phys. Rev. Lett. 113, 233001 (2014)
J. Schätti, P. Rieser, U. Sezer, G. Richter, P. Geyer, G.G. Rondina, D. Häussinger, M. Mayor, A. Shayeghi, V. Köhler, M. Arndt, Commun. Chem. 1(1), 93 (2018)
A. Shayeghi, P. Rieser, G. Richter, U. Sezer, J. Rodewald, P. Geyer, T. Martinez, M. Arndt, Nat. Commun. 11, 144 (2020)
Y.Y. Fein, P. Geyer, P. Zwick, F. Kiałka, S. Pedalino, M. Mayor, S. Gerlich, M. Arndt, Nat. Phys. (2019)
J. Schätti, M. Kriegleder, M. Debiossac, M. Kerschbaum, P. Geyer, M. Mayor, M. Arndt, V. Köhler, Chem. Commun. (Camb) 55(83), 12507 (2019)
F. Kiałka, B. Stickler, K. Hornberger, Y.Y. Fein, P. Geyer, L. Mairhofer, S. Gerlich, M. Arndt, Physica Scripta (2018)
R. Schäfer, S. Schlecht, J. Woenckhaus, J.A. Becker, Phys. Rev. Lett. 76(3), 471 (1996)
K. Bonin, V. Kresin, Electric-Dipole Polarizabilities of Atoms, Molecules and Clusters (World Scientific, 1997)
R. Antoine, I. Compagnon, D. Rayane, M. Broyer, P. Dugourd, N. Sommerer, M. Rossignol, D. Pippen, F.C. Hagemeister, M.F. Jarrold, Anal. Chem. 75, 5512 (2003)
W.A. de Heer, V.V. Kresin, Electric and Magnetic Dipole Moments of Free Nanoclusters (CRC Press, 2011), pp. 10/1–13
T.M. Fuchs, R. Schäfer, Phys. Rev. A 98, 6 (2018)
F. Filsinger, J. Kupper, G. Meijer, J.L. Hansen, J. Maurer, J.H. Nielsen, L. Holmegaard, H. Stapelfeldt, Angew Chem. Int. Ed. Engl. 48(37), 6900 (2009)
Y.P. Chang, K. Dlugolecki, J. Küpper, D. Rösch, D. Wild, S. Willitsch, Science 342(6154), 98 (2013)
E. Gershnabel, M. Shapiro, I. Averbukh, J. Chem. Phys. 135(19), 194310 (2011)
M. Abd El Rahim, R. Antoine, L. Arnaud, M. Barbaire, M. Broyer, C. Clavier, I. Compagnon, P. Dugourd, J. Maurelli, D. Rayane, Rev. Sci. Instrum. 75(12), 5221 (2004)
M.K. Oberthaler, S. Bernet, E.M. Rasel, J. Schmiedmayer, A. Zeilinger, Phys. Rev. A 54, 3165 (1996)
J.F. Clauser, S. Li, Phys. Rev. A 49, R2213 (1994)
K. Hornberger, S. Gerlich, P. Haslinger, S. Nimmrichter, M. Arndt, Rev. Mod. Phys. 84, 157 (2012)
Y.Y. Fein, A. Shayeghi, L. Mairhofer, F. Kiałka, P. Rieser, P. Geyer, S. Gerlich, M. Arndt, Phys. Rev. X 10, 011014 (2020)
M.A. Bouchiat, C. Bouchiat, Phys. Rev. A 83, 5 (2011)
K. Zeiske, G. Zinner, F. Riehle, J. Helmcke, Appl. Phys. B 60, 205 (1995)
E. Cohen, H. Larocque, F. Bouchard, F. Nejadsattari, Y. Gefen, E. Karimi, Nat. Rev. Phys. 1(7), 437 (2019)
S. Lepoutre, A. Gauguet, M. Büchner, J. Vigué, Phys. Rev. A 88, 4 (2013)
U. Sezer, L. Wörner, J. Horak, L. Felix, J. Tüxen, C. Götz, A. Vaziri, M. Mayor, M. Arndt, Anal. Chem. 87, 5614–5619 (2015)
M. Gring, S. Gerlich, S. Eibenberger, S. Nimmrichter, T. Berrada, M. Arndt, H. Ulbricht, K. Hornberger, M. Müri, M. Mayor, M. Böckmann, N.L. Doltsinis, Phys. Rev. A 81, 031604(R) (2010)
J. Tüxen, S. Eibenberger, S. Gerlich, M. Arndt, M. Mayor, Eur. J. Organ. Chem. (25), 4823 (2011)
P. Schmid, F. Stöhr, M. Arndt, J. Tüxen, M. Mayor, J. Am. Soc. Mass Spectrom. 24(4), 602 (2013)
J. Tüxen, S. Gerlich, S. Eibenberger, M. Arndt, M. Mayor, Chem. Commun. 46(23), 4145 (2010)
B.C. Das, S.D. Gero, E. Lederer, Biochem. Biophys. Res. Commun. 29(2), 211 (1967)
J. Schätti, V. Köhler, M. Mayor, Y.Y. Fein, P. Geyer, L. Mairhofer, S. Gerlich, M. Arndt, J. Mass Spectrom. 55(6), e4514 (2020). E4514 JMS-19-0196.R2
A. Akhmetov, J.F. Moore, G.L. Gasper, P.J. Koin, L. Hanley, J. Mass Spectrom. 45(2), 137 (2010)
O. Nairz, M. Arndt, A. Zeilinger, J. Modern Opt. 47(14–15), 2811 (2000)
M. Marksteiner, P. Haslinger, M. Sclafani, H. Ulbricht, M. Arndt, J. Phys. Chem. A 113(37), 9952 (2009)
J. Schätti, U. Sezer, S. Pedalino, J.P. Cotter, M. Arndt, M. Mayor, V. Köhler, J. Mass Spectrom. 52, 550 (2017)
M. Debiossac, J. Schätti, M. Kriegleder, P. Geyer, A. Shayeghi, M. Mayor, M. Arndt, V. Köhler, Phys. Chem. Chem. Phys. 20, 11412 (2018)
A. Stefanov, M. Berninger, M. Arndt, Meas. Sci. Technol. 19, 5 (2008)
L. Mairhofer, S. Eibenberger, A. Shayeghi, M. Arndt, Entropy 20, 516 (2018)
M. Berninger, A. Stefanov, S. Deachapunya, M. Arndt, Phys. Rev. A 76, 013607 (2007)
Y.Y. Fein, P. Geyer, F. Kiałka, S. Gerlich, M. Arndt, Phys. Rev. Res. 1, 033158 (2019)
L. Mairhofer, S. Eibenberger, J.P. Cotter, M. Romirer, A. Shayeghi, M. Arndt, Angew. Chem. Int. Ed. 56, 10947 (2017)
S. Eibenberger, S. Gerlich, M. Arndt, J. Tüxen, M. Mayor, New J. Phys. 13(4), 043033 (2011)
S. Gerlich, M. Gring, H. Ulbricht, K. Hornberger, J. Tüxen, M. Mayor, M. Arndt, Angew Chem. Int. Ed. Engl. 47(33), 6195 (2008)
Y.Y. Fein, A. Shayeghi, F. Kiałka, P. Geyer, S. Gerlich, M. Arndt, Phys. Chem. Chem. Phys. pp. 14,036–14,041 (2020)
L. Hackermüller, K. Hornberger, S. Gerlich, M. Gring, H. Ulbricht, M. Arndt, Appl. Phys. B 89(4), 469 (2007)
K. Hornberger, S. Gerlich, H. Ulbricht, L. Hackermüller, S. Nimmrichter, I. Goldt, O. Boltalina, M. Arndt, New J. Phys. 11, 043032 (2009)
J.P. Cotter, S. Eibenberger, L. Mairhofer, X. Cheng, P. Asenbaum, M. Arndt, K. Walter, S. Nimmrichter, K. Hornberger, Nat. Commun. 6, 7336 (2015)
S. Eibenberger, X. Cheng, J.P. Cotter, M. Arndt, Phys. Rev. Lett. 112, 250402 (2014)
C. Brand, B.A. Stickler, C. Knobloch, A. Shayegh, K. Hornberger, M. Arndt, Phys. Rev. Lett. 121, 173002 (2018)
J. Rodewald, P. Haslinger, N. Dörre, B.A. Stickler, A. Shayeghi, K. Hornberger, M. Arndt, Appl. Phys. B 123(1), 3 (2017)
J. Rodewald, N. Dörre, A. Grimaldi, P. Geyer, L. Felix, M. Mayor, A. Shayeghi, M. Arndt, New J. Phys. 20, 033016 (2018)
Y.Y. Fein, F. Kiałka, P. Geyer, S. Gerlich, M. Arndt, New J. Phys. 22, 033013 (2020)
M. Gregoire, N. Brooks, R. Trubko, A. Cronin, Atoms 4(3), 21 (2016)
J.V. Vleck, The Theory of Electric and Magnetic Susceptibilities (Oxford University Press London, 1965)
H. Ulbricht, M. Berninger, S. Deachapunya, A. Stefanov, M. Arndt, Nanotechnology 19, 045502 (2008)
W.D. Knight, R. Monot, E.R. Dietz, A.R. George, Phys. Rev. Lett. 40, 1324 (1978)
U. Rohrmann, R. Schafer, Phys. Rev. Lett. 111(13), 133401 (2013)
O. Amit, Y. Margalit, O. Dobkowski, Z. Zhou, Y. Japha, M. Zimmermann, M.A. Efremov, F.A. Narducci, E.M. Rasel, W.P. Schleich, R. Folman, Phys. Rev. Lett. 123, 083601 (2019)
Y.Y. Fein, Long-baseline universal matter-wave interferometry. Thesis (2020)
S. Nimmrichter, K. Hornberger, Phys. Rev. A 78, 023612 (2008)
R.P. Cameron, S.M. Barnett, A.M. Yao, New J. Phys. 16 (2014)
Acknowledgements
We acknowledge funding by the European Research Council (Project No. 320694), the Austrian Science Funds (FWF P-30176, P-32543-N), the Swiss National Funds (Project No. 200020159730), the SNI PhD School (P1403) as well as the tireless contribution of many master and PhD students as well as postdocs who contributed over the years to various molecule interferometers and interferometer applications for molecular science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Gerlich, S., Fein, Y.Y., Shayeghi, A., Köhler, V., Mayor, M., Arndt, M. (2021). Otto Stern’s Legacy in Quantum Optics: Matter Waves and Deflectometry. In: Friedrich, B., Schmidt-Böcking, H. (eds) Molecular Beams in Physics and Chemistry. Springer, Cham. https://doi.org/10.1007/978-3-030-63963-1_24
Download citation
DOI: https://doi.org/10.1007/978-3-030-63963-1_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63962-4
Online ISBN: 978-3-030-63963-1
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)