Abstract
Challenge on energy resources exists, especially when the fossil resources are limited. Fuel cells, as an alternative replacement, can be used. Fuel cells with coated bipolar plates are the interest of this paper. Current research is concerned with the effects of CrN/TiN coatings on interfacial contact resistance (ICR). Stainless steel 410 was selected as a base metal, and the coating process was performed using chromium nitride and titanium nitride by cathodic arc evaporation method. It was found that the surface roughness and ICR values of CrN-coated sample are lower than the TiN-coated sample. The concluded that the CrN layer could be replaced with the TiN layer for better performance of bipolar plates.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Bipolar plate
- Titanium nitride coating
- Chromium nitride coating
- Interfacial contact resistance
- Surface roughness
1 Introduction
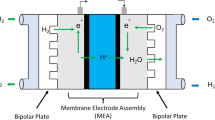
One of the systems that produce clean energy is the fuel cell. A fuel cell can converts the chemical energy of a fuel directly into electrical energy. In contrast to batteries, fuel cells can generate electricity without the need for recharging until the fuel is fed to the cell. The general reactants of fuel cells are hydrogen and oxygen. Fuel cells as a new generation of clean energy producers have more beneficiaries; including higher performance than other traditional energy sources, simpler design, utilization of hydrogen as a reactor (without environmental pollution), its widespread applications on a small and large scale can be concluded that the designing and optimization of these systems are significant [1]. In this regard, bipolar plates are one of the most significant segments of a fuel cell, which are responsible for separating cells, connecting the cathode to the anode from one cell to another with appropriate electrical conductivity, and feeding reactive gases through gas channels, etc. Accordingly, bipolar plates must have unique electrical, material, and mechanical properties (e.g., high electrical conductivity, high mechanical strength, high corrosion resistance, low surface contact resistance), non-permeability of gas, and low cost [2]. Hence, stainless steel is one of the appropriate choices for making bipolar plates [3].
A considerable improvement on Interfacial Contact Resistance (ICR) of 316L steel bipolar plates with TiN coating has been reported [4]. The published results indicated that the TiN-coated samples had better corrosion resistance compared to non-coated samples. Moreover, TiN and ZrN coatings have been deposited on 316L stainless steel by magnetron sputtering to improve material and electrical properties [5]. The main aim of this research is to study the effects of CrN and TiN coatings on the efficiency of stainless-steel 410 bipolar plates which is our novelty in this study. To achieve this purpose, the ICR and surface roughness of coated samples was compared to that of non-coated samples.
2 Material and Specimens
In this research, stainless steel 410 with a thickness of 3 mm was selected as the base material for the bipolar plates. The chemical composition of 410 SS, which was used in this survey was compared with the standard case in Table 17.1. Accordingly, the mechanical properties can be considered as the AISI 410 SS.
To fabricate samples, steel plates were quenched at 1050 °C in an austenitic salt furnace. Furthermore, the tempering operation was performed at 120 °C for 10 min to stress-relief the sheet. Samples with the dimensions of 10*10 mm2 were cut by utilizing automatic wire cutting machine. At the final stage, a magnetic grinding machine was employed to smooth the surface. The CrN/TiN coatings were deposited by the PVD technique with cathodic-arc evaporation technology and employing one cathode arc head. The settings for this purpose were as described in Table 17.2. Prior to the coating process, the samples were under argon ion bombing for 30 min utilizing level 5 argon gas at a pressure of \({10}^{-2}\) mbar and −1000 V bias. Also, the temperature of samples and bias voltage during the coating process were 250 °C and −80 V, respectively. Furthermore, the rotational speed of the samples around the central axis of the chamber and its central axis was 5 and 30 rpm, respectively.
3 Experimental Procedure
The Three-Dimensional Atomic Force Microscopy (3D AFM), DME-(DualscopeTM C-26), was used to characterize the morphology of the roughness accurately. Surface roughness determination procedure was obtained using the Lainović study [7]. After that, the interfacial contact resistance between the bipolar plates and the Gas Diffusion Layer (GDL) was measured using Lutron MO-2013 milliohm meter with an accuracy of 1 µΩ. In this test, a gas diffusion layer (Toray TGP-H-060) with a thickness of 190 µm was used to simulate the fuel cell environment. The samples were put between two GDLs and then between two copper plates. Figure 17.1 presents the mechanical setup which is used to apply force (35–150 N cm−2) to this system. Also, each GDL layer was used just once. To ensure the reliability, each experiment was repeated three times, and the average of the surface roughness and ICR were reported. The coating process was applied to one side of the targets. For uncoated samples, the obtained values are divided by 2.
4 Result and Discussion
The 3D AFM surface images for both coated and uncoated specimens are demonstrated in Fig. 17.2. The measured data are reported in Table 17.3 that \({R}_{a}\) is the average of surfaces roughness. The results proved that the non-coated samples, TiN-coated samples, and CrN-coated samples have the highest roughness, respectively. Figure 17.3 depicts the ICR changes in terms of pressure for both coated and non-coated samples. From this figure, it is clear that for all cases, the ICR values decrease by raising the applied pressure. It is because of an increase in the effective contact surface. In addition, the ICR of both coated samples (CrN and TiN-coated) is higher than that of the non-coated sample (Table 17.3). USA Department Energy is recommended ICR values of less than 10 mΩ cm2 at the pressure of 150 N/cm2 [7]. Based on statistical analysis, although the average ICR value of the TiN-coated group was higher than CrN-coated group, their differences were insignificant, with 95% of confidence interval (P-Value = 0.336). Moreover, the differences between the ICR values of the Non-coated material group and the TiN-coated group were significant (P-Value = 0.004). Also, the differences between the ICR values of the Non-coated material group and the CrN-coated group were significant, with 95% of confidence interval (P-Value = 0.008). The trend of ICR is a function of surface roughness for coating samples; it means that the CrN-coated bipolar plates have lower ICR values than TiN-coated. The ICR of the CrN-coated sample in the present study is almost 50% of ICR of the CrN-coated steel obtained by PF [8] and PVD methods. Also, the ICR of TiN-coated steel in the present study is approximately 33% of the ICR of TiN-coated steel by EBPVD method.
5 Conclusion
In the present research, stainless steel 410 was considered as a base metal for the bipolar plates, and chromium nitride and titanium nitride solid films with the same thickness were deposited on it by the CAE method. The highlight conclusion can be mentioned below:
-
Based on the statistical analysis, the ICR value differences of coated samples were significant compared to uncoated samples. It showed that the coated samples increased the ICR values.
-
The 3D AFM images, and surface morphology showed that the CrN-coated samples had the minimum values of averaged surface roughness.
-
The differences between ICR values of CrN and TiN coated were insignificant, but the average values of ICR and surface roughness of CrN-Coated were lower than that of TiN-Coated group.
-
Eventually, it is evident that the coating can improve the corrosion resistance of metals; the lowest values of ICR is required for the efficiency of a fuel cell. Therefore, it can be claimed that the CrN-coated layer is more efficient than a non-coated or TiN-coated layer.
References
P.P. Edwards, V.L. Kuznetsov, W.I. David, N.P. Brandon, Hydrogen and fuel cells: towards a sustainable energy future. Energy Policy 36(12), 4356–4362 (2008)
Y. Song, C. Zhang, C.Y. Ling, M. Han, R.Y. Yong, D. Sun, J. Chen, Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrog. Energy (2019)
K. Feng, Y. Shen, H. Sun, D. Liu, Q. An, X. Cai, P.K. Chu, Conductive amorphous carbon-coated 316L stainless steel as bipolar plates in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 34(16), 6771–6777 (2009)
E. Cho, U.S. Jeon, S.A. Hong, I.H. Oh, S.G. Kang, Performance of a 1 kW-class PEMFC stack using TiN-coated 316 stainless steel bipolar plates. J. Power Sources 142(1–2), 177–183 (2005)
P. Yi, L. Zhu, C. Dong, K. Xiao, Corrosion and interfacial contact resistance of 316L stainless steel coated with magnetron sputtered ZrN and TiN in the simulated cathodic environment of a proton-exchange membrane fuel cell. Surf. Coat. Technol. 363, 198–202 (2019)
A.T. Krawczynska, W. Chrominski, E. Ura-Binczyk, M. Kulczyk, M. Lewandowska, Mechanical properties and corrosion resistance of ultrafine grained austenitic stainless steel processed by hydrostatic extrusion. Mater. Des. 136, 34–44 (2017)
T. Lainović, M. Vilotić, L. Blažić, D. Kakaš, D. Marković, A. Ivanišević, Determination of surface roughness and topography of dental resin-based nanocomposites using AFM analysis. Bosn. J. Basic Med. Sci. 13, 34 (2013)
S.J. Lee, C.H. Huang, Y.P. Chen, Investigation of PVD coating on corrosion resistance of metallic bipolar plates in PEM fuel cell. J. Mater. Process. Technol. 140(1–3), 688–693 (2003)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this paper
Cite this paper
Forouzanmehr, M., Reza Kashyzadeh, K., Borjali, A., Jafarnode, M., Chizari, M. (2021). Effects of CrN/TiN Coatings on Interfacial Contact Resistance of Stainless Steel 410 Bipolar Plates in Fuel Cells. In: Mporas, I., Kourtessis, P., Al-Habaibeh, A., Asthana, A., Vukovic, V., Senior, J. (eds) Energy and Sustainable Futures. Springer Proceedings in Energy. Springer, Cham. https://doi.org/10.1007/978-3-030-63916-7_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-63916-7_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63915-0
Online ISBN: 978-3-030-63916-7
eBook Packages: EnergyEnergy (R0)