Abstract
Background: Eloquent location of a brain arteriovenous malformation (BAVM) is known to increase the surgical risk. Surgical removal of such BAVMs is challenging. Useful indicators for the safe removal of eloquent BAVMs are needed. The aim of this study was to determine the surgical risk factors for these challenging entities.
Methods: The authors retrospectively reviewed 29 motor and/or sensory BAVM patients who underwent surgeries. The risk factors for surgical morbidity were analyzed. As a new risk factor, maximum nidus depth, was evaluated.
Results: Complete obliteration was achieved in 28 patients (96.6%). Postoperative transient and permanent neurological deteriorations were seen in nine patients (31.0%) and five patients (17.2%), respectively. In univariate analysis, maximum nidus depth (p = 0.0204) and asymptomatic onset (p = 0.0229) were significantly correlated with the total morbidity. In multivariate analysis, only maximum nidus depth was significantly correlated with total morbidity (p = 0.0357; odds ratio, 2.78598; 95% confidence interval, 0.8866–8.7535). The cut-off value for the maximum nidus depth was 36 mm for total morbidity (area under the curve [AUC], 0.7428) and 41 mm for permanent morbidity (AUC, 0.8833). The cutoff value of the maximum nidus size was 30 mm for total morbidity (AUC, 0.5785) and 30 mm for permanent morbidity (AUC, 0.7625). AUC was higher for the maximum nidus depth than it was for the maximum nidus size.
Conclusions: Maximum nidus depth was significantly associated with surgical morbidity of eloquent BAVMs. The maximum nidus depth is a novel and a simpler indicator of the risk of surgical morbidity.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Surgical treatment of brain arteriovenous malformations (BAVMs) is a radical treatment modality. Safe and radical removal is expected to help prevent future hemorrhage and symptomatic deterioration. However, there are BAVMs that are unsuitable for surgical intervention and an eloquently located BAVM is known to increase the surgical risk [1,2,3,4]. In particular, in the Rolandic area, i.e., the motor and sensory areas, surgical morbidity is directly linked to the independence of the patient. Hence, it is still challenging to surgically treat these entities. Indicators for safe removal of Rolandic BAVMs are necessary but have not been determined thus far.
In addition to factors such as well-known hemorrhagic onset, unruptured BAVM, size, and deep venous drainage that are included in the Spetzler-Martin (S-M) grading scale [1,2,3,4], several surgical risk factors for eloquent BAVM have been reported in recent years, such as cortical reorganization and plasticity of motor area visualized via functional magnetic resonance imaging (fMRI) [5], BAVM lesion-to-corticospinal tract (CST) distance assessed by diffusion tensor imaging (DTI) [6, 7], and BAVM lesion-to-activation area distance (LAD) assessed by fMRI [8]. They can help predict postoperative neurological outcomes in the eloquent area and these factors contributed to acceptable long-term outcomes [6, 9], along with transient but finally reversible surgical morbidity. Thus, there is a possibility of plasticity and cortical reorganization of the cortical region. Impairment of the CST and deep white fibers may be more critical and irreversible in the Rolandic area. However, production of a uniform image is sometimes difficult with DTI and fMRI because of various methodological limitations [10]. The imaging results may differ between institutions and the type of imaging method. Therefore, predictors of surgical risk that can be evaluated easily with general imaging modalities are necessary. In this regard, we focused on the maximum nidus depth as a new indicator, which is a factor measurable on general imaging.
The aim of this study was to determine the surgical risk factors in patients with eloquent BAVMs, especially in the Rolandic area. Furthermore, we analyzed maximum nidus depth as a novel indicator and predictor of the surgical outcome in patients with this challenging entity.
Material and Methods
The institutional review board of Tokyo Women’s Medical University approved this retrospective study. All patients provided written informed consent for the surgical procedures.
Patient Selection
This retrospective study included 297 consecutive patients with diagnosed BAVMs who underwent treatment at Tokyo Women’s Medical University between January 2002 and August 2017. Of these, 202 patients were diagnosed with BAVM localized in an eloquent area. Furthermore, of these, 128 patients underwent gamma knife surgery (GKS) alone and 74 patients underwent surgical nidus removal. In these 74 patients who underwent surgical treatment, there were 29 patients who had BAVMs in the Rolandic motor or sensory area, or both. Data of these 29 patients were finally analyzed in this study.
Diagnosis and Treatment Strategy
The diagnoses and locations of BAVMs were established based on digital subtraction angiography (DSA) combined with magnetic resonance imaging (MRI) and computed tomography angiography (CTA) by a board-certified neurosurgeon of the Japan Neurosurgical Society. Data were collected from the medical records. The characteristics of BAVM included the size, depth, diffuseness, anatomic location, and venous drainage. These parameters were evaluated with a combination of imaging modalities. The size and depth of the nidus were also measured using the imaging modalities mentioned above. The maximum diameter was defined as the maximum nidus size measured from various angles. The maximum depth was defined as the distance from the brain surface to the deepest part of the nidus vertically. The nidus depth, if wider than other measurements, can be the same as the maximum diameter as well. BAVMs were further classified based on the S-M grading scale [1].
Uncontrollable symptoms, including hemorrhage, epilepsy, and severe headache, were considered as the indications for surgical nidus removal of the BAVM. However, asymptomatic BAVMs were also surgically removed if the patients strongly requested it after they were fully informed of the natural history and treatment options, including observation. The same surgical team performed the microsurgical nidus removal and managed the patients postoperatively. Preoperative endovascular interventions were performed as necessary. Each BAVM resection was completed while taking special care to preserve the functional reaction that was assessed using motor-evoked potentials, somatosensory-evoked potentials, and cortical/subcortical electrical stimulation.
Postoperatively, the patients were maintained in a hypotensive state under sedation. In principle, DSA was performed before and after the surgeries and complete removal was evaluated by postoperative DSA.
Outcome Parameters
Postoperative follow-ups were performed at our institution, and the degree of excision and the presence or absence of complications were evaluated. In this study, morbidity was defined as deterioration in the neurological findings related to the surgery and its associated complications. The final morbidity was assessed at 3 months postoperatively to evaluate whether the morbidity was a transient or permanent symptom. Furthermore, the modified Rankin Scale (mRS) score was assessed at 3 months postoperatively to evaluate whether it was intact or had worsened postoperatively. Permanent morbidity was defined as persistent neurological deterioration, and we classified it into two groups: “mRS change<2” and “2≤mRS change” compared with preoperative mRS. Transient morbidity was defined as neurological deterioration that occurred postoperatively and resolved within 3 months. Any death was determined as mortality.
Statistical Analysis
All statistical analyses were performed using JMP Pro13 (SAS Institute, NC, USA). Continuous variables were expressed as mean ± standard deviation. Univariate analysis was performed to identify the risk factors associated with postoperative surgical morbidity at 3 months in Rolandic eloquent BAVM. When comparing two groups, categorical variables were evaluated by Fisher’s exact test and continuous variables were evaluated by Student’s t-test. Significant risk factors and variables that were associated with morbidity, defined as p < 0.20 in univariate analysis, were included in multivariate logistic regression analysis. Since nidus size, eloquent location, deep venous drainage, age, ruptured presentation and diffuseness were already included as univariate factors in statistical analysis, S-M grade and Lawton-Young supplementary grading system containing these factors were excluded because they were confounding factors.
Odds ratio (OR) for surgical morbidity was estimated from logistic regression analysis, and receiver-operator characteristic (ROC) curves were constructed to obtain the optimal cutoff values for the maximum nidus size and depth in predicting surgical morbidity. To confirm the predictive and diagnostic ability of the ROC curves, areas under the ROC curves (AUCs) were also determined. The significance level was set at p < 0.05 and all p values reported in this study are two-sided.
Results
Patient Demographics and BAVM Characteristics
A total of 29 patients with BAVMs in the Rolandic area who underwent surgical nidus removal at our institution were analyzed in this study. The patient demographics and BAVM characteristics are summarized in Table 1. With respect to clinical presentation, uncontrollable symptomatic BAVM was seen in 22 patients (75.9%) and hemorrhagic onset was seen in 11 (37.3%). Four patients (13.8%) underwent pre-surgical embolization and six patients (20.7%) pre-surgical GKS. The overall median S-M grading scale score was 3 (2—4), and the most frequent SM grade was grade III (51.7%). All BAVMs were in the eloquent location; in 12 patients (41.4%) they were related to the motor area, while in 11 patients (37.9%), they were related to the sensory area, and in six patients (20.7%), they were related to both. The mean maximum nidus size was 33 ± 17 mm, and the mean maximum nidus depth was 37 ± 17 mm. BAVMs with deep venous drainage were seen in only a few (13.8%).
Outcomes
The outcomes after surgical nidus removal are summarized in Table 2. Complete removal was achieved in 28 patients (96.6%). Partial removal was achieved in one patient; the patient suffered from perioperative hemorrhage and required reoperation. Postoperative hemorrhage due to normal perfusion pressure breakthrough visualized by cold-Xenon computed tomography was recognized in two patients. There was no incidence of hemorrhage after the perioperative period. Overall, 14 patients (48.3%) suffered from postoperative morbidity, including transient morbidity in nine patients (31.0%) and permanent morbidity in five patients (17.2%). The deterioration in neurological findings related to the surgery was often resolved during the follow-up after a few weeks. Motor and sensory disorders, dysarthria, and higher brain dysfunction were recognized as neurological complications. Although renal failure occurred in one patient, there were no major systemic complications leading to mRS deterioration. There was no mortality. Deterioration of two or more on mRS was observed in two patients.
Univariate and Multivariate Analyses of Predictors of Surgical Morbidity for Rolandic BAVM
To identify the risk factor for surgical morbidity in Rolandic area BAVMs, several variables were analyzed between the total morbidity group and no morbidity group using univariate analysis (Table 3). The patients in both groups were well-matched with respect to age, sex, and preoperative mRS. Asymptomatic BAVM was a significant risk factor for surgical morbidity (p = 0.0229), but hemorrhagic onset was not (p = 0.3156). Of the BAVM characteristics, the maximum nidus size, deep venous drainage, diffuseness, and laterality were not significant risk factors, but maximum nidus depth was a significant risk factor for surgical morbidity (p = 0.0204).
For multivariate analysis (Table 4), significant variables and two variables that were associated with morbidity based on p value <0.20 in univariate analysis (preoperative mRS, laterality) were included in the multivariate logistic regression analysis. As mentioned in the methods section, S-M grade and Lawton-Young supplementary grading systems were excluded because they were confounding factors. The maximum nidus depth was the only risk factor of surgical morbidity in this analysis (OR = 2.78598, 95% confidence interval [CI]: 0.8866–8.7535, p = 0.0357).
Optimal Cut-off Values for Surgical Morbidity
From the results of logistic regression analysis (Table 5, Fig. 1a, b), maximum nidus depth was identified as a predictor of total morbidity (OR = 2.82190, p = 0.015) and permanent morbidity (OR = 8.34984, p = 0.002), and maximum nidus size was identified as a predictor of permanent morbidity (OR = 2.46749, p = 0.027). The optimal cutoff values identified from the ROC curve analysis included maximum nidus size of 30 mm for permanent morbidity, maximum nidus depth of 36 mm for total morbidity, and 41 mm for permanent morbidity (Table 5). AUC of total morbidity, indicating the predictive accuracy of the ROC curve, was 0.7428 for maximum nidus depth and 0.5785 for maximum nidus size. AUC for permanent morbidity was 0.8833 for maximum nidus depth and 0.7625 for maximum nidus size. AUC was higher for the maximum nidus depth compared with that for the maximum nidus size, for both total and permanent morbidities (Table 5, Fig. 1c, d). As illustrative cases, the patient (case 1) with sensory and motor area BAVM (S-M grade II) presented with headache, with maximum nidus size of 21 mm and maximum nidus depth of 35 mm (Fig. 2a, b). Activation was found adjacent to the nidus on fMRI (Fig. 2c, d). Transient morbidity was observed after the surgery. In the patient (case 2) with asymptomatic sensory area BAVM (S-M grade III), maximum nidus size was 36 mm and maximum nidus depth was 44 mm (Fig. 3a, b). Permanent morbidity was observed after the surgery.
Surgical risk factor and cutoff values in Rolandic brain arteriovenous malformations (BAVMs). (a and b) Maximum nidus depth was significantly correlated with surgical morbidity (p = 0.02), but maximum nidus size was not a significant factor. The distribution charts demonstrate the relationship between the maximum nidus size and surgical morbidity (a), and the maximum nidus depth and surgical morbidity (b). Green rhomboids indicate the mean and 95% confident intervals. (c and d) Receiver operating characteristic curve analyses for maximum nidus depth and surgical morbidity; total morbidity (c) and permanent morbidity (d). The area under the curve was 0.74 and the cutoff value was 36 mm (c). The area under the curve was 0.88 and the cutoff value was 41 mm (d)
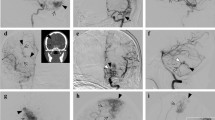
Illustrative case 1. Axial T2-weighted image (a) and coronal maximum intensity projection image of time of flight magnetic resonance angiography (b) showing sensory motor area brain arteriovenous malformation (Spetzler-Martin grade II). The maximum nidus depth was 35 mm. The yellow dotted line indicates the maximum nidus depth relative to the tangential line of the brain’s surface. The activations related to left-hand gripping (c) and right-hand gripping were found adjacent to the nidus (d) on fMRI
Illustrative case 2. Axial T2-weighted image (a) and axial and coronal maximum intensity projection image of time of flight magnetic resonance angiography (b and c) showing brain arteriovenous malformation (Spetzler-Martin grade III) in the sensory area. The maximum nidus depth was 44 mm. The yellow dotted line indicates the maximum nidus depth relative to the tangential line of the brain’s surface
Discussion
In this series of 29 patients with BAVM who underwent surgical nidus removal, we found that nidus depth may be more useful than nidus size as a predictor of the surgical risk for postoperative surgery-related morbidity in eloquent BAVM, especially with BAVMs in the motor and sensory areas. As the maximum nidus depth from the brain surface increased, the morbidity rate elevated, suggesting that the morbidity was permanent.
Many grading scales and scores to indicate the surgical risk associated with BAVM removal, on behalf of S-M grading scale, have been previously reported [1,2,3,4, 11,12,13]. From these reports, it is well known that the surgical risk is higher with eloquent BAVM. Most of the grading scores include nidus size as a surgical risk factor, and its importance has been previously reported [1,2,3,4, 11,12,13]. There is no doubt that the surgical risk increases with increasing size of the BAVM nidus. However, regardless of the nidus size, there are reports of morbidity due to surgery even with a smaller nidus. Thus, in BAVMs in eloquent areas, the surgical risk is higher from the very beginning [1, 2]. Furthermore, there are no reports that have evaluated the assessment of eloquence in detail. Therefore, certain problems still remain, such as the lack of inclusion of white matter eloquent fibers in the grading systems, lack of definition of the width and depth of the eloquent area, and lack of quantitative variables to describe the relationship between the nidus and eloquence [14].
Following the findings of a randomized control trial (RCT) for unruptured BAVM [15], the treatment for eloquent BAVM is still considered challenging. However, there are also some cases wherein BAVM resulted in hemorrhage with high morbidity and presented with uncontrollable symptoms, suggesting that surgical treatment should be considered for eloquent BAVM. As was reported previously, although symptoms appear transiently after surgical removal, they often resolve during follow-up [5, 8, 16, 17]. It appears that there may be risk factors other than nidus size. If there is a factor that can predict whether eloquent BAVM is treatable or not, then the decision for surgical intervention can be better judged.
Nidus Depth as a New Indicator of Surgical Risk for Eloquent BAVM
In previous reports, the following variables have been reported as risk factors for surgical removal in BAVM: BAVM size, eloquence, deep venous drainage, diffuseness, perforating artery supply, and unruptured presentation [1,2,3,4, 11,12,13]. It is also known that relatively good outcomes are obtained with surgical treatment of BAVM with hemorrhagic onset [5, 18]. Particularly with regard to the nidus size, it is easy to predict that BAVM localization will dominate the critical area as it gets bigger, and it will lead to an increase in the risk. However, in measuring the size, the obtained values and implications vary depending on the direction used to measure on the image since the size can be measured in all directions. There are no uniform guidelines on assessing this measurement. Therefore, we focused on the nidus depth and performed the analyses for surgical morbidity for eloquent BAVM in this study. Although it seems like common sense that more extensive and deeper AVMs in eloquent locations will be associated with more surgical morbidity, to the best of our knowledge there are no reports in the literature that investigated and mentioned BAVM depth. Various factors that were previously reported to be significantly correlated with postoperative morbidity were not found to be significantly correlated in this study; the p values of the factors, though relatively low, were not significant. It is suggested that the maximum nidus depth may be more useful as a predictor in the surgical treatment of BAVM in the Rolandic area (Table 3 and Fig. 2).
Optimal Cutoff Value of Maximum Nidus Depth for Surgical Morbidity
To determine the optimal cutoff values for the maximum nidus depth and size in relationship with surgical morbidity, ROC curves were generated. The cutoff value for the maximum nidus depth was 36 mm for total morbidity and 41 mm for permanent morbidity (Table 4). Therefore, the deeper the maximum nidus depth, the higher the surgical risk. The cutoff value for the maximum nidus size for permanent morbidity was 30 mm. The AUC of the maximum depth (total morbidity: 0.74, permanent morbidity: 0.88) was larger than that of the maximum size (total morbidity: 0.58, permanent morbidity: 0.76); therefore, maximum nidus depth is a more useful predictor than the maximum nidus size (Table 4, Fig. 2).
These findings raise the question of the underlying mechanism that can explain how the nidus depth affects the surgical risk. Frequently this is due to small arterioles arriving at the deep part of the AVM nidus, or due to small draining venules, which are bleeding notoriously and are difficult to coagulate at the same time. It is also already known that the deeper a BAVM is, the more the nidus involves the CST [9] and is closer to the perforating supply area. In previous reports, lesion-to-CST distance measured by DTI [6, 7] and LAD measured by fMRI [8] have been reported as surgical risk factors for vascular malformations of the eloquent area. Furthermore, the plasticity and cortical reorganization of the eloquent cortical area were also reported [5, 16, 17], and it was suggested, based on fMRI, that significant activation did not lie within the nidus of BAVM [16]. The anatomic location alone of a BAVM may not provide any information on the functional reorganization. In fact, we have encountered cases where translocation of the eloquent area was found on intraoperative direct stimulation. Thus, the plasticity may contribute to the transient deterioration in the cortical regions after the surgery. On the other hand, impairment of CST and deep white fibers may be more critical and irreversible, resulting in permanent deterioration. Therefore, the nidus depth can be a risk factor as it reflects the association between a BAVM and the deep white fibers.
Although DTI and fMRI are useful for the prediction of the surgical outcomes, they are often complicated. It is often difficult to perform imaging accurately because of methodological limitations and technical difficulties like different stepwise procedures between institutions, false-positives and false-negatives, and hemorrhage and edema that can affect the results [10]. On the other hand, nidus depth, as proposed in this study, is an easily measurable parameter that does not require special images. It can be measured with routine images that do not vary significantly between institutions.
ARUBA trial was the only RCT in unruptured BAVMs with a negative stance on interventions [15], and it suggested that interventions for unruptured BAVM should be cautious; however, there have been objections to that report [19, 20]. In this study, asymptomatic presentation was a significant risk factor in univariate analysis but not in multivariate analysis. Maximum nidus depth was the only significant factor in multivariate analysis. Its cutoff value obtained in this study does not strongly recommend the surgical removal of eloquent BAVMs; however, it can be one of the indicators of the surgical risk in cases that require treatment. This study does not deny the importance of the size of the nidus as a risk factor since there were cases wherein the measured maximum size was in the same direction as the depth. Therefore, the direction in which the maximum size is measured is also important.
Many outstanding questions and issues pertinent to this study remain. The results obtained in this study are from limited locations of eloquent BAVMs, specifically the Rolandic area. However, it is unclear as to which area is better—the motor or sensory area. The significance of the depth in other eloquent areas like the language area remains unknown. Furthermore, we do not discuss the relations between DTI tractography and closeness of the nidus. Although the effectiveness of GKS and multimodal treatment for high-grade BAVM has been reported in recent years [21,22,23], the current study is not a comparative one between the modalities of treatment. The question remains whether surgical removal is superior to GKS or another multimodal strategy. As another limitation, this study was a retrospective one and the sample size was small. Further analyses and experiments are needed in this regard. This small subset of patients may overestimate the value of nidus depth compared with other variables that are well known to be factors in development of perioperative morbidity, including hemorrhagic status and size, primarily, and others. However, this is an interesting case series of an uncommon entity: surgically operated motorsensory cortex. We think that it is a meaningful report derived from rare cases.
From our results, the radicality of surgical removal was extremely high and the requirement for surgery cannot be completely ruled out. In the future, downgrading using multimodal treatment may also be promising as a new strategy to prevent surgical morbidities in such challenging entities. Thus, critical deep lesions or eloquent locations should first be eliminated with relatively noninvasive treatment modalities with higher priority [24], resulting in downgrading by decreasing the maximum nidus depth and affected eloquent area followed by surgical treatment. It is necessary to consider new treatment strategies for safe and highly radical BAVM treatment. Maximum nidus depth is more likely to be associated with surgical morbidity in BAVMs in the eloquent Rolandic areas. Although surgical treatment should be carefully considered, the maximum nidus depth is a simpler and stronger predictor of the outcome than maximum nidus size in patients with this challenging entity.
Abbreviations
- AUC:
-
Areas under the receiver-operator characteristic curves
- BAVM:
-
Brain arteriovenous malformations
- CI:
-
Confidence interval
- CST:
-
Corticospinal tract
- CTA:
-
Computed tomography angiography
- DSA:
-
Digital subtraction angiography
- DTI:
-
Diffusion tensor imaging
- fMRI:
-
Functional magnetic resonance imaging
- GKS:
-
Gamma knife surgery
- LAD:
-
Lesion-to-activation area distance
- MRI:
-
Magnetic resonance imaging
- mRS:
-
Modified Rankin Scale
- OR:
-
Odds ratio
- RCT:
-
Randomized control trial
- ROC:
-
Receiver-operator characteristic
- S-M:
-
Spetzler-Martin
References
Hamilton MG, Spetzler RF (1994) The prospective application of a grading system for arteriovenous malformations. Neurosurgery 34:2–6. discussion 6–7
Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL (2010) A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 66:702–713. discussion 713
Ryu B, Ishikawa T, Kawamata T (2017) Multimodal treatment strategy for Spetzler-Martin grade III arteriovenous malformations of the brain. Neurol Med Chir (Tokyo) 57:73–81
Spetzler RF, Ponce FA (2011) A 3-tier classification of cerebral arteriovenous malformations. Clinical article. J Neurosurg 114:842–849
Lee L, Sitoh YY, Ng I, Ng WH (2013) Cortical reorganization of motor functional areas in cerebral arteriovenous malformations. J Clin Neurosci 20:649–653
Lin F, Zhao B, Wu J, Wang L, Jin Z, Cao Y, Wang S (2016) Risk factors for worsened muscle strength after the surgical treatment of arteriovenous malformations of the eloquent motor area. J Neurosurg 125:289–298
Lin Y, Lin F, Kang D, Jiao Y, Cao Y, Wang S (2018) Supratentorial cavernous malformations adjacent to the corticospinal tract: surgical outcomes and predictive value of diffusion tensor imaging findings. J Neurosurg 128:541–552
Tuntiyatorn L, Wuttiplakorn L, Laohawiriyakamol K (2011) Plasticity of the motor cortex in patients with brain tumors and arteriovenous malformations: a functional MR study. J Med Assoc Thai 94:1134–1140
Lin F, Wu J, Zhao B, Tong X, Jin Z, Cao Y, Wang S (2016) Preoperative functional findings and surgical outcomes in patients with motor cortical arteriovenous malformation. World Neurosurg 85:273–281
Okada T, Miki Y, Kikuta K, Mikuni N, Urayama S, Fushimi Y, Yamamoto A, Mori N, Fukuyama H, Hashimoto N, Togashi K (2007) Diffusion tensor fiber tractography for arteriovenous malformations: quantitative analyses to evaluate the corticospinal tract and optic radiation. AJNR Am J Neuroradiol 28:1107–1113
Davidson ASM, Morgan MK (2010) How safe is arteriovenous malformation surgery? A prospective, observational study of surgery as first-line treatment for brain arteriovenous malformations. Neurosurgery 66:498–505
de Oliveira E, Tedeschi H, Raso J (1998) Comprehensive management of arteriovenous malformations. Neurol Res 20:673–683
Pandey P, Marks MP, Harraher CD, Westbroek EM, Chang SD, Do HM, Levy RP, Dodd RL, Steinberg GK (2012) Multimodality management of Spetzler-Martin grade III arteriovenous malformations. J Neurosurg 116:1279–1288
Jiao Y, Lin F, Wu J, Li H, Wang L, Jin Z, Wang S, Cao Y (2018) A supplementary grading scale combining lesion-to-eloquence distance for predicting surgical outcomes of patients with brain arteriovenous malformations. J Neurosurg 128:530–540
Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart E, Cordonnier C, Stefani MA, Hartmann A, von Kummer R, Biondi A, Berkefeld J, Klijn CJ, Harkness K, Libman R, Barreau X, Moskowitz AJ (2014) Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383:614–621
Alkadhi H, Kollias SS, Crelier GR, Golay X, Hepp-Reymond MC, Valavanis A (2000) Plasticity of the human motor cortex in patients with arteriovenous malformations: a functional MR imaging study. AJNR Am J Neuroradiol 21:1423–1433
Ding D, Starke RM, Liu KC, Crowley RW (2015) Cortical plasticity in patients with cerebral arteriovenous malformations. J Clin Neurosci 22:1857–1861
Lawton MT, Du R, Tran MN, Achrol AS, McCulloch CE, Johnston SC, Quinnine NJ, Young WL (2005) Effect of presenting hemorrhage on outcome after microsurgical resection of brain arteriovenous malformations. Neurosurgery 56:485–493. discussion 485–493
Nerva JD, Mantovani A, Barber J, Kim LJ, Rockhill JK, Hallam DK, Ghodke BV, Sekhar LN (2015) Treatment outcomes of unruptured arteriovenous malformations with a subgroup analysis of ARUBA (a randomized trial of unruptured brain arteriovenous malformations)-eligible patients. Neurosurgery 76:563–570
Rutledge WC, Abla AA, Nelson J, Halbach VV, Kim H, Lawton MT (2014) Treatment and outcomes of ARUBA-eligible patients with unruptured brain arteriovenous malformations at a single institution. Neurosurg Focus 37:E8
Derdeyn CP, Zipfel GJ, Albuquerque FC, Cooke DL, Feldmann E, Sheehan JP, Torner JC (2017) Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48:e200–e224
Ding D, Yen CP, Starke RM, Xu Z, Sun X, Sheehan JP (2014) Radiosurgery for Spetzler-Martin grade III arteriovenous malformations. J Neurosurg 120:959–969
Kano H, Flickinger JC, Yang HC, Flannery TJ, Tonetti D, Niranjan A, Lunsford LD (2014) Stereotactic radiosurgery for Spetzler-Martin grade III arteriovenous malformations. J Neurosurg 120:973–981
Abla AA, Rutledge WC, Seymour ZA, Guo D, Kim H, Gupta N, Sneed PK, Barani IJ, Larson D, McDermott MW, Lawton MT (2015) A treatment paradigm for high-grade brain arteriovenous malformations: volume-staged radiosurgical downgrading followed by microsurgical resection. J Neurosurg 122:419–432
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We would like to thank Editage (www.editage.jp) for English language editing.
Funding: No funding was received for this research.
Conflict of interest: The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Tokyo Women’s Medical University) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: The requirement for written informed consent was waived because of the retrospective design.
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this paper
Cite this paper
Ryu, B. et al. (2021). Maximum Nidus Depth as a Risk Factor of Surgical Morbidity in Eloquent Brain Arteriovenous Malformations. In: Esposito, G., Regli, L., Cenzato, M., Kaku, Y., Tanaka, M., Tsukahara, T. (eds) Trends in Cerebrovascular Surgery and Interventions. Acta Neurochirurgica Supplement, vol 132. Springer, Cham. https://doi.org/10.1007/978-3-030-63453-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-63453-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63452-0
Online ISBN: 978-3-030-63453-7
eBook Packages: MedicineMedicine (R0)