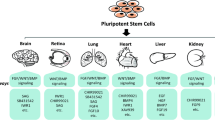
Abstract
Cardiac function is a summation of multiple facets of physiology including electrophysiology, mechanics, fluid dynamics, and cardiac structure. Each of these parameters can modulate cardiac physiology and pathophysiology and are thus crucial components in understanding this complex organ. Thus, the study of cardiac physiology requires multiscale approaches and current models of cardiac tissue used in research can be divided into in vivo, in vitro, and in silico models. Several animal models have been used in in vivo studies including mice, rats, guinea pigs, rabbits, sheep, pigs, and dogs. While these in vivo models can give a complete understanding of systemic effects of diseases and drugs on cardiac physiology, significant species-dependent differences have impeded the progress of this knowledge to benefit human health. To address this concern, in vitro human cardiac models that mimic cardiac physiology including Langendorff-perfused whole heart and wedge preparations, cardiac organotypic slices, as well as two- and three-dimensional cell cultures with multiple cardiac cell types were developed. In vivo conditions such as electrical and mechanical stimulation and structural anisotropy have also been incorporated in these cell culture models. Finally, mechanistic in silico models and their applications in cardiac pathophysiology and drug response predictions are also discussed in this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Allam AH, Thompson RC, Wann LS, et al. Atherosclerosis in ancient Egyptian mummies: the horus study. JACC Cardiovasc Imaging. 2011;4:315. https://doi.org/10.1016/j.jcmg.2011.02.002.
Salama G, London B. Mouse models of long QT syndrome. J Physiol. 2007;578:43. https://doi.org/10.1113/jphysiol.2006.118745.
Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;58:5.61.1. https://doi.org/10.1002/0471141755.ph0561s58.
Camacho P, Fan H, Liu Z, He JQ. Small mammalian animal models of heart disease. Am J Cardiovasc Dis. 2016;6(3):70–80.
DeMartini T, Nowell M, James J, Williamson L, Lahni P, Shen H, Kaplan JM. High fat diet-induced obesity increases myocardial injury and alters cardiac STAT3 signaling in mice after polymicrobial sepsis. Biochim Biophys Acta Mol basis Dis. 2017;1863:2654. https://doi.org/10.1016/j.bbadis.2017.06.008.
Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol. 2008;295:H1206. https://doi.org/10.1152/ajpheart.00319.2008.
Alarcon G, Roco J, Medina M, Medina A, Peral M, Jerez S. High fat diet-induced metabolically obese and normal weight rabbit model shows early vascular dysfunction: mechanisms involved. Int J Obes. 2018;42:1535. https://doi.org/10.1038/s41366-018-0020-6.
Dhungel S, Sinha R, Sinha M, Paudel BH, Bhattacharya N, Mandal MB. High fat diet induces obesity in British Angora Rabbit: a model for experimental obesity. Indian J Physiol Pharmacol. 2009;53(1):55–60.
Fitzgerald SM, Henegar JR, Brands MW, Henegar LK, Hall JE. Cardiovascular and renal responses to a high-fat diet in Osborne-Mendel rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R547.
Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:1.
Han C, Pogwizd SM, Killingsworth CR, He B. Noninvasive reconstruction of the three-dimensional ventricular activation sequence during pacing and ventricular tachycardia in the rabbit heart. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:1684–7. https://doi.org/10.1109/IEMBS.2011.6090484.
Orini M, Taggart P, Lambiase PD. In vivo human sock-mapping validation of a simple model that explains unipolar electrogram morphology in relation to conduction-repolarization dynamics. J Cardiovasc Electrophysiol. 2018;29:990. https://doi.org/10.1111/jce.13606.
Ando K, Takahara A, Nakamura Y, et al. Changes of electrocardiogram and hemodynamics in response to dipyridamole: in vivo comparative analyses using anesthetized beagle dogs and microminipigs. J Pharmacol Sci. 2018;136:86. https://doi.org/10.1016/j.jphs.2018.01.002.
Lee P, Quintanilla JG, Alfonso-Almazán JM, et al. In vivo ratiometric optical mapping enables high-resolution cardiac electrophysiology in pig models. Cardiovasc Res. 2019;115:1659. https://doi.org/10.1093/cvr/cvz039.
Zimmer H-G. The isolated perfused heart and its pioneers. Physiology. 1998;13:203. https://doi.org/10.1152/physiologyonline.1998.13.4.203.
Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff-Still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55:113. https://doi.org/10.1016/j.vascn.2006.05.006.
Langendorff O. Untersuchungen am überlebenden Säugethierherzen. Pflüger. 1895;61:291. https://doi.org/10.1007/BF01812150.
Liao R, Podesser BK, Lim CC. The continuing evolution of the Langendorff and ejecting murine heart: new advances in cardiac phenotyping. Am J Physiol Heart Circ Physiol. 2012;303:H156. https://doi.org/10.1152/ajpheart.00333.2012.
Lateef R, Al-Masri A, Alyahya A. Langendorff’s isolated perfused rat heart technique: a review. Int J Basic Clin Pharmacol. 2015;4:1314–22. https://doi.org/10.18203/2319-2003.ijbcp20151381.
Yeo JM, Tse V, Kung J, Lin HY, Lee YT, Kwan J, Yan BP, Tse G. Isolated heart models for studying cardiac electrophysiology: a historical perspective and recent advances. J Basic Clin Physiol Pharmacol. 2017;28(3):191–200. https://doi.org/10.1515/jbcpp-2016-0110.
Milani-Nejad N, Janssen PML. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther. 2014;141:235. https://doi.org/10.1016/j.pharmthera.2013.10.007.
Benforado JM. The effects of vagal stimulation on the isolated perfused rat heart. J Physiol. 1959;145:266. https://doi.org/10.1113/jphysiol.1959.sp006141.
Buschman HP, Storm CJ, Duncker DJ, Verdouw PD, et al. Heart rate control via vagus nerve stimulation. Neuromodulation. 2006;9:214. https://doi.org/10.1111/j.1525-1403.2006.00062.x.
Antzelevitch C. Cellular basis for the repolarization waves of the ECG. Ann N Y Acad Sci. 2006;1080:268. https://doi.org/10.1196/annals.1380.021.
Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372. https://doi.org/10.1161/01.CIR.93.2.372.
Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation. 1998;98:1921. https://doi.org/10.1161/01.CIR.98.18.1921.
Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res. 2010;106:981. https://doi.org/10.1161/CIRCRESAHA.109.204891.
Valderrábano M, Lee MH, Ohara T, Lai AC, Fishbein MC, Lin SF, Karagueuzian HS, Chen PS. Dynamics of intramural and transmural reentry during ventricular fibrillation in isolated swine ventricles. Circ Res. 2001;88:839. https://doi.org/10.1161/hh0801.089259.
Liu T, Brown BS, Wu Y, Antzelevitch C, Kowey PR, Yan GX. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm. 2006;3:948. https://doi.org/10.1016/j.hrthm.2006.04.021.
Shenasa M, Hindricks G, Borggrefe M, Breithardt G. Cardiac mapping. Card Mapp. 2009; https://doi.org/10.1002/9781444303438.
Brandenburger M, Wenzel J, Bogdan R, Richardt D, Nguemo F, Reppel M, Hescheler J, Terlau H, Dendorfer A. Organotypic slice culture from human adult ventricular myocardium. Cardiovasc Res. 2012;93:50–9.
Habeler W, Peschanski M, Monville C. Organotypic heart slices for cell transplantation and physiological studies. Organogenesis. 2009;5:62. https://doi.org/10.4161/org.5.2.9091.
Kang C, Qiao Y, Li G, Baechle K, Camelliti P, Rentschler S, Efimov IR. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials. Sci Rep. 2016;6:28798. https://doi.org/10.1038/srep28798.
Ou Q, Jacobson Z, Abouleisa RRE, et al. Physiological biomimetic culture system for pig and human heart slices. Circ Res. 2019;125:628–42.
Qiao Y, Dong Q, B L, et al. (18AD) Multiparametric slice culture platform for the investigation of human cardiac tissue physiology. Prog Biophys Mol Biol. 2019;144:139–50.
Veldhuizen J, Migrino RQ, Nikkhah M. Three-dimensional microengineered models of human cardiac diseases. J Biol Eng. 2019;13:29. https://doi.org/10.1186/s13036-019-0155-6.
Lyon AR, Joudrey PJ, Jin D, Nass RD, Aon MA, Rourke B, Akar FG. Optical imaging of mitochondrial function uncovers actively propagating waves of mitochondrial membrane potential collapse across intact heart. J Mol Cell Cardiol. 2010;49:565. https://doi.org/10.1016/j.yjmcc.2010.07.002.
Venable PW, Taylor TG, Sciuto KJ, Zhao J, Shibayama J, Warren M, Spitzer KW, Zaitsev AV. Detection of mitochondrial depolarization/recovery during ischaemia-reperfusion using spectral properties of confocally recorded TMRM fluorescence. J Physiol. 2013;591:2781. https://doi.org/10.1113/jphysiol.2012.248153.
Moretti A, Bellin M, Welling A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397. https://doi.org/10.1056/NEJMoa0908679.
Liang P, Sallam K, Wu H, et al. Patient-specific and genome-edited induced pluripotent stem cell–derived cardiomyocytes elucidate single-cell phenotype of brugada syndrome. J Am Coll Cardiol. 2016;68:2086. https://doi.org/10.1016/j.jacc.2016.07.779.
Wei H, Zhang XH, Clift C, Yamaguchi N, Morad M. CRISPR/Cas9 Gene editing of RyR2 in human stem cell-derived cardiomyocytes provides a novel approach in investigating dysfunctional Ca2+ signaling. Cell Calcium. 2018;73:104. https://doi.org/10.1016/j.ceca.2018.04.009.
Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song LJ, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239. https://doi.org/10.1038/s41586-018-0016-3.
Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813. https://doi.org/10.1016/j.biomaterials.2013.04.026.
Zhao Y, Rafatian N, Feric NT, et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. 2019;176:913. https://doi.org/10.1016/j.cell.2018.11.042.
Yan Y, Bejoy J, Xia J, Griffin K, Guan J, Li Y. Cell population balance of cardiovascular spheroids derived from human induced pluripotent stem cells. Sci Rep. 2019;9:1–12. https://doi.org/10.1038/s41598-018-37686-1.
Zuppinger C. 3D cardiac cell culture: a critical review of current technologies and applications. Front Cardiovasc Med. 2019;6:87. https://doi.org/10.3389/fcvm.2019.00087.
Polonchuk L, Chabria M, Badi L, Hoflack JC, Figtree G, Davies MJ, Gentile C. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep. 2017;7(1):1–12. https://doi.org/10.1038/s41598-017-06385-8.
El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob Cardiol Sci Pract. 2013;2013:38. https://doi.org/10.5339/gcsp.2013.38.
Huang S, Yang Y, Yang Q, Zhao Q, Ye X. Engineered circulatory scaffolds for building cardiac tissue. J Thorac Dis. 2018;10:S2312. https://doi.org/10.21037/jtd.2017.12.92.
Schaaf S, Shibamiya A, Mewe M, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6:e26397. https://doi.org/10.1371/journal.pone.0026397.
Mannhardt I, Breckwoldt K, Letuffe-Brenière D, et al. Human engineered heart tissue: analysis of contractile force. Stem Cell Reports. 2016;7:29. https://doi.org/10.1016/j.stemcr.2016.04.011.
Tiburcy M, Hudson JE, Balfanz P, et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 2017;135:1832. https://doi.org/10.1161/CIRCULATIONAHA.116.024145.
Zhang YS, Aleman J, Arneri A, Bersini S, Piraino F, Shin SR, Dokmeci MR, Khademhosseini A. From cardiac tissue engineering to heart-on-a-chip: beating challenges. Biomed Mater. 2015;10:034006. https://doi.org/10.1088/1748-6041/10/3/034006.
Qian F, Huang C, Lin YD, et al. Simultaneous electrical recording of cardiac electrophysiology and contraction on chip. Lab Chip. 2017;17:1732. https://doi.org/10.1039/c7lc00210f.
Kitsara M, Kontziampasis D, Agbulut O, Chen Y. Heart on a chip: micro-nanofabrication and microfluidics steering the future of cardiac tissue engineering. Microelectron Eng. 2019;203-204:44. https://doi.org/10.1016/j.mee.2018.11.001.
Noble D. A modification of the Hodgkin—Huxley equations applicable to Purkinje fibre action and pacemaker potentials. J Physiol. 1962;160:317. https://doi.org/10.1113/jphysiol.1962.sp006849.
O’Hara T, Virág L, Varró A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol. 2011;7:e1002061. https://doi.org/10.1371/journal.pcbi.1002061.
Yarov-Yarovoy V, Allen TW, Clancy CE. Computational models for predictive cardiac ion channel pharmacology. Drug Discov Today Dis Model. 2014;14:3. https://doi.org/10.1016/j.ddmod.2014.04.001.
Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004;110:3168. https://doi.org/10.1161/01.CIR.0000147231.69595.D3.
Zhang Y, Barocas VH, Berceli SA, et al. Multi-scale modeling of the cardiovascular system: disease development, progression, and clinical intervention. Ann Biomed Eng. 2016;44:2642. https://doi.org/10.1007/s10439-016-1628-0.
Abriel H, de Lange E, Kucera JP, Loussouarn G, Tarek M. Computational tools to investigate genetic cardiac channelopathies. Front Physiol. 2013;4:390. https://doi.org/10.3389/fphys.2013.00390.
Musgaard M, Paramo T, Domicevica L, Andersen OJ, Biggin PC. Insights into channel dysfunction from modelling and molecular dynamics simulations. Neuropharmacology. 2018;132:20. https://doi.org/10.1016/j.neuropharm.2017.06.030.
Jafri MS. Models of excitation-contraction coupling in cardiac ventricular myocytes. Methods Mol Biol. 2012;910:309–35. https://doi.org/10.1007/978-1-61779-965-5_14.
Bell MM, Cherry EM. Computational cardiac electrophysiology: implementing mathematical models of cardiomyocytes to simulate action potentials of the heart. Methods Mol Biol. 2015;1299:65–74. https://doi.org/10.1007/978-1-4939-2572-8_5.
Kheyfets VO, Rios L, Smith T, et al. Patient-specific computational modeling of blood flow in the pulmonary arterial circulation. Comput Methods Prog Biomed. 2015;120:88. https://doi.org/10.1016/j.cmpb.2015.04.005.
Trayanova NA. Computational cardiology: the heart of the matter. ISRN Cardiol. 2012;2012:1. https://doi.org/10.5402/2012/269680.
Lopez-Perez A, Sebastian R, Izquierdo M, Ruiz R, Bishop M, Ferrero JM. Personalized cardiac computational models: from clinical data to simulation of infarct-related ventricular tachycardia. Front Physiol. 2019;10:580. https://doi.org/10.3389/fphys.2019.00580.
Szopos M, Poussineau N, Maday Y, Canniffe C, Celermajer DS, Bonnet D, Ou P. Computational modeling of blood flow in the aorta – insights into eccentric dilatation of the ascending aorta after surgery for coarctation. J Thorac Cardiovasc Surg. 2014;148:1572. https://doi.org/10.1016/j.jtcvs.2013.11.055.
Mayourian J, Sobie EA, Costa KD. An introduction to computational modeling of cardiac electrophysiology and arrhythmogenicity. Methods Mol Biol. 2018;1816:17–35. https://doi.org/10.1007/978-1-4939-8597-5_2.
Pathmanathan P, Gray RA. Validation and trustworthiness of multiscale models of cardiac electrophysiology. Front Physiol. 2018;9:106. https://doi.org/10.3389/fphys.2018.00106.
Chi KR. Revolution dawning in cardiotoxicity testing. Nat Rev Drug Discov. 2013;12:565. https://doi.org/10.1038/nrd4083.
Gintant G, Sager PT, Stockbridge N. Evolution of strategies to improve preclinical cardiac safety testing. Nat Rev Drug Discov. 2016;15:457. https://doi.org/10.1038/nrd.2015.34.
Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011;51:288. https://doi.org/10.1016/j.yjmcc.2011.06.012.
Di Diego JM, Sicouri S, Myles RC, et al. Optical and electrical recordings from isolated coronary-perfused ventricular wedge preparations. J Mol Cell Cardiol. 2013;54:53–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lin, Z., George, S.A. (2021). Newer Models of Cardiac Tissue. In: Efimov, I.R., Ng, F.S., Laughner, J.I. (eds) Cardiac Bioelectric Therapy. Springer, Cham. https://doi.org/10.1007/978-3-030-63355-4_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-63355-4_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63354-7
Online ISBN: 978-3-030-63355-4
eBook Packages: MedicineMedicine (R0)