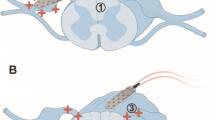
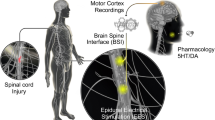
Abstract
After SCI, deficits of motor, sensory, and autonomic functions are commensurate with injury severity and level (Jaja et al. 2019; Failli et al. 2012). Advances in clinical care have reduced morbidities, increased survival, and improved neurological recovery after SCI (Freed et al. 1966; Stauffer 1975; Closson et al. 1991; Badhiwala et al. 2021). These advances have shifted the focus from complication management to improved life quality and independence with increasingly greater emphasis on recovery. For this review, by recovery, we specifically mean a measurable enduring improvement linked to improved spinal cord circuit function. This review does not focus on technological substitution of function using bypass technology such as a brain-machine interface, as covered in other chapters. Rather, we wish to discuss methods by which additional function can be obtained from limited residual connections.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Adams MM, Hicks AL (2005) Spasticity after spinal cord injury. Spinal Cord 43(10):577–586
Aimetti AA et al (2019) Natural history of neurological improvement following complete (AIS A) thoracic spinal cord injury across three registries to guide acute clinical trial design and interpretation. Spinal Cord 57(9):753–762
Angeli CA et al (2014) Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137(5):1394–1409
Aslan SC et al (2018) Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury-induced cardiovascular deficits. Front Physiol 9:565
Badhiwala JH et al (2021) The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol 20(2):117–126
Baker SN, Perez MA (2017) Reticulospinal contributions to gross hand function after human spinal cord injury. J Neurosci 37(40):9778–9784
Bareyre FM et al (2004) The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7(3):269–277
Behrman AL, Bowden MG, Nair PM (2006) Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther 86(10):1406–1425
Behrman AL, Ardolino EM, Harkema SJ (2017) Activity-based therapy: from basic science to clinical application for recovery after spinal cord injury. J Neurol Phys Ther 41(Suppl 3):S39–S45
Belanger M et al (2000) Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 81(8):1090–1098
Brown JO, McCouch GP (1947) Abortive regeneration of the transected spinal cord. J Comp Neurol 87(2):131–137
Bui TV et al (2016) Spinal microcircuits comprising dI3 interneurons are necessary for motor functional recovery following spinal cord transection. eLife 5:e21715
Bussel B et al (1988) Myoclonus in a patient with spinal cord transection: possible involvement of the spinal stepping generator. Brain 111(5):1235–1245
Calancie B (1991) Interlimb reflexes following cervical spinal cord injury in man. Exp Brain Res 85(2):458–469
Calancie B (2006) Spinal myoclonus after spinal cord injury. J Spinal Cord Med 29(4):413–424
Calancie B et al (1994) Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain 117(Pt 5):1143–1159
Calancie B, Lutton S, Broton JG (1996) Central nervous system plasticity after spinal cord injury in man: interlimb reflexes and the influence of cutaneous stimulation. Electroencephalogr Clin Neurophysiol 101(4):304–315
Calvert JS et al (2019) Emergence of epidural electrical stimulation to facilitate sensorimotor network functionality after spinal cord injury. Neuromodulation 22(3):244–252
Campos RJ et al (1987) Epidural spinal cord stimulation in spastic spinal cord injury patients. Appl Neurophysiol 50(1–6):453–454
Cardenas DD et al (2007) Phase 2 trial of sustained-release fampridine in chronic spinal cord injury. Spinal Cord 45(2):158–168
Cardenas DD et al (2014) Two phase 3, multicenter, randomized, placebo-controlled clinical trials of fampridine-SR for treatment of spasticity in chronic spinal cord injury. Spinal Cord 52(1):70–76
Ceto S et al (2020) Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after spinal cord injury. Cell Stem Cell 27(3):430–440.e5
Chau C, Barbeau H, Rossignol S (1998) Effects of intrathecal alpha(1)- and alpha(2)-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol 79(6):2941–2963
Chen MS et al (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403(6768):434–439
Chen B et al (2018) Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell 174(6):1599
Cioni B et al (1986) Voluntary supraspinal suppression of spinal reflex activity in paralyzed muscles of spinal cord injury patients. Exp Neurol 93(3):574–583
Closson JB et al (1991) Rehabilitation in spinal cord disorders. 3. Comprehensive management of spinal cord injury. Arch Phys Med Rehabil 72(4-S):S298–S308
Cohen J (1992) A power primer. Psychol Bull 112(1):155–159
Courtine G et al (2008) Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14(1):69–74
Courtine G et al (2009) Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12(10):1333–U167
Cowley KC, Zaporozhets E, Schmidt BJ (2008) Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol 586(6):1623–1635
Crone SA et al (2009) In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci 29(21):7098–7109
Curtis E et al (2018) A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell 22(6):941–950.e6
Darrow D et al (2019) Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma 36(15):2325–2336
de Leon RD et al (1998) Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol 79(3):1329–1340
Dietz V, Colombo G, Jensen L (1994) Locomotor activity in spinal man. Lancet 344(8932):1260–1263
Dimitrijević MR (1988) Residual motor functions in spinal cord injury. Adv Neurol 47:138–155
Dimitrijevic MR et al (1983) Motor control in man after partial or complete spinal cord injury. Adv Neurol 39:915–926
Dimitrijevic MR et al (1984) Suprasegmentally induced motor unit activity in paralyzed muscles of patients with established spinal cord injury. Ann Neurol 16(2):216–221
Dimitrijevic MR et al (1987) Epidural spinal cord stimulation and carry-over effect in chronic spinal cord injury patients. Stereotact Funct Neurosurg 50(1–6):449–450
Dimitrijevic MR, Gerasimenko Y, Pinter MM (1998a) Evidence for a spinal central pattern generator in humans. In: Kiehn O et al (eds) Neuronal mechanisms for generating locomotor activity. New York Acad Sciences, New York, pp 360–376
Dimitrijevic MR, Gerasimenko Y, Pinter MM (1998b) Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci 860(1):360–376
Dougherty KJ et al (2013) Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 80(4):920–933
Dubner R, Ruda MA (1992) Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci 15(3):96–103
Eccles JC (1976) The plasticity of the mammalian central nervous system with special reference to new growths in response to lesions. Naturwissenschaften 63(1):8–15
Edgerton VR et al (2001a) Retraining the injured spinal cord. J Physiol 533(1):15–22
Edgerton VR, Roy RR, de Leon RD (2001b) Neural Darwinism in the mammalian spinal cord. In: Patterson MM, Grau JW (eds) Spinal cord plasticity: alterations in reflex function. Springer, Boston, MA, pp 185–206
Edgerton VR et al (2008) Training locomotor networks. Brain Res Rev 57(1):241–254
Erb DE, Mora RJ, Bunge RP (1993) Reinnervation of adult rat gastrocnemius muscle by embryonic motoneurons transplanted into the axotomized tibial nerve. Exp Neurol 124(2):372–376
Failli V et al (2012) Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain 135(11):3238–3250
Fehlings MG et al (2018) Rho inhibitor VX-210 in acute traumatic subaxial cervical spinal cord injury: design of the SPinal Cord Injury Rho INhibition InvestiGation (SPRING) clinical trial. J Neurotrauma 35(9):1049–1056
Feigin I, Geller EH, Wolf A (1951) Absence of regeneration in the spinal cord of the young rat. J Neuropathol Exp Neurol 10(4):420–425
Filli L et al (2014) Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci 34(40):13399–13410
Flynn JR et al (2011) The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 60(5):809–822
Forssberg H et al (1980) The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand 108(3):283–295
Freed MM, Bakst HJ, Barrie DL (1966) Life expectancy, survival rates, and causes of death in civilian patients with spinal cord trauma. Arch Phys Med Rehabil 47(7):457–463
Fung J, Stewart JE, Barbeau H (1990) The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal-cord injured subjects. J Neurol Sci 100(1–2):85–93
Gad P et al (2018) Non-invasive activation of cervical spinal networks after severe paralysis. J Neurotrauma 35(18):2145–2158
Gallien P et al (1995) Restoration of gait by functional electrical stimulation for spinal cord injured patients. Paraplegia 33(11):660–664
Geisler FH et al (2001) The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976) 26(24 Suppl):S87–S98
Gerasimenko YP et al (2007) Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol 98(5):2525–2536
Gerasimenko YP et al (2015) Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma 32(24):1968–1980
Gill ML et al (2018) Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 24(11):1677–1682
Gottlieb GL et al (1985) Evaluation of cervical stimulation for chronic treatment of spasticity. Neurology 35(5):699–704
Goulding MJNRN (2009) Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10(7):507–518
Green JB et al (1999) Cortical motor reorganization after paraplegia: an EEG study. Neurology 53(4):736–743
Grillner S (2006) Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52(5):751–766
Grillner S, Jessell TM (2009) Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19(6):572–586
Grumbles RM et al (2013) Acute stimulation of transplanted neurons improves motoneuron survival, axon growth, and muscle reinnervation. J Neurotrauma 30(12):1062–1069
Guertin PA (2009) The mammalian central pattern generator for locomotion. Brain Res Rev 62(1):45–56
Hägglund M et al (2013) Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl Acad Sci U S A 110(28):11589–11594
Hansebout RR et al (1993) 4-Aminopyridine in chronic spinal cord injury: a controlled, double-blind, crossover study in eight patients. J Neurotrauma 10(1):1–18
Harkema SJ et al (1997) Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 77(2):797–811
Harkema S et al (2011) Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377(9781):1938–1947
Harkema SJ et al (2012) Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil 93(9):1508–1517
Harkema SJ et al (2018) Normalization of blood pressure with spinal cord epidural stimulation after severe spinal cord injury. Front Hum Neurosci 12:83
Hebb DO (2005) The organization of behavior: a neuropsychological theory. Psychology Press, New York
Herrity AN et al (2018) Lumbosacral spinal cord epidural stimulation improves voiding function after human spinal cord injury. Sci Rep 8(1):8688
Hoshimiya N et al (1989) A multichannel FES system for the restoration of motor functions in high spinal cord injury patients: a respiration-controlled system for multijoint upper extremity. IEEE Trans Biomed Eng 36(7):754–760
Hugenholtz H et al (1988) Cervical spinal cord stimulation for spasticity in cerebral palsy. Neurosurgery 22(4):707–714
Inanici F et al (2018) Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng 26(6):1272–1278
Inanici F et al (2021) Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans Neural Syst Rehabil Eng 29:310–319
Jacobson PB et al (2021) Elezanumab, a human anti-RGMa monoclonal antibody, promotes neuroprotection, neuroplasticity, and neurorecovery following a thoracic hemicompression spinal cord injury in non-human primates. Neurobiol Dis 155:105385
Jain N, Catania KC, Kaas JH (1997) Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature 386(6624):495–498
Jain N et al (2000) Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci U S A 97(10):5546–5550
Jaja BNR et al (2019) Association of pneumonia, wound infection, and sepsis with clinical outcomes after acute traumatic spinal cord injury. J Neurotrauma 36(21):3044–3050
Järvinen TAH et al (2002) Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. J Muscle Res Cell Motil 23(3):245–254
Jones EG (2000) Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex. Annu Rev Neurosci 23:1–37
Jones LA et al (2010) A phase 2 autologous cellular therapy trial in patients with acute, complete spinal cord injury: pragmatics, recruitment, and demographics. Spinal Cord 48(11):798–807
Kemler MA et al (2000) Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med 343(9):618–624
Kern H et al (2002) Denervated muscles in humans: limitations and problems of currently used functional electrical stimulation training protocols. Artif Organs 26(3):216–218
Kiehn O (2011) Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol 21(1):100–109
Kiehn O (2016) Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17(4):224
Kirshblum S et al (2021) Characterizing natural recovery after traumatic spinal cord injury. J Neurotrauma
Klose KJ et al (1997) Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 1. Ambulation performance and anthropometric measures. Arch Phys Med Rehabil 78(8):789–793
Knutson JS et al (2002) Electrode fracture rates and occurrences of infection and granuloma associated with percutaneous intramuscular electrodes in upper-limb functional electrical stimulation applications. J Rehabil Res Dev 39(6):671–683
Krassioukov A, Claydon VE (2006) The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res 152:223–229
Kucher K et al (2018) First-in-man intrathecal application of neurite growth-promoting anti-Nogo-A antibodies in acute spinal cord injury. Neurorehabil Neural Repair 32(6–7):578–589
Kwon BK et al (2010) Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma 27(4):669–682
Laliberte AM et al (2019) Propriospinal neurons: essential elements of locomotor control in the intact and possibly the injured spinal cord. Front Cell Neurosci 13:512
Lance JW (1980) The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 30:1303–1313
Levy WJ Jr et al (1990) Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Res 510(1):130–134
Lovely RG et al (1986) Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol 92(2):421–435
Malisoux L et al (2007) Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol 102(1):340–349
Martinez M, Rossignol S (2011) Changes in CNS structures after spinal cord lesions: implications for BMI. In: Schouenborg J, Garwicz M, Danielsen N (eds) Brain machine interfaces: implications for science, clinical practice and society. Elsevier Science Bv, Amsterdam, pp 191–202
Martinez M et al (2012) Effect of locomotor training in completely spinalized cats previously submitted to a spinal hemisection. J Neurosci 32(32):10961
May Z et al (2017) Following spinal cord injury transected reticulospinal tract axons develop new collateral inputs to spinal interneurons in parallel with locomotor recovery. Neural Plast 2017:1932875
Meglio M et al (1980) Epidural spinal cord stimulation for the treatment of neurogenic bladder. Acta Neurochir 54(3–4):191–199
Melzack R, Wall PDJS (1965) Pain mechanisms: a new theory. Science 150(3699):971–979
Merletti R, Knaflitz M, De Luca CJ (1992) Electrically evoked myoelectric signals. Crit Rev Biomed Eng 19(4):293–340
Minassian K et al (2004) Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42(7):401–416
Mirbagheri MM et al (2002) The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. IEEE Trans Neural Syst Rehabil Eng 10(4):280–289
Moe JH, Post HW (1962) Functional electrical stimulation for ambulation in hemiplegia. J Lancet 82:285–288
Morrison SA et al (2012) NeuroRecovery Network provides standardization of locomotor training for persons with incomplete spinal cord injury. Arch Phys Med Rehabil 93(9):1574–1577
Morrison SA et al (2018) Longitudinal recovery and reduced costs after 120 sessions of locomotor training for motor incomplete spinal cord injury. Arch Phys Med Rehabil 99(3):555–562
Mortimer JT, Shealy CN, Wheeler C (1970) Experimental nondestructive electrical stimulation of the brain and spinal cord. J Neurosurg 32(5):553–559
Mulcahey MJ et al (2004) Implantation of the freehand system during initial rehabilitation using minimally invasive techniques. Spinal Cord 42(3):146–155
Nadeau S et al (2010) Spontaneous motor rhythms of the back and legs in a patient with a complete spinal cord transection. Neurorehabil Neural Repair 24(4):377–383
Olsson MC et al (2006) Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J Physiol 577(Pt 1):339–352
Orlovskii GN, Fel'dman AG (1972) Classification of lumbosacral neurons by their discharge pattern during evoked locomotion. Neurophysiology 4(4):311–317
Ottersen OP, Helm PJ (2002) How hardwired is the brain? Nature 420(6917):751–752
Peckham PH (1987) Functional electrical stimulation: current status and future prospects of applications to the neuromuscular system in spinal cord injury. Paraplegia 25(3):279–288
Peckham PH, Knutson JS (2005) Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng 7:327–360
Peckham PH, Mortimer JT, Marsolais EB (1976) Upper and lower motor neuron lesions in the upper extremity muscles of tetraplegics. Paraplegia 14(2):115–121
Penn RD et al (1989) Intrathecal baclofen for severe spinal spasticity. N Engl J Med 320(23):1517–1521
Peschanski M, Leforestier N, Rapisardi S (1993) Neuroplasticity as a basis for therapeutics in spinal-cord injuries and diseases. Restor Neurol Neurosci 5(1):87–97
Pinter MM, Gerstenbrand F, Dimitrijevic MR (2000) Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord 38(9):524–531
Prentice SD (1999) Biological neural networks: hierarchical concept of brain function. Konstantin V Baev 74(2):247–247
Prochazka A et al (1997) The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil 78(6):608–614
Raineteau O, Schwab ME (2001) Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2(4):263–273
Rejc E et al (2017a) Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci Rep 7(1):13476
Rejc E et al (2017b) Effects of stand and step training with epidural stimulation on motor function for standing in chronic complete paraplegics. J Neurotrauma 34(9):1787–1802
Remy-Neris O et al (1999) Effects of intrathecal clonidine injection on spinal reflexes and human locomotion in incomplete paraplegic subjects. Exp Brain Res 129(3):433–440
Richardson RR, McLone DG (1978) Percutaneous epidural neuro-stimulation for paraplegic spasticity. Surg Neurol 9(3):153–155
Richardson RR et al (1979) Percutaneous epidural neurostimulation in modulation of paraplegic spasticity. Six case reports. Acta Neurochir (Wien) 49(3–4):235–243
Rosenzweig ES et al (2010) Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci 13(12):1505–1510
Rosenzweig ES et al (2019) Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat Neurosci 22(8):1269–1275
Rossignol S, Dubuc R, Gossard JP (2006) Dynamic sensorimotor interactions in locomotion. Physiol Rev 86(1):89–154
Roy RR, Acosta L Jr (1986) Fiber type and fiber size changes in selected thigh muscles six months after low thoracic spinal cord transection in adult cats: exercise effects. Exp Neurol 92(3):675–685
Roy RR et al (1998) Training effects on soleus of cats spinal cord transected (T12–13) as adults. Muscle Nerve 21(1):63–71
Roy RR et al (1999) Differential response of fast hindlimb extensor and flexor muscles to exercise in adult spinalized cats. Muscle Nerve 22(2):230–241
Sagi Y et al (2012) Learning in the fast lane: new insights into neuroplasticity. Neuron 73(6):1195–1203
Sangari S, Perez MA (2019) Imbalanced corticospinal and reticulospinal contributions to spasticity in humans with spinal cord injury. J Neurosci 39(40):7872–7881
Sangari S et al (2019) Residual descending motor pathways influence spasticity after spinal cord injury. Ann Neurol 86(1):28–41
Sangari S et al (2021) Distinct patterns of spasticity and corticospinal connectivity following complete spinal cord injury. J Physiol
Santamaria AJ et al (2020) Neurophysiological changes in the first year after cell transplantation in sub-acute complete paraplegia. Front Neurol 11:514181
Schleip R et al (2006) Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med Hypotheses 66(1):66–71
Shealy CN, Mortimer JT, Reswick JB (1967) Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg 46(4):489–491
Sherrington CS, Laslett EE (1903) Observations on some spinal reflexes and the interconnection of spinal segments. J Physiol 29(1):58–96
Sherwood AM, Dimitrijevic MR, McKay WB (1992) Evidence of subclinical brain influence in clinically complete spinal-cord injury—discomplete SCI. J Neurol Sci 110(1–2):90–98
Stauffer ES (1975) Spinal cord injuries. Clin Orthop Relat Res 112:2–3
Stein RB, Mushahwar V (2005) Reanimating limbs after injury or disease. Trends Neurosci 28(10):518–524
Tabary JC et al (1972) Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol 224(1):231–244
Tanadini LG et al (2014) Identifying homogeneous subgroups in neurological disorders: unbiased recursive partitioning in cervical complete spinal cord injury. Neurorehabil Neural Repair 28(6):507–515
Tigchelaar S et al (2019) MicroRNA biomarkers in cerebrospinal fluid and serum reflect injury severity in human acute traumatic spinal cord injury. J Neurotrauma 36(15):2358–2371
Topka H et al (1991) Reorganization of corticospinal pathways following spinal cord injury. Neurology 41(8):1276–1283
Vidalsanz M et al (1987) Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion-cells in the adult-rat. J Neurosci 7(9):2894–2909
Wagner FB et al (2018) Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563(7729):65–71
Wang X et al (2020) Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury. Brain 143(6):1697–1713
Weidner N et al (2001) Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A 98(6):3513–3518
Weiss P (1941) Autonomous versus reflexogenous activity of the central nervous system. Proc Am Phil Soc 84(1):53–64
Wernig A et al (1995) Laufband therapy based on rules of spinal locomotion is effective in spinal-cord injured persons. Eur J Neurosci 7(4):823–829
Wirth ED 3rd et al (2001) Feasibility and safety of neural tissue transplantation in patients with syringomyelia. J Neurotrauma 18(9):911–929
Wirz M et al (2005) Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil 86(4):672–680
Wolpaw JR (2018) The negotiated equilibrium model of spinal cord function. J Physiol 596(16):3469–3491
Yokoyama H et al (2016) Distinct sets of locomotor modules control the speed and modes of human locomotion. Sci Rep 6:14
Zhang J et al (2014) V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 82(1):138–150
Zholudeva LV et al (2018) The neuroplastic and therapeutic potential of spinal interneurons in the injured spinal cord. Trends Neurosci 41(9):625–639
Acknowledgments
The ultrasound data were collected at the University of Louisville within the SCI neuromodulation program. Data was analyzed by J Guest at the University of Miami. Specific contributors are Drs. Susan Harkema, Jill Wecht, Alex Ovechkin, and Bonnie Legg, Jessie Fisher, and Shelly Wade.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Santamaria, A.J., Saraiva, P.M., Chang, S.J., Opris, I., Noga, B.R., Guest, J.D. (2021). Spinal Cord Injury and Epidural Spinal Cord Stimulation. In: Opris, I., A. Lebedev, M., F. Casanova, M. (eds) Modern Approaches to Augmentation of Brain Function. Contemporary Clinical Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-030-54564-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-54564-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54563-5
Online ISBN: 978-3-030-54564-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)