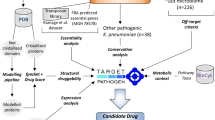
Abstract
This review focuses on efforts toward target prioritization from several pathogenic bacteria applying integrated multi-omics approaches. In an integrative scheme, diverse layers of multi-omics data, genome-scale models (GSMs), and structural/functional data related to any pathogenic species can be used to prioritize genes and proteins with attractive target characteristics for the development of new antimicrobials agents. The reconstruction of genome-scale metabolic models (GSMMs) and transcriptional regulatory networks (TRNs) is described in detail. Also, we discuss the methods for the integration of GSMs and diverse web servers for drug targeting in pathogens. Structural approaches are also illustrated. We stress the clinical importance of the drug-resistant isolates related to severe nosocomial or community infections belonging to the species Klebsiella pneumoniae and Staphylococcus aureus, two of the six ESKAPE pathogens, as well as Mycobacterium tuberculosis, the causative agent of tuberculosis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AMR:
-
Antimicrobial resistance
- BEC:
-
Brazilian Epidemic Clone
- BIGG:
-
Biochemical, Genetic and Genomic knowledge base
- CAI:
-
Community-acquired infections
- CA-MRSA:
-
Community-associated MRSA strains
- cis-RE:
-
cis-regulatory elements
- CCR:
-
Carbon Catabolite Repression
- ChIP-seq:
-
Chromatin immunoprecipitation followed by sequencing
- CG:
-
Clonal group
- COBRA:
-
Constraint-based reconstruction and analysis (COBRA), a wide class of methods to analyze possible flux distributions in metabolic network
- CP:
-
Choke-points
- CRKp:
-
carbapenem-resistant Klebsiella pneumoniae
- CRISPR-Cas:
-
Clustered Regularly Interspaced Short Palindromic Repeats
- CWD:
-
Crystallized With Drugs
- D:
-
Druggable
- DBD:
-
Domain-Based Druggable
- DCW:
-
Dry Cell Weight
- DS:
-
Druggability Score
- ESKAPE:
-
acronym encompassing the names of six bacterial pathogens commonly associated with antimicrobial resistance belonging to species Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp
- FBA:
-
Flux Balance Analysis
- FVA:
-
Flux Variability Analysis
- GCNs:
-
Gene Co-expression Networks
- GENRE:
-
Genome-Scale Reconstruction
- GIMME:
-
Gene Inactivity Moderated by Metabolism and Expression
- GIM3E:
-
Gene Inactivation Moderated by Metabolism, Metabolomics and Expression
- GPRs:
-
Gene–Protein–Reactions
- GSM:
-
Genome-scale Model also referred to as GEM
- GSMM :
-
Genome-scale Metabolic Model also referred to as GEMM
- GRN:
-
Gene Regulatory Network also referred to as TRN
- HA-MRSA:
-
Healthcare-associated Methicillin-Resistant Staphylococcus aureus
- HAIs:
-
Healthcare-associated infections
- HD:
-
Highly Druggable
- HIV:
-
Human Immunodeficiency Virus
- HMM:
-
Hidden Markov Models
- iFBA:
-
integrated Flux Balance Analysis
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- KL:
-
K-Locus, referred to as in silico predicted capsular serotype
- KPC:
-
Klebsiella pneumoniae carbapenemase, also referred to as KPC-2
- Kp13-MN:
-
Kp13 metabolic network
- LPS:
-
Lipopolysaccharide
- mAb:
-
monoclonal antibody
- MADE:
-
Metabolic Adjustment by Differential Expression
- MDR:
-
Multidrug-resistant, an organism resistant to multiple drugs
- MRSA:
-
Methicillin-Resistant Staphylococcus aureus
- MSSA:
-
Methicillin-Susceptible Staphylococcus aureus
- MTB:
-
Mycobacterium tuberculosis
- Mtb-MN:
-
Mycobacterium tuberculosis metabolic network
- mRNA:
-
messenger RNA
- PDB:
-
Protein Data Bank
- PB:
-
Polymyxin B
- PFAM:
-
Protein Family Database
- PROM:
-
Probabilistic Regulation Of Metabolism
- PWM:
-
Position Weight Matrix
- rFBA:
-
regulated Flux Balance Analysis
- RNAi:
-
RNA interference
- RNA-seq:
-
RNA sequencing
- RNOS:
-
Reactive Nitrogen and Oxygen Species
- RSA:
-
Regulatory Steady-state Analysis
- SBML:
-
Systems Biology Markup Language
- SBML-qual:
-
Systems Biology Markup Language Qualitative
- ST:
-
Sequence Type
- SR-FBA:
-
Steady-State Regulated Flux Balance Analysis
- TB:
-
Tuberculosis
- TF:
-
Transcription Factor
- TP:
-
Target-Pathogen database
- TFBS:
-
Transcription Factor Binding Site
- TRN:
-
Transcriptional Regulatory Network
- TU:
-
Transcription Unit
- WGCNA:
-
Weighted Gene Co-expression Network Analysis
- WHO:
-
World Health Organization’s
References
Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10:S122–S129
New report calls for urgent action to avert antimicrobial resistance crisis, 11 Jun 2019. http://www.who.int/news-room/detail/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis
Wenzel RP (2004) The antibiotic pipeline—challenges, costs, and values. N Engl J Med 523–526. http://dx.doi.org/10.1056/nejmp048093
Blundell TL, Sibanda BL, Montalvão RW, Brewerton S, Chelliah V, Worth CL et al (2006) Structural biology and bioinformatics in drug design: opportunities and challenges for target identification and lead discovery. Philos Trans R Soc Lond B Biol Sci 361:413–423
Pires DP, Cleto S, Sillankorva S, Azeredo J, Lu TK (2016) Genetically engineered phages: a review of advances over the last decade. Microbiol Mol Biol Rev 80:523–543
Ekins S, Freundlich JS (2013) Computational models for tuberculosis drug discovery. Methods Mol Biol 993:245–262
Galizzi J-P, Lockhart BP, Bril A (2013) Applying systems biology in drug discovery and development. Drug Metabol Drug Interact 28:67–78
Radusky LG, Hassan S, Lanzarotti E, Tiwari S, Jamal S, Ali J et al (2015) An integrated structural proteomics approach along the druggable genome of Corynebacterium pseudotuberculosis species for putative druggable targets. BMC Genom 16(Suppl 5):S9
Defelipe LA, Do Porto DF, Pereira Ramos PI, Nicolás MF, Sosa E, Radusky L et al (2016) A whole genome bioinformatic approach to determine potential latent phase specific targets in Mycobacterium tuberculosis. Tuberculosis. 97:181–192
Kaur D, Kutum R, Dash D, Brahmachari SK (2017) Data intensive genome level analysis for identifying novel, non-toxic drug targets for multi drug resistant mycobacterium tuberculosis. Sci Rep. 7:46595
Wadood A, Ghufran M, Khan A, Azam SS, Uddin R, Waqas M, et al (2017) The methicillin-resistant S. epidermidis strain RP62A genome mining for potential novel drug targets identification. Gene Rep 8:88–93
Uddin R, Jamil F (2018) Prioritization of potential drug targets against P. aeruginosa by core proteomic analysis using computational subtractive genomics and Protein-Protein interaction network. Comput Biol Chem 115–122. http://dx.doi.org/10.1016/j.compbiolchem.2018.02.017
Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, et al (2011) Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother 5134–5142. http://dx.doi.org/10.1128/aac.05028-11
Bergen PJ, Bulman ZP, Landersdorfer CB, Smith N, Lenhard JR, Bulitta JB et al (2015) Optimizing polymyxin combinations against resistant gram-negative bacteria. Infect Dis Ther 4:391–415
Wang Q, Chang C-S, Pennini M, Pelletier M, Rajan S, Zha J et al (2016) Target-agnostic identification of functional monoclonal antibodies against klebsiella pneumoniae multimeric MrkA fimbrial subunit. J Infect Dis 213:1800–1808
Szijártó V, Guachalla LM, Visram ZC, Hartl K, Varga C, Mirkina I et al (2015) Bactericidal monoclonal antibodies specific to the lipopolysaccharide O antigen from multidrug-resistant Escherichia coli clone ST131-O25b:H4 elicit protection in mice. Antimicrob Agents Chemother 59:3109–3116
Szijártó V, Nagy E, Nagy G (2018) Directly bactericidal anti-escherichia coli antibody. Trends Microbiol 642–644
Storek KM, Auerbach MR, Shi H, Garcia NK, Sun D, Nickerson NN et al (2018) Monoclonal antibody targeting the β-barrel assembly machine of Escherichia coli is bactericidal. Proc Natl Acad Sci U S A 115:3692–3697
Steiner B, Swart AL, Hilbi H (2019) Perturbation of legionella cell infection by RNA interference. Methods Mol Biol 1921:221–238
de la Fuente-Núñez C, Lu TK (2017) CRISPR-Cas9 technology: applications in genome engineering, development of sequence-specific antimicrobials, and future prospects. Integr Biol 9:109–122
Ligon BL (2004) Penicillin: its discovery and early development. Semin Pediatr Infect Dis 15:52–57
Brown ED, Wright GD (2016) Antibacterial drug discovery in the resistance era. Nature 336–343. http://dx.doi.org/10.1038/nature17042
Wright GD (2012) Antibiotics: a new hope. Chem Biol 19:3–10
Kolter R, van Wezel GP (2016) Goodbye to brute force in antibiotic discovery? Nat Microbiol 1:15020
Reymond J-L, van Deursen R, Blum LC, Ruddigkeit L (2010) Chemical space as a source for new drugs. Med Chem Comm 30. http://dx.doi.org/10.1039/c0md00020e
Selzer PM, Brutsche S, Wiesner P, Schmid P, Müllner H (2000) Target-based drug discovery for the development of novel antiinfectives. Int J Med Microbiol 191–201. http://dx.doi.org/10.1016/s1438-4221(00)80090-9
Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6:29–40
Projan SJ (2003) Why is big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol 6:427–430
Hackbarth CJ, Chen DZ, Lewis JG, Clark K, Mangold JB, Cramer JA et al (2002) N-alkyl urea hydroxamic acids as a new class of peptide deformylase inhibitors with antibacterial activity. Antimicrob Agents Chemother 46:2752–2764
Flores A, Quesada E (2013) Entry inhibitors directed towards glycoprotein gp120: an overview on a promising target for HIV-1 therapy. Curr Med Chem 20:751–771
Farha MA, Leung A, Sewell EW, D’Elia MA, Allison SE, Ejim L et al (2013) Inhibition of WTA synthesis blocks the cooperative action of PBPs and sensitizes MRSA to β-lactams. ACS Chem Biol 8:226–233
Starkey M, Lepine F, Maura D, Bandyopadhaya A, Lesic B, He J, et al (2014) Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathogens e1004321. http://dx.doi.org/10.1371/journal.ppat.1004321
Feist AM, Palsson BØ (2008) The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli. Nat Biotechnol 26:659–667
Kim TY, Sohn SB, Kim YB, Kim WJ, Lee SY (2012) Recent advances in reconstruction and applications of genome-scale metabolic models. Curr Opin Biotechnol 23:617–623
Kim HU, Kim TY, Lee SY (2010) Genome-scale metabolic network analysis and drug targeting of multi-drug resistant pathogen Acinetobacter baumannii AYE. Mol BioSyst 6:339–348
Lewis NE, Schramm G, Bordbar A, Schellenberger J, Andersen MP, Cheng JK et al (2010) Large-scale in silico modeling of metabolic interactions between cell types in the human brain. Nat Biotechnol 28:1279–1285
Kim HU, Kim SY, Jeong H, Kim TY, Kim JJ, Choy HE et al (2011) Integrative genome-scale metabolic analysis of Vibrio vulnificus for drug targeting and discovery. Mol Syst Biol 7:460
Bidkhori G, Benfeitas R, Elmas E, Kararoudi MN, Arif M, Uhlen M et al (2018) Metabolic network-based identification and prioritization of anticancer targets based on expression data in hepatocellular carcinoma. Front Physiol 9:916
Thiele I, Palsson BØ (2010) A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat Protoc 5:93–121
Varma A, Palsson BO (1994) Metabolic Flux Balancing: Basic Concepts, Scientific and Practical Use. Nat Biotechnol 12:994–998
Seif Y, Kavvas E, Lachance J-C, Yurkovich JT, Nuccio S-P, Fang X et al (2018) Genome-scale metabolic reconstructions of multiple Salmonella strains reveal serovar-specific metabolic traits. Nat Commun 9:3771
Price ND, Papin JA, Schilling CH, Palsson BO (2003) Genome-scale microbial in silico models: the constraints-based approach. Trends Biotechnol 21:162–169
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K (2017) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45:D353–D361
Karp PD, Billington R, Caspi R, Fulcher CA, Latendresse M, Kothari A et al (2019) The BioCyc collection of microbial genomes and metabolic pathways. Brief Bioinform 20:1085–1093
Jassal B, Matthews L, Viteri G, Gong C, Lorente P, Fabregat A et al (2020) The reactome pathway knowledgebase. Nucleic Acids Res 48:D498–D503
Butt AM, Tahir S, Nasrullah I, Idrees M, Lu J, Tong Y (2012) Mycoplasma genitalium: a comparative genomics study of metabolic pathways for the identification of drug and vaccine targets. Infect Genet Evol 12:53–62
Shanmugham B, Pan A (2013) Identification and characterization of potential therapeutic candidates in emerging human pathogen Mycobacterium abscessus: a novel hierarchical in silico approach. PLoS ONE 8:e59126
Belda E, Sekowska A, Le Fèvre F, Morgat A, Mornico D, Ouzounis C, et al (2013) An updated metabolic view of the Bacillus subtilis 168 genome. Microbiology 757–770. http://dx.doi.org/10.1099/mic.0.064691-0
Scaria J, Mao C, Chen J-W, McDonough SP, Sobral B, Chang Y-F (2013) Differential stress transcriptome landscape of historic and recently emerged hypervirulent strains of Clostridium difficile strains determined using RNA-seq. PLoS ONE 8:e78489
Machado D, Andrejev S, Tramontano M, Patil KR (2018) Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic Acids Res 46:7542–7553
Lacroix V, Cottret L, Thebault P, Sagot MF (2008) An introduction to metabolic networks and their structural analysis. IEEE/ACM Trans Comput Biol Bioinform 594–617. http://dx.doi.org/10.1109/tcbb.2008.79
Montañez R, Medina MA, Solé RV, Rodríguez-Caso C (2010) When metabolism meets topology: reconciling metabolite and reaction networks. BioEssays 32:246–256
Cottret L, Jourdan F (2010) Graph methods for the investigation of metabolic networks in parasitology. Parasitology 137:1393–1407
Jeong H, Mason SP, Barabási AL, Oltvai ZN (2001) Lethality and centrality in protein networks. Nature 41–42. http://dx.doi.org/10.1038/35075138
Ramos PIP, Arge LWP, Lima NCB, Fukutani KF, de Queiroz ATL (2019) Leveraging user-friendly network approaches to extract knowledge from high-throughput omics datasets. Front Genet 10:1120
Giuliani S, Silva ACE, Borba JVVB, Ramos PIP, Paveley RA, Muratov EN, et al (2018) Computationally-guided drug repurposing enables the discovery of kinase targets and inhibitors as new schistosomicidal agents. PLoS Comput Biol (no prelo)
Zhang M, Su S, Bhatnagar RK, Hassett DJ, Lu LJ (2012) Prediction and analysis of the protein interactome in Pseudomonas aeruginosa to enable network-based drug target selection. PLoS One 7:e41202
Watowich AF, Muskus S, Current C (2012) Advances in computational strategies for drug discovery in leishmaniasis. Curr Top Trop Med http://dx.doi.org/10.5772/28292
Ochoa R, Martínez-Pabón MC, Arismendi-Echeverri MA, Rendón-Osorio WL, Muskus-López CE (2017) In silico search of inhibitors of Streptococcus mutans for the control of dental plaque. Arch Oral Biol 83:68–75
Gupta SK, Gross R, Dandekar T (2016) An antibiotic target ranking and prioritization pipeline combining sequence, structure and network-based approaches exemplified for Serratia marcescens. Gene 591:268–278
Yeh I, Hanekamp T, Tsoka S, Karp PD, Altman RB (2004) Computational analysis of Plasmodium falciparum metabolism: organizing genomic information to facilitate drug discovery. Genome Res 14:917–924
Singh S, Malik BK, Sharma DK (2007) Choke point analysis of metabolic pathways in E. histolytica: a computational approach for drug target identification. Bioinformation 68–72 http://dx.doi.org/10.6026/97320630002068
Rahman SA, Schomburg D (2006) Observing local and global properties of metabolic pathways: “load points” and “choke points” in the metabolic networks. Bioinformatics 22:1767–1774
Jadhav A, Ezhilarasan V, Prakash Sharma O, Pan A (2013) Clostridium-DT(DB): a comprehensive database for potential drug targets of Clostridium difficile. Comput Biol Med 43:362–367
Sharma A, Pan A (2012) Identification of potential drug targets in Yersinia pestis using metabolic pathway analysis: MurE ligase as a case study. Eur J Med Chem 57:185–195
Gupta M, Prasad Y, Sharma SK, Jain CK (2017) Identification of phosphoribosyl-AMP cyclohydrolase, as drug target and its inhibitors in Brucella melitensis bv. 1 16 M using metabolic pathway analysis. J Biomol Struct Dyn 35:287–299
Ramos PIP, Fernández Do Porto D, Lanzarotti E, Sosa EJ, Burguener G, Pardo AM, et al (2018) An integrative, multi-omics approach towards the prioritization of Klebsiella pneumoniae drug targets. Sci Rep 8:10755
Heavner BD, Smallbone K, Barker B, Mendes P, Walker LP (2012) Yeast 5—an expanded reconstruction of the Saccharomyces cerevisiae metabolic network. BMC Syst Biol 55. http://dx.doi.org/10.1186/1752-0509-6-55
Schellenberger J, Park JO, Conrad TM, Palsson BØ (2010) BiGG: a biochemical genetic and genomic knowledgebase of large scale metabolic reconstructions. BMC Bioinform 11:213
Norsigian CJ, Pusarla N, McConn JL, Yurkovich JT, Dräger A, Palsson BO et al (2020) BiGG models 2020: multi-strain genome-scale models and expansion across the phylogenetic tree. Nucleic Acids Res 48:D402–D406
Raman K, Chandra N (2009) Flux balance analysis of biological systems: applications and challenges. Brief Bioinform 10:435–449
Lee JM, Gianchandani EP, Papin JA (2006) Flux balance analysis in the era of metabolomics. Brief Bioinform 7:140–150
Orth JD, Thiele I, Palsson BØ (2010) What is flux balance analysis? Nat Biotechnol 28:245–248
Lewis NE, Nagarajan H, Palsson BO (2012) Constraining the metabolic genotype-phenotype relationship using a phylogeny of in silico methods. Nat Rev Microbiol 10:291–305
Edwards JS, Palsson BO (2000) The Escherichia coli MG1655 in silico metabolic genotype: its definition, characteristics, and capabilities. Proc Natl Acad Sci U S A 97:5528–5533
Gudmundsson S, Thiele I (2010) Computationally efficient flux variability analysis. BMC Bioinform 11:489
Schellenberger J, Que R, Fleming RMT, Thiele I, Orth JD, Feist AM, et al (2011) Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox v2.0. Nat Protoc 6:1290–1307
Ebrahim A, Lerman JA, Palsson BO, Hyduke DR (2013) COBRApy: constraints-based reconstruction and analysis for python. BMC Syst Biol 7:74
Karlebach G, Shamir R (2008) Modelling and analysis of gene regulatory networks. Nat Rev Mol Cell Biol 9:770–780
Browning DF, Busby SJ (2004) The regulation of bacterial transcription initiation. Nat Rev Microbiol 2:57–65
Barabási A-L, Oltvai ZN (2004) Network biology: understanding the cell’s functional organization. Nat Rev Genet 5:101–113
Flores-Bautista E, Cronick CL, Fersaca AR, Martinez-Nuñez MA, Perez-Rueda E (2018) Functional prediction of hypothetical transcription factors of escherichia coli K-12 based on expression data. Comput Struct Biotechnol J 16:157–166
Gama-Castro S, Salgado H, Santos-Zavaleta A, Ledezma-Tejeida D, Muñiz-Rascado L, García-Sotelo JS, et al (2016) RegulonDB version 9.0: high-level integration of gene regulation, coexpression, motif clustering and beyond. Nucleic Acids Res 44:D133–D143
Moreno-Campuzano S, Janga SC, Pérez-Rueda E (2006) Identification and analysis of DNA-binding transcription factors in Bacillus subtilis and other Firmicutes–a genomic approach. BMC Genom 7:147
Kobayashi H, Akitomi J, Fujii N, Kobayashi K, Altaf-Ul-Amin M, Kurokawa K et al (2007) The entire organization of transcription units on the Bacillus subtilis genome. BMC Genom 8:197
Brune I, Brinkrolf K, Kalinowski J, Pühler A, Tauch A (2005) The individual and common repertoire of DNA-binding transcriptional regulators of Corynebacterium glutamicum, Corynebacterium efficiens, Corynebacterium diphtheriae and Corynebacterium jeikeium deduced from the complete genome sequences. BMC Genom 6:86
Brinkrolf K, Brune I, Tauch A (2006) Transcriptional regulation of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Genet Mol Res 5:773–789
Ibarra JA, Pérez-Rueda E, Carroll RK, Shaw LN (2013) Global analysis of transcriptional regulators in Staphylococcus aureus. BMC Genom 14:126
Pandurangan AP, Stahlhacke J, Oates ME, Smithers B, Gough J (2019) The SUPERFAMILY 2.0 database: a significant proteome update and a new webserver. Nucleic Acids Res 47:D490–D494
El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC et al (2019) The Pfam protein families database in 2019. Nucleic Acids Res 47:D427–D432
Imam S, Schäuble S, Brooks AN, Baliga NS, Price ND (2015) Data-driven integration of genome-scale regulatory and metabolic network models. Front Microbiol 6:409
Madan Babu M, Teichmann SA, Aravind L (2006) Evolutionary dynamics of prokaryotic transcriptional regulatory networks. J Mol Biol 358:614–633
Medeiros Filho F, do Nascimento APB, Dos Santos MT, Carvalho-Assef APD, da Silva FAB (2019) Gene regulatory network inference and analysis of multidrug-resistant Pseudomonas aeruginosa. Mem Inst Oswaldo Cruz 114:e190105
Tsoy OV, Ravcheev DA, Čuklina J, Gelfand MS (2016) Nitrogen fixation and molecular oxygen: comparative genomic reconstruction of transcription regulation in alphaproteobacteria. Front Microbiol 7:1343
Pérez AG, Angarica VE, Vasconcelos ATR, Collado-Vides J. Tractor_DB (2007) (version 2.0): a database of regulatory interactions in gamma-proteobacterial genomes. Nucleic Acids Res 35:D132–D136
Lozada-Chávez I, Janga SC, Collado-Vides J (2006) Bacterial regulatory networks are extremely flexible in evolution. Nucleic Acids Res 34:3434–3445
Santos-Zavaleta A, Pérez-Rueda E, Sánchez-Pérez M, Velázquez-Ramírez DA, Collado-Vides J (2019) Tracing the phylogenetic history of the crl regulon through the bacteria and archaea genomes. BMC Genom 20:299
Stuart JM, Segal E, Koller D, Kim SK (2003) A gene-coexpression network for global discovery of conserved genetic modules. Science 302:249–255
Law KL, Su GY, Lin N, Lin HY, Jang WT, Chi CS (1989) Acute peritoneal dialysis in low birth weight infants. Zhonghua Yi Xue Za Zhi 43:119–124
van Noort V, Snel B, Huynen MA (2004) The yeast coexpression network has a small-world, scale-free architecture and can be explained by a simple model. EMBO Rep 5:280–284
Tsaparas P, Mariño-Ramírez L, Bodenreider O, Koonin EV, Jordan IK (2006) Global similarity and local divergence in human and mouse gene co-expression networks. BMC Evol Biol 6:70
Zhang B, Horvath S (2005) A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 4(17)
Mueller AJ, Canty-Laird EG, Clegg PD, Tew SR (2017) Cross-species gene modules emerge from a systems biology approach to osteoarthritis. NPJ Syst Biol Appl 3:13
van Dam S, Võsa U, van der Graaf A, Franke L, de Magalhães JP (2018) Gene co-expression analysis for functional classification and gene-disease predictions. Brief Bioinform 19:575–592
Hosseinkhan N, Mousavian Z, Masoudi-Nejad A (2018) Comparison of gene co-expression networks in Pseudomonas aeruginosa and Staphylococcus aureus reveals conservation in some aspects of virulence. Gene 639:1–10
Bakhtiarizadeh MR, Hosseinpour B, Shahhoseini M, Korte A, Gifani P (2018) Weighted gene co-expression network analysis of endometriosis and identification of functional modules associated with its main hallmarks. Front Genet 9:453
Yang Y, Han L, Yuan Y, Li J, Hei N, Liang H (2014) Gene co-expression network analysis reveals common system-level properties of prognostic genes across cancer types. Nat Commun 5:3231
Amar D, Safer H, Shamir R (2013) Dissection of regulatory networks that are altered in disease via differential co-expression. PLoS Comput Biol 9:e1002955
Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J et al (2008) Genetics of gene expression and its effect on disease. Nature 452:423–428
Carlson MRJ, Zhang B, Fang Z, Mischel PS, Horvath S, Nelson SF (2006) Gene connectivity, function, and sequence conservation: predictions from modular yeast co-expression networks. BMC Genom 7:40
Hartwell LH, Hopfield JJ, Leibler S, Murray AW (1999) From molecular to modular cell biology. Nature 402:C47–C52
Jeong H, Tombor B, Albert R, Oltvai ZN, Barabási AL (2000) The large-scale organization of metabolic networks. Nature 407:651–654
Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabási AL (2002) Hierarchical organization of modularity in metabolic networks. Science 297:1551–1555
Potapov AP (2008) Signal Transduction and Gene Regulation Networks. In: Junker BH, Schreiber F (eds) Analysis of biological networks. Wiley, Hoboken, NJ, USA, pp 181–206
Junker BH, Schreiber F (2011) Analysis of biological networks. books.google.com, https://books.google.com/books?hl=en&lr=&id=YeXLbClh1SIC&oi=fnd&pg=PT4&dq=Junker+BH+Schreiber+F+Analysis+of+biological+networks+1st+ed+Wiley+Interscience+2008+ISBN+978-0-470-04144-4&ots=0El1K8ARJZ&sig=y2bn9u3wWL3bNNdxk7ZTyOgVa3M
Greenfield A, Madar A, Ostrer H, Bonneau R (2010) DREAM4: combining genetic and dynamic information to identify biological networks and dynamical models. PLoS One 5:e13397
Lee JM, Gianchandani EP, Eddy JA, Papin JA (2008) Dynamic analysis of integrated signaling, metabolic, and regulatory networks. PLoS Comput Biol 4:e1000086
Price ND, Shmulevich I (2007) Biochemical and statistical network models for systems biology. Curr Opin Biotechnol 18:365–370
Palsson B (2002) In silico biology through “omics”. Nat Biotechnol 20:649–650
Chavali AK, D’Auria KM, Hewlett EL, Pearson RD, Papin JA (2012) A metabolic network approach for the identification and prioritization of antimicrobial drug targets. Trends Microbiol 20:113–123
Covert MW, Knight EM, Reed JL, Herrgard MJ, Palsson BO (2004) Integrating high-throughput and computational data elucidates bacterial networks. Nature 429:92–96
Covert MW, Palsson BØ (2002) Transcriptional regulation in constraints-based metabolic models of Escherichia coli. J Biol Chem 277:28058–28064
Zhang W, Li F, Nie L (2010) Integrating multiple “omics” analysis for microbial biology: application and methodologies. Microbiology 156:287–301
Covert MW, Schilling CH, Palsson B (2001) Regulation of gene expression in flux balance models of metabolism. J Theor Biol 213:73–88
Covert MW, Xiao N, Chen TJ, Karr JR (2008) Integrating metabolic, transcriptional regulatory and signal transduction models in Escherichia coli. Bioinformatics 24:2044–2050
Shlomi T, Eisenberg Y, Sharan R, Ruppin E (2007) A genome-scale computational study of the interplay between transcriptional regulation and metabolism. Mol Syst Biol 3:101
Chandrasekaran S, Price ND (2010) Probabilistic integrative modeling of genome-scale metabolic and regulatory networks in Escherichia coli and Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 107:17845–17850
Blazier AS, Papin JA (2012) Integration of expression data in genome-scale metabolic network reconstructions. Front Physiol 3:299
Ma S, Minch KJ, Rustad TR, Hobbs S, Zhou S-L, Sherman DR et al (2015) Integrated modeling of gene regulatory and metabolic networks in mycobacterium tuberculosis. PLoS Comput Biol 11:e1004543
Jensen PA, Papin JA (2011) Functional integration of a metabolic network model and expression data without arbitrary thresholding. Bioinformatics 27:541–547
Presta L, Bosi E, Mansouri L, Dijkshoorn L, Fani R, Fondi M (2017) Constraint-based modeling identifies new putative targets to fight colistin-resistant A. baumannii infections. Sci Rep 7:3706
Becker SA, Palsson BO (2008) Context-specific metabolic networks are consistent with experiments. PLoS Comput Biol 4:e1000082
Schmidt BJ, Ebrahim A, Metz TO, Adkins JN, Palsson BØ, Hyduke DR (2013) GIM3E: condition-specific models of cellular metabolism developed from metabolomics and expression data. Bioinformatics 29:2900–2908
Marmiesse L, Peyraud R, Cottret L (2015) FlexFlux: combining metabolic flux and regulatory network analyses. BMC Syst Biol 9:93
Chaouiya C, Bérenguier D, Keating SM, Naldi A, van Iersel MP, Rodriguez N et al (2013) SBML qualitative models: a model representation format and infrastructure to foster interactions between qualitative modelling formalisms and tools. BMC Syst Biol 7:135
Jacob F, Monod J (1961) Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol 3:318–356
Feist AM, Henry CS, Reed JL, Krummenacker M, Joyce AR, Karp PD et al (2007) A genome-scale metabolic reconstruction for Escherichia coli K-12 MG1655 that accounts for 1260 ORFs and thermodynamic information. Mol Syst Biol 3:121
Busby S, Ebright RH (1999) Transcription activation by catabolite activator protein (CAP). J Mol Biol 293:199–213
Banos DT, Trébulle P, Elati M (2017) Integrating transcriptional activity in genome-scale models of metabolism. BMC Syst Biol 11:134
Koonin EV, Wolf YI, Karev GP (2002) The structure of the protein universe and genome evolution. Nature 420:218–223
Röntgen WC (1896) On a new kind of rays. Science 3:227–231
Friedrich W, Knipping P, Laue M (1913) Interferenzerscheinungen bei Röntgenstrahlen. Annalen der Physik 971–988 http://dx.doi.org/10.1002/andp.19133461004
Bragg WL (1913) The diffraction of short electromagnetic waves by a crystal. Proc Camb Philos Soc, 17:43–57 (1913). Communicated by Professor Sir Thomson JJ (1966) Read 11 November 1912. X-ray and neutron diffraction 109–125. http://dx.doi.org/10.1016/b978-0-08-011999-1.50015-8
Bernal JD, Crowfoot D (1934) X-Ray photographs of crystalline pepsin. Nature 794–795. http://dx.doi.org/10.1038/133794b0
El-Mehairy MM, Shaker A, Ramadan M, Hamza S, Tadros SS (1981) Control of essential hypertension with captopril, an angiotensin converting enzyme inhibitor. Br J Clin Pharmacol 469–475. http://dx.doi.org/10.1111/j.1365-2125.1981.tb01152.x
Renaud J (2020) The evolving role of structural biology in drug discovery. Struct Biol Drug Discov 1–22. http://dx.doi.org/10.1002/9781118681121.ch1
Berman HM, Battistuz T, Bhat TN, Bluhm WF, Bourne PE, Burkhardt K, et al (2002) The protein data bank. Acta Crystallogr Sect D Biol Crystallogr 899–907. http://dx.doi.org/10.1107/s0907444902003451
UniProt Consortium T (2018) UniProt: the universal protein knowledgebase. Nucleic Acids Res 46:2699
Li W, Godzik A (2006) Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22:1658–1659
Eswar N, Eramian D, Webb B, Shen M-Y, Sali A (2008) Protein structure modeling with MODELLER. Methods Mol Biol 145–159. http://dx.doi.org/10.1007/978-1-60327-058-8_8
Melo F, Sali A (2007) Fold assessment for comparative protein structure modeling. Protein Sci 2412–2426. http://dx.doi.org/10.1110/ps.072895107
Benkert P, Tosatto SCE, Schomburg D (2008) QMEAN: a comprehensive scoring function for model quality assessment. Proteins: Struct, Funct, Bioinform 261–277. http://dx.doi.org/10.1002/prot.21715
Hopkins AL, Groom CR (2002) The druggable genome. Nat Rev Drug Discov 727–730. http://dx.doi.org/10.1038/nrd892
Cheng AC, Coleman RG, Smyth KT, Cao Q, Soulard P, Caffrey DR, et al (2007) Structure-based maximal affinity model predicts small-molecule druggability. Nat Biotechnol 71–75. http://dx.doi.org/10.1038/nbt1273
Radusky L, Defelipe LA, Lanzarotti E, Luque J, Barril X, Marti MA, et al (2014) TuberQ: a Mycobacterium tuberculosis protein druggability database. Database 2014:bau035
An J, Totrov M, Abagyan R (2005) Pocketome via comprehensive identification and classification of ligand binding envelopes. Mol Cell Proteomics 4:752–761
Hendlich M, Rippmann F, Barnickel G (1997) LIGSITE: automatic and efficient detection of potential small molecule-binding sites in proteins. J Mol Graph Model 15:359–363, 389
Weisel M, Proschak E, Schneider G (2007) PocketPicker: analysis of ligand binding-sites with shape descriptors. Chem Cent J 1:7
Le Guilloux V, Schmidtke P, Tuffery P (2009) Fpocket: an open source platform for ligand pocket detection. BMC Bioinform 10:168
Coleman RG, Sharp KA (2006) Travel depth, a new shape descriptor for macromolecules: application to ligand binding. J Mol Biol 362:441–458
Goodford P (2005) The basic principles of GRID. Methods Princ Med Chem 1–25. http://dx.doi.org/10.1002/3527607676.ch1
Laurie ATR, Jackson RM (2005) Q-SiteFinder: an energy-based method for the prediction of protein-ligand binding sites. Bioinformatics 21:1908–1916
Hernandez M, Ghersi D, Sanchez R (2009) SITEHOUND-web: a server for ligand binding site identification in protein structures. Nucleic Acids Res 37:W413–W416
Jiménez J, Doerr S, Martínez-Rosell G, Rose AS, De Fabritiis G (2017) DeepSite: protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 33:3036–3042
Levitt DG, Banaszak LJ (1992) POCKET: a computer graphics method for identifying and displaying protein cavities and their surrounding amino acids. J Mol Graph 10:229–234
Venkatachalam CM, Jiang X, Oldfield T, Waldman M (2003) LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J Mol Graph Model 21:289–307
Zhang Z, Li Y, Lin B, Schroeder M, Huang B (2011) Identification of cavities on protein surface using multiple computational approaches for drug binding site prediction. Bioinformatics 27:2083–2088
Schmidtke P, Le Guilloux V, Maupetit J, Tufféry P (2010) fpocket: online tools for protein ensemble pocket detection and tracking. Nucleic Acids Res 38:W582–W589
Schmidtke P, Barril X (2010) Understanding and predicting druggability. A high-throughput method for detection of drug binding sites. J Med Chem 53:5858–5867
Sosa EJ, Burguener G, Lanzarotti E, Defelipe L, Radusky L, Pardo AM et al (2018) Target-pathogen: a structural bioinformatic approach to prioritize drug targets in pathogens. Nucleic Acids Res 46:D413–D418
Karp PD, Ivanova N, Krummenacker M, Kyrpides N, Latendresse M, Midford P et al (2019) A comparison of microbial genome web portals. Front Microbiol 10:208
Jensen PA (2018) Coupling fluxes, enzymes, and regulation in genome-scale metabolic models. Methods Mol Biol 1716:337–351
Mienda BS, Salihu R, Adamu A, Idris S (2018) Genome-scale metabolic models as platforms for identification of novel genes as antimicrobial drug targets. Future Microbiol 13:455–467
Gu C, Kim GB, Kim WJ, Kim HU, Lee SY (2019) Current status and applications of genome-scale metabolic models. Genome Biol 20:121
Merigueti TC, Carneiro MW, Carvalho-Assef APD, Silva-Jr FP, da Silva FAB (2019) FindTargetsWEB: a user-friendly tool for Identification of potential therapeutic targets in metabolic networks of bacteria. Front Genet 10:633
Arkin AP, Cottingham RW, Henry CS, Harris NL, Stevens RL, Maslov S et al (2018) KBase: the united states department of energy systems biology knowledgebase. Nat Biotechnol 36:566–569
Cottret L, Frainay C, Chazalviel M, Cabanettes F, Gloaguen Y, Camenen E, et al (2018) MetExplore: collaborative edition and exploration of metabolic networks. Nucleic Acids Res 46:W495–W502
Gao Z, Li H, Zhang H, Liu X, Kang L, Luo X et al (2008) PDTD: a web-accessible protein database for drug target identification. BMC Bioinform 9:104
Li H, Gao Z, Kang L, Zhang H, Yang K, Yu K et al (2006) TarFisDock: a web server for identifying drug targets with docking approach. Nucleic Acids Res 34:W219–W224
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, et al (2017) PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucl Acids Res W356–W360. http://dx.doi.org/10.1093/nar/gkx374
Yang H, Qin C, Li YH, Tao L, Zhou J, Yu CY, et al (2016) Therapeutic target database update 2016: enriched resource for bench to clinical drug target and targeted pathway information. Nucl Acids Res D1069–D1074. http://dx.doi.org/10.1093/nar/gkv1230
Chen L, Oughtred R, Berman HM, Westbrook J (2004) TargetDB: a target registration database for structural genomics projects. Bioinformatics 20:2860–2862
Chanumolu SK, Rout C, Chauhan RS (2012) UniDrug-target: a computational tool to identify unique drug targets in pathogenic bacteria. PLoS One 7:e32833
Wang L, Ma C, Wipf P, Liu H, Su W, Xie X-Q (2013) TargetHunter: an in silico target identification tool for predicting therapeutic potential of small organic molecules based on chemogenomic database. AAPS J 395–406. http://dx.doi.org/10.1208/s12248-012-9449-z
Singh NK, Selvam SM, Chakravarthy P (2006) T-iDT: tool for identification of drug target in bacteria and validation by Mycobacterium tuberculosis. Silico Biol 6:485–493
Luo H, Lin Y, Gao F, Zhang C-T, Zhang R (2014) DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res 42:D574–D580
Magariños MP, Carmona SJ, Crowther GJ, Ralph SA, Roos DS, Shanmugam D et al (2012) TDR Targets: a chemogenomics resource for neglected diseases. Nucleic Acids Res 40:D1118–D1127
Raman K, Yeturu K, Chandra N (2008) targetTB: a target identification pipeline for Mycobacterium tuberculosis through an interactome, reactome and genome-scale structural analysis. BMC Syst Biol 2:109
Panjkovich A, Gibert I, Daura X (2014) antibacTR: dynamic antibacterial-drug-target ranking integrating comparative genomics, structural analysis and experimental annotation. BMC Genom 15:36
Shanmugam A, Natarajan J (2010) Computational genome analyses of metabolic enzymes in Mycobacterium leprae for drug target identification. Bioinformation 4:392–395
Oany AR, Mia M, Pervin T, Hasan MN, Hirashima A (2018) Identification of potential drug targets and inhibitor of the pathogenic bacteria Shigella flexneri 2a through the subtractive genomic approach. Silico Pharmacol 6:11
Kumar Jaiswal A, Tiwari S, Jamal SB, Barh D, Azevedo V, Soares SC (2017) An in silico identification of common putative vaccine candidates against treponema pallidum: a reverse vaccinology and subtractive genomics based approach. Int J Mol Sci 18. http://dx.doi.org/10.3390/ijms18020402
de Sarom A, Kumar Jaiswal A, Tiwari S, de Castro Oliveira L, Barh D, Azevedo V, et al (2018) Putative vaccine candidates and drug targets identified by reverse vaccinology and subtractive genomics approaches to control Haemophilus ducreyi, the causative agent of chancroid. J R Soc Interface 15. http://dx.doi.org/10.1098/rsif.2018.0032
Mukherjee S, Gangopadhay K, Mukherjee SB (2019) Identification of potential new vaccine candidates in Salmonella typhi using reverse vaccinology and subtractive genomics-based approach. bioRxiv. biorxiv.org. https://www.biorxiv.org/content/10.1101/521518v1.abstract
Song J-H, Ko KS (2008) Detection of essential genes in Streptococcus pneumoniae using bioinformatics and allelic replacement mutagenesis. Methods Mol Biol 416:401–408
Hasan S, Daugelat S, Rao PSS, Schreiber M (2006) Prioritizing genomic drug targets in pathogens: application to Mycobacterium tuberculosis. PLoS Comput Biol 2:e61
Cloete R, Oppon E, Murungi E, Schubert W-D, Christoffels A (2016) Resistance related metabolic pathways for drug target identification in Mycobacterium tuberculosis. BMC Bioinform 17:75
Lee D-Y, Chung BKS, Yusufi FNK, Selvarasu S (2011) In silico genome-scale modeling and analysis for identifying anti-tubercular drug targets. Drug Dev Res 121–129. http://dx.doi.org/10.1002/ddr.20408
Neelapu NRR, Mutha NVR (2015) Identification of potential drug targets in Helicobacter pylori strain HPAG1 by in silico genome analysis. Infect Disord-Drug. ingentaconnect.com, https://www.ingentaconnect.com/content/ben/iddt/2015/00000015/00000002/art00006
Mondal SI, Ferdous S, Jewel NA, Akter A, Mahmud Z, Islam MM et al (2015) Identification of potential drug targets by subtractive genome analysis of Escherichia coli O157:H7: an in silico approach. Adv Appl Bioinform Chem 8:49–63
Hossain T, Kamruzzaman M, Choudhury TZ, Mahmood HN, Nabi AHMN, Hosen MI (2017) Application of the subtractive genomics and molecular docking analysis for the identification of novel putative drug targets against salmonella enterica subsp. enterica serovar Poona. Biomed Res Int 2017:3783714
Pradeepkiran JA, Kumar KK, Kumar YN, Bhaskar M (2015) Modeling, molecular dynamics, and docking assessment of transcription factor rho: a potential drug target in Brucella melitensis 16M. Drug Des Devel Ther 9:1897–1912
Muhammad SA, Ahmed S, Ali A, Huang H, Wu X, Yang XF et al (2014) Prioritizing drug targets in Clostridium botulinum with a computational systems biology approach. Genomics 104:24–35
Bhardwaj T, Somvanshi P (2017) Pan-genome analysis of Clostridium botulinum reveals unique targets for drug development. Gene 623:48–62
Kumar A, Thotakura PL, Tiwary BK, Krishna R (2016) Target identification in Fusobacterium nucleatum by subtractive genomics approach and enrichment analysis of host-pathogen protein-protein interactions. BMC Microbiol 16:84
Rahman A, Noore S, Hasan A, Ullah R, Rahman H, Hossain A et al (2014) Identification of potential drug targets by subtractive genome analysis of Bacillus anthracis A0248: An in silico approach. Comput Biol Chem 52:66–72
David MZ, Daum RS (2010) Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23:616–687
McCarthy AJ, Lindsay JA (2010) Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol 10:173
Le Loir Y, Baron F, Gautier M (2003) Staphylococcus aureus and food poisoning. Genet Mol Res 2:63–76
Plata K, Rosato AE, Wegrzyn G (2009) Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochim Pol 56:597–612
Eriksen KR (1961) “Celbenin”-resistant staphylococci. Ugeskr Laeger 123:384–386
Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A et al (2018) Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers 4:18033
Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG (2002) The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U S A 99:7687–7692
Nübel U, Roumagnac P, Feldkamp M, Song J-H, Ko KS, Huang Y-C et al (2008) Frequent emergence and limited geographic dispersal of methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 105:14130–14135
Pavillard R, Harvey K, Douglas D, Hewstone A, Andrew J, Collopy B et al (1982) Epidemic of hospital-acquired infection due to methicillin-resistant Staphylococcus aureus in major Victorian hospitals. Med J Aust 1:451–454
Aires De Sousa M, Miragaia M, Sanches IS, Avila S, Adamson I, Casagrande ST et al (2001) Three-year assessment of methicillin-resistant Staphylococcus aureus clones in Latin America from 1996 to 1998. J Clin Microbiol 39:2197–2205
Aires de Sousa M, Sanches IS, Ferro ML, Vaz MJ, Saraiva Z, Tendeiro T, et al (1998) Intercontinental spread of a multidrug-resistant methicillin-resistant Staphylococcus aureus clone. J Clin Microbiol 36:2590–2596
Szczepanik A, Kozioł-Montewka M, Al-Doori Z, Morrison D, Kaczor D (2007) Spread of a single multiresistant methicillin-resistant Staphylococcus aureus clone carrying a variant of staphylococcal cassette chromosome mec type III isolated in a university hospital. Eur J Clin Microbiol Infect Dis 26:29–35
Botelho AMN, Cerqueira E Costa MO, Moustafa AM, Beltrame CO, Ferreira FA, Côrtes MF, et al (2019) Local diversification of methicillin- resistant staphylococcus aureus ST239 in South America after its rapid worldwide dissemination. Front Microbiol 10:82
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Otto M (2018) Staphylococcal biofilms. Microbiol Spectr 6. http://dx.doi.org/10.1128/microbiolspec.GPP3-0023-2018
Otto M (2013) Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188
Otto M (2019) Staphylococcal biofilms. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Braunstein M, Rood JI (eds) Gram-positive pathogens. ASM Press, Washington, DC, USA, pp 699–711
McCarthy H, Rudkin JK, Black NS, Gallagher L, O’Neill E, O’Gara JP (2015) Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front Cell Infect Microbiol 5:1
Costa MOC (2018) Modelo metabólico em escala genômica integrado com as vias regulatórias associadas ao biofilme de Staphylococcus aureus ST239-SCCmecIII (Bmb9393). Petrópolis. Thesis [Ph.D in Computational Modeling]—Laboratório Nacional de Computação Científica
Costa MOC, Beltrame CO, Ferreira FA, Botelho AMN, Lima NCB, Souza RC, et al (2013) Complete genome sequence of a variant of the methicillin-resistant staphylococcus aureus ST239 lineage, strain BMB9393, displaying superior ability to accumulate ica-independent biofilm. Genome Announc 1. http://dx.doi.org/10.1128/genomeA.00576-13
Botelho AMN, Costa MOC, Beltrame CO, Ferreira FA, Côrtes MF, Bandeira PT et al (2016) Complete genome sequence of an agr-dysfunctional variant of the ST239 lineage of the methicillin-resistant Staphylococcus aureus strain GV69 from Brazil. Stand Genomic Sci 11:34
Almeida LGP, Paixão R, Souza RC, da Costa GC, Barrientos FJA, dos Santos MT et al (2004) A system for automated bacterial (genome) integrated annotation–SABIA. Bioinformatics 20:2832–2833
Lister JL, Horswill AR (2014) Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol 4:178
Ravcheev DA, Best AA, Tintle N, Dejongh M, Osterman AL, Novichkov PS et al (2011) Inference of the transcriptional regulatory network in Staphylococcus aureus by integration of experimental and genomics-based evidence. J Bacteriol 193:3228–3240
Nagarajan V, Elasri MO (2007) SAMMD: Staphylococcus aureus microarray meta-database. BMC Genom 8:351
Lechner M, Findeiss S, Steiner L, Marz M, Stadler PF, Prohaska SJ (2011) Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinform 12:124
Bosi E, Monk JM, Aziz RK, Fondi M, Nizet V, Palsson BØ (2016) Comparative genome-scale modelling of Staphylococcus aureus strains identifies strain-specific metabolic capabilities linked to pathogenicity. Proc Natl Acad Sci U S A 113:E3801–E3809
Helikar T, Kowal B, McClenathan S, Bruckner M, Rowley T, Madrahimov A et al (2012) The cell collective: toward an open and collaborative approach to systems biology. BMC Syst Biol 6:96
Görke B, Stülke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624
Oskouian B, Stewart GC (1990) Repression and catabolite repression of the lactose operon of Staphylococcus aureus. J Bacteriol 172:3804–3812
Brückner R, Titgemeyer F (2002) Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett 209:141–148
Somerville GA, Proctor RA (2009) At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol Mol Biol Rev 73:233–248
Lindgren JK, Thomas VC, Olson ME, Chaudhari SS, Nuxoll AS, Schaeffer CR et al (2014) Arginine deiminase in Staphylococcus epidermidis functions to augment biofilm maturation through pH homeostasis. J Bacteriol 196:2277–2289
Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ et al (2004) Global gene expression in Staphylococcus aureus biofilms. J Bacteriol 186:4665–4684
Resch A, Rosenstein R, Nerz C, Götz F (2005) Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol 71:2663–2676
Makhlin J, Kofman T, Borovok I, Kohler C, Engelmann S, Cohen G et al (2007) Staphylococcus aureus ArcR controls expression of the arginine deiminase operon. J Bacteriol 189:5976–5986
Fernández M, Zúñiga M (2006) Amino acid catabolic pathways of lactic acid bacteria. Crit Rev Microbiol 32:155–183
Podschun R, Pietsch S, Höller C, Ullmann U (2001) Incidence of Klebsiella species in surface waters and their expression of virulence factors. Appl Environ Microbiol 67:3325–3337
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603
Rendueles O (2020) Deciphering the role of the capsule of Klebsiella pneumoniae during pathogenesis: a cautionary tale. Mol Microbiol http://dx.doi.org/10.1111/mmi.14474
Martin RM, Bachman MA (2018) Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol 8:4
Wyres KL, Lam MMC, Holt KE (2020) Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol http://dx.doi.org/10.1038/s41579-019-0315-1
Ko W-C, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S et al (2002) Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 8:160–166
Paczosa MK, Mecsas J (2016) Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661
Meatherall BL, Gregson D, Ross T, Pitout JDD, Laupland KB (2009) Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med 122:866–873
Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D et al (2015) Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A 112:E3574–E3581
Navon-Venezia S, Kondratyeva K, Carattoli A (2017) Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev 41:252–275
Wyres KL, Holt KE (2016) Klebsiella pneumoniae population genomics and antimicrobial-resistant clones. Trends Microbiol 24:944–956
Ambler RP (1980) The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci 289:321–331
Escandón-Vargas K, Reyes S, Gutiérrez S, Villegas MV (2017) The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev Anti Infect Ther 15:277–297
Lee C-R, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH (2016) Global dissemination of carbapenemase-producing klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol 7:895
Wyres KL, Wick RR, Judd LM, Froumine R, Tokolyi A, Gorrie CL et al (2019) Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae. PLoS Genet 15:e1008114
Braun G, Cayô R, Matos AP, de Mello Fonseca J, Gales AC (2018) Temporal evolution of polymyxin B-resistant Klebsiella pneumoniae clones recovered from blood cultures in a teaching hospital during a 7-year period. Int J Antimicrob Agents 51:522–527
Tiwari V, Tiwari M, Solanki V (2017) Polyvinylpyrrolidone-capped silver nanoparticle inhibits infection of carbapenem-resistant strain of acinetobacter baumannii in the human pulmonary epithelial cell. Front Immunol 8:973
Ramos PIP, Picão RC, de Almeida LGP, Lima NCB, Girardello R, Vivan ACP, et al (2014) Comparative analysis of the complete genome of KPC-2-producing Klebsiella pneumoniae Kp13 reveals remarkable genome plasticity and a wide repertoire of virulence and resistance mechanisms. BMC Genomics 54. http://dx.doi.org/10.1186/1471-2164-15-54
NIH HMP Working Group, Peterson J, Garges S, Giovanni M, McInnes P, Wang L, et al (2009) The NIH human microbiome project. Genome Res 19:2317–2323
Ramage B, Erolin R, Held K, Gasper J, Weiss E, Brittnacher M, et al (2017) Comprehensive arrayed transposon mutant library of Klebsiella pneumoniae outbreak strain KPNIH1. J Bacteriol 199. http://dx.doi.org/10.1128/JB.00352-17
Liao Y-C, Huang T-W, Chen F-C, Charusanti P, Hong JSJ, Chang H-Y et al (2011) An experimentally validated genome-scale metabolic reconstruction of Klebsiella pneumoniae MGH 78578, iYL1228. J Bacteriol 193:1710–1717
Payne DJ, Miller WH, Berry V, Brosky J, Burgess WJ, Chen E et al (2002) Discovery of a novel and potent class of FabI-directed antibacterial agents. Antimicrob Agents Chemother 46:3118–3124
Joo SH (2015) Lipid a as a drug target and therapeutic molecule. Biomol Ther 23:510–516
Lemaître N, Liang X, Najeeb J, Lee C-J, Titecat M, Leteurtre E, et al (2017) Curative treatment of severe gram-negative bacterial infections by a new class of antibiotics targeting LpxC. MBio 8. http://dx.doi.org/10.1128/mBio.00674-17
Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582
Daugelavicius R, Bakiene E, Bamford DH (2000) Stages of polymyxin B interaction with the Escherichia coli cell envelope. Antimicrob Agents Chemother 44:2969–2978
Ramos PIP, Custódio MGF, Quispe Saji GDR, Cardoso T, da Silva GL, Braun G et al (2016) The polymyxin B-induced transcriptomic response of a clinical, multidrug-resistant Klebsiella pneumoniae involves multiple regulatory elements and intracellular targets. BMC Genom 17:737
Heuston S, Begley M, Gahan CGM, Hill C (2012) Isoprenoid biosynthesis in bacterial pathogens. Microbiology 158:1389–1401
Masini T, Hirsch AKH (2014) Development of inhibitors of the 2C-methyl-D-erythritol 4-phosphate (MEP) pathway enzymes as potential anti-infective agents. J Med Chem 57:9740–9763
Saggu GS, Pala ZR, Garg S, Saxena V (2016) New insight into isoprenoids biosynthesis process and future prospects for drug designing in plasmodium. Front Microbiol 7:1421
Kadian K, Vijay S, Gupta Y, Rawal R, Singh J, Anvikar A et al (2018) Structural modeling identifies Plasmodium vivax 4-diphosphocytidyl-2C-methyl-d-erythritol kinase (IspE) as a plausible new antimalarial drug target. Parasitol Int 67:375–385
Tang M, Odejinmi SI, Allette YM, Vankayalapati H, Lai K (2011) Identification of novel small molecule inhibitors of 4-diphosphocytidyl-2-C-methyl-D-erythritol (CDP-ME) kinase of Gram-negative bacteria. Bioorg Med Chem 19:5886–5895
Zhang Y-M, Rock CO (2008) Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233
Bukata L, Altabe S, de Mendoza D, Ugalde RA, Comerci DJ (2008) Phosphatidylethanolamine synthesis is required for optimal virulence of Brucella abortus. J Bacteriol 190:8197–8203
Postma TM, Liskamp RMJ (2016) Triple-targeting Gram-negative selective antimicrobial peptides capable of disrupting the cell membrane and lipid A biosynthesis. RSC Adv R Soc Chem 6:65418–65421
Design and synthesis of novel Azatidinones analogues as potential antimicrobials. J Chem, Biol Phys Sci (2017) http://dx.doi.org/10.24214/jcbps.a.7.3.68187
Bommineni GR, Kapilashrami K, Cummings JE, Lu Y, Knudson SE, Gu C et al (2016) Thiolactomycin-based inhibitors of bacterial β-Ketoacyl-ACP synthases with in vivo activity. J Med Chem 59:5377–5390
Serio AW, Kubo A, Lopez S, Gomez M, Corey VC, Andrews L, et al (2013) Structure, potency and bactericidal activity of ACHN-975, a first-in-class LpxC inhibitor. In: 53rd interscience conference on antimicrobial agents and chemotherapy. Achaogen, pp 10–13
Pahal V (2018) Significance of apigenin and rosmarinic acid mediated inhibition pathway of MurG, MurE and DNA adenine methylase enzymes with antibacterial potential derived from the methanolic extract of Ocimum sanctum. MOJ Drug Des Dev & Ther http://dx.doi.org/10.15406/mojddt.2018.02.00031
World Health Organization (2018) Global Tuberculosis Report 2018. World Health Organization
Lillebaek T, Dirksen A, Vynnycky E, Baess I, Thomsen VØ, Andersen ÅB (2003) Stability of DNA patterns and evidence ofmycobacterium tuberculosisreactivation occurring decades after the initial infection. J Infect Dis 1032–1039. http://dx.doi.org/10.1086/378240
Ascenzi P, Visca P (2008) Scavenging of reactive nitrogen species by mycobacterial truncated hemoglobins. Methods Enzymol 436:317–337
Rhee KY, Erdjument-Bromage H, Tempst P, Nathan CF (2005) S-nitroso proteome of mycobacterium tuberculosis: enzymes of intermediary metabolism and antioxidant defense. Proc Natl Acad Sci 467–472 http://dx.doi.org/10.1073/pnas.0406133102
Schopfer MP, Mondal B, Lee D-H, Sarjeant AAN, Karlin KD (2009) Heme/O2/•NO Nitric Oxide Dioxygenase (NOD) reactivity: phenolic nitration via a putative heme-peroxynitrite intermediate. J Am Chem Soc 11304–11305. http://dx.doi.org/10.1021/ja904832j
Buchmeier NA, Newton GL, Koledin T, Fahey RC (2003) Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics. Mol Microbiol 1723–1732 http://dx.doi.org/10.1046/j.1365-2958.2003.03416.x
Lunardi J, Nunes J, Bizarro C, Basso L, Santos D, Machado P (2013) Targeting the histidine pathway in mycobacterium tuberculosis. Curr Top Med Chem 2866–2884. http://dx.doi.org/10.2174/15680266113136660203
Barry CE, Crick DC, McNeil MR (2007) Targeting the formation of the cell wall core of M. tuberculosis. Infect Disord Drug Targets 7:182–202
Chan K, Knaak T, Satkamp L, Humbert O, Falkow S, Ramakrishnan L (2002) Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proc Natl Acad Sci U S A 99:3920–3925
Scandurra GM, Ryan AA, Pinto R, Britton WJ, Triccas JA (2006) Contribution ofL-alanine dehydrogenase toin vivopersistence and protective efficacy of the BCG vaccine. Microbiol Immunol 805–810. http://dx.doi.org/10.1111/j.1348-0421.2006.tb03856.x
Acknowledgments
This work was supported by fellowships from CNPq (process no. 306894/2019-0) and grant by CAPES (process no. 88887.368759/2019-00) to M.F.N. E.P-R was supported by Dirección General de Asuntos del Personal Académico-Universidad Nacional Autónoma de México (IN-209620) and Programa Iberoamericano de Ciencia y Tecnologìa para el Desarrollo (P918PTE0261).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nicolás, M.F. et al. (2020). Integrating Omics Data to Prioritize Target Genes in Pathogenic Bacteria. In: da Silva, F.A.B., Carels, N., Trindade dos Santos, M., Lopes, F.J.P. (eds) Networks in Systems Biology. Computational Biology, vol 32. Springer, Cham. https://doi.org/10.1007/978-3-030-51862-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-51862-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51861-5
Online ISBN: 978-3-030-51862-2
eBook Packages: Computer ScienceComputer Science (R0)