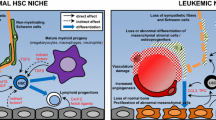
Abstract
Hematopoietic stem cells (HSCs) rely on instructive cues from the marrow microenvironment for their maintenance and function. Accumulating evidence indicates that the survival and proliferation of hematopoietic neoplasms are dependent not only on cell-intrinsic, genetic mutations, and other molecular alterations present within neoplastic stem cells, but also on the ability of the surrounding microenvironmental cells to nurture and promote the malignancy. It is anticipated that a better understanding of the molecular and cellular events responsible for these microenvironmental features of neoplastic hematopoiesis will lead to improved treatment for patients. This review will focus on the myeloproliferative neoplasms (MPNs), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), in which an acquired signaling kinase mutation (JAK2V617F) plays a central, pathogenetic role in 50–100% of patients with these disorders. Evidence is presented that the development of an MPN requires both an abnormal, mutation-bearing (i.e., neoplastic) HSC and an abnormal, mutation-bearing microenvironment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481:457–462
Ding L, Morrison SJ (2013) Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495:231–235
Greenbaum A, Hsu YM, Day RB et al (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495:227–230
Oguro H, Ding L, Morrison SJ (2013) SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13:102–116
Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ (2014) Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15:154–168
Bruns I, Lucas D, Pinho S et al (2014) Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20:1315–1320
Zhao M, Perry JM, Marshall H et al (2014) Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20:1321–1326
Asada N, Kunisaki Y, Pierce H et al (2017) Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19:214–223
Mendez-Ferrer S, Michurina TV, Ferraro F et al (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834
Nakamura-Ishizu A, Takubo K, Fujioka M, Suda T (2014) Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem Biophys Res Commun 454:353–357
Heazlewood SY, Neaves RJ, Williams B, Haylock DN, Adams TE, Nilsson SK (2013) Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res 11:782–792
Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K, Suda T (2015) CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med 212:2133–2146
Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121
Acar M, Kocherlakota KS, Murphy MM et al (2015) Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526:126–130
Zhao M, Ross JT, Itkin T et al (2012) FGF signaling facilitates postinjury recovery of mouse hematopoietic system. Blood 120:1831–1842
Calvi LM, Adams GB, Weibrecht KW et al (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846
Zhang J, Niu C, Ye L et al (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425:836–841
Winkler IG, Sims NA, Pettit AR et al (2010) Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116:4815–4828
Ludin A, Itkin T, Gur-Cohen S et al (2012) Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol 13:1072–1082
Mendez-Ferrer S, Lucas D, Battista M, Frenette PS (2008) Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452:442–447
Yamazaki S, Ema H, Karlsson G et al (2011) Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147:1146–1158
Chow A, Lucas D, Hidalgo A et al (2011) Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208:261–271
Walkley CR, Olsen GH, Dworkin S et al (2007) A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 129:1097–1110
Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH (2007) Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129:1081–1095
Kim YW, Koo BK, Jeong HW et al (2008) Defective notch activation in microenvironment leads to myeloproliferative disease. Blood 112:4628–4638
Raaijmakers MH, Mukherjee S, Guo S et al (2010) Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464:852–857
Kode A, Manavalan JS, Mosialou I et al (2014) Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature 506:240–244
Mager LF, Riether C, Schurch CM et al (2015) IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J Clin Invest 125:2579–2591
Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, Hoffman R (2009) The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 113:5246–5249
Rosti V, Villani L, Riboni R et al (2013) Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 121:360–368
Helman R, Pereira WO, Marti LC et al (2018) Granulocyte whole exome sequencing and endothelial JAK2V617F in patients with JAK2V617F positive Budd-Chiari Syndrome without myeloproliferative neoplasm. Br J Haematol 180:443–445
Yoder MC, Mead LE, Prater D et al (2007) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109:1801–1809
Teofili L, Martini M, Iachininoto MG et al (2011) Endothelial progenitor cells are clonal and exhibit the JAK2(V617F) mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood 117:2700–2707
Sozer S, Ishii T, Fiel MI et al (2009) Human CD34+ cells are capable of generating normal and JAK2V617F positive endothelial like cells in vivo. Blood Cells Mol Dis 43:304–312
Piaggio G, Rosti V, Corselli M et al (2009) Endothelial colony-forming cells from patients with chronic myeloproliferative disorders lack the disease-specific molecular clonality marker. Blood 114:3127–3130
Streubel B, Chott A, Huber D et al (2004) Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N Engl J Med 351:250–259
Fang B, Zheng C, Liao L et al (2005) Identification of human chronic myelogenous leukemia progenitor cells with hemangioblastic characteristics. Blood 105:2733–2740
Rigolin GM, Fraulini C, Ciccone M et al (2006) Neoplastic circulating endothelial cells in multiple myeloma with 13q14 deletion. Blood 107:2531–2535
Schepers K, Pietras EM, Reynaud D et al (2013) Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 13:285–299
Arranz L, Sanchez-Aguilera A, Martin-Perez D et al (2014) Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512:78–81
Zhang B, Ho YW, Huang Q et al (2012) Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell 21:577–592
Schmidt T, Kharabi Masouleh B, Loges S et al (2011) Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer Cell 19:740–753
Krevvata M, Silva BC, Manavalan JS et al (2014) Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood 124:2834–2846
Ciurea SO, Merchant D, Mahmud N et al (2007) Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 110:986–993
Medinger M, Skoda R, Gratwohl A et al (2009) Angiogenesis and vascular endothelial growth factor-/receptor expression in myeloproliferative neoplasms: correlation with clinical parameters and JAK2-V617F mutational status. Br J Haematol 146:150–157
Boveri E, Passamonti F, Rumi E et al (2008) Bone marrow microvessel density in chronic myeloproliferative disorders: a study of 115 patients with clinicopathological and molecular correlations. Br J Haematol 140:162–168
Gianelli U, Vener C, Raviele PR et al (2007) VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol 128:966–973
Zetterberg E, Vannucchi AM, Migliaccio AR et al (2007) Pericyte coverage of abnormal blood vessels in myelofibrotic bone marrows. Haematologica 92:597–604
Tiedt R, Hao-Shen H, Sobas MA et al (2008) Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 111:3931–3940
Constien R, Forde A, Liliensiek B et al (2001) Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis 30:36–44
Etheridge SL, Roh ME, Cosgrove ME et al (2014) JAK2V617F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc Natl Acad Sci U S A 111:2295–2300
Zhan H, Lin CHS, Segal Y, Kaushansky K (2018) The JAK2V617F-bearing vascular niche promotes clonal expansion in myeloproliferative neoplasms. Leukemia 32:462–469
Lin CH, Kaushansky K, Zhan H (2016) JAK2V617F-mutant vascular niche contributes to JAK2V617F clonal expansion in myeloproliferative neoplasms. Blood Cells Mol Dis 62:42–48
Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG (2006) Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 107:4274–4281
Mullally A, Lane SW, Ball B et al (2010) Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 17:584–596
Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG (2010) Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 115:3589–3597
Li J, Spensberger D, Ahn JS et al (2010) JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 116:1528–1538
Kent DG, Li J, Tanna H et al (2013) Self-renewal of single mouse hematopoietic stem cells is reduced by JAK2V617F without compromising progenitor cell expansion. PLoS Biol 11:e1001576
Li J, Kent DG, Godfrey AL et al (2014) JAK2V617F homozygosity drives a phenotypic switch in myeloproliferative neoplasms, but is insufficient to sustain disease. Blood 123:3139–3151
Gale RE, Allen AJ, Nash MJ, Linch DC (2007) Long-term serial analysis of X-chromosome inactivation patterns and JAK2 V617F mutant levels in patients with essential thrombocythemia show that minor mutant-positive clones can remain stable for many years. Blood 109:1241–1243
Lambert JR, Gale RE, Linch DC (2009) The production of JAK2 wild-type platelets is not downregulated in patients with JAK2 V617F mutant-positive essential thrombocythaemia. Br J Haematol 145:128–130
Stein BL, Williams DM, Wang NY et al (2010) Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica 95:1090–1097
Kroger N, Holler E, Kobbe G et al (2009) Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 114:5264–5270
Patriarca F, Bacigalupo A, Sperotto A et al (2008) Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Haematologica 93:1514–1522
Guardiola P, Anderson JE, Bandini G et al (1999) Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Societe Francaise de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood 93:2831–2838
Ballen KK, Shrestha S, Sobocinski KA et al (2010) Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant 16:358–367
Lin CHS, Zhang Y, Kaushansky K, Zhan H (2018) JAK2V617F-bearing vascular niche enhances malignant hematopoietic regeneration following radiation injury. Haematologica 103:1160–1168
Doan PL, Himburg HA, Helms K et al (2013) Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med 19:295–304
Himburg HA, Muramoto GG, Daher P et al (2010) Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med 16:475–482
Junt T, Schulze H, Chen Z et al (2007) Dynamic visualization of thrombopoiesis within bone marrow. Science 317:1767–1770
Tiedt R, Schomber T, Hao-Shen H, Skoda RC (2007) Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 109:1503–1506
Zhan H, Ma Y, Lin CH, Kaushansky K (2016) JAK2(V617F)-mutant megakaryocytes contribute to hematopoietic stem/progenitor cell expansion in a model of murine myeloproliferation. Leukemia 30:2332–2341
Zhang Y, Lin CHS, Kaushansky K, Zhan H (2018) JAK2V617F megakaryocytes promote hematopoietic stem/progenitor cell expansion in mice through thrombopoietin/MPL signaling. Stem Cells 36:1676–1684
Kaushansky K, Lok S, Holly RD et al (1994) Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature 369:568–571
Fox N, Priestley G, Papayannopoulou T, Kaushansky K (2002) Thrombopoietin expands hematopoietic stem cells after transplantation. J Clin Invest 110:389–394
Yoshihara H, Arai F, Hosokawa K et al (2007) Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1:685–697
Qian H, Buza-Vidas N, Hyland CD et al (2007) Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1:671–684
Sitnicka E, Lin N, Priestley GV et al (1996) The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood 87:4998–5005
Kimura S, Roberts AW, Metcalf D, Alexander WS (1998) Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A 95:1195–1200
Solar GP, Kerr WG, Zeigler FC et al (1998) Role of c-mpl in early hematopoiesis. Blood 92:4–10
Cardier JE, Dempsey J (1998) Thrombopoietin and its receptor, c-mpl, are constitutively expressed by mouse liver endothelial cells: evidence of thrombopoietin as a growth factor for liver endothelial cells. Blood 91:923–929
Brizzi MF, Battaglia E, Montrucchio G et al (1999) Thrombopoietin stimulates endothelial cell motility and neoangiogenesis by a platelet-activating factor-dependent mechanism. Circ Res 84:785–796
Eguchi M, Masuda H, Kwon S et al (2008) Lesion-targeted thrombopoietin potentiates vasculogenesis by enhancing motility and enlivenment of transplanted endothelial progenitor cells via activation of Akt/mTOR/p70S6kinase signaling pathway. J Mol Cell Cardiol 45:661–669
Sangkhae V, Etheridge SL, Kaushansky K, Hitchcock IS (2014) The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm. Blood 124:3956–3963
Araki M, Yang Y, Masubuchi N et al (2016) Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood 127:1307–1316
Chachoua I, Pecquet C, El-Khoury M et al (2016) Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 127:1325–1335
Elf S, Abdelfattah NS, Chen E et al (2016) Mutant calreticulin requires both its mutant C-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov 6:368–381
Marty C, Pecquet C, Nivarthi H et al (2016) Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood 127:1317–1324
Ng AP, Kauppi M, Metcalf D et al (2014) Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc Natl Acad Sci U S A 111:5884–5889
Privratsky JR, Newman PJ (2014) PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res 355:607–619
Itkin T, Gur-Cohen S, Spencer JA et al (2016) Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532:323–328
Niswander LM, Fegan KH, Kingsley PD, McGrath KE, Palis J (2014) SDF-1 dynamically mediates megakaryocyte niche occupancy and thrombopoiesis at steady state and following radiation injury. Blood 124:277–286
Avecilla ST, Hattori K, Heissig B et al (2004) Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 10:64–71
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zhan, H., Kaushansky, K. (2020). The Hematopoietic Microenvironment in Myeloproliferative Neoplasms: The Interplay Between Nature (Stem Cells) and Nurture (the Niche). In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1273. Springer, Cham. https://doi.org/10.1007/978-3-030-49270-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-49270-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49269-4
Online ISBN: 978-3-030-49270-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)