Abstract
Our idea of the interior of a cell at the molecular scale is often rather naïf. If one could see the interior of a cell with molecular resolution, an aqueous solution of molecules with the cellular organelles suspended would not be seen. The molecular crowding, particularly the macromolecular crowding, inside a cell is such that the interior of a cell is more like a gel than a solution. Molecular packing is so dense that it is hard for macromolecules to diffuse freely. The ubiquitous presence of the cytoskeleton and macromolecular assemblies in a space that is highly restricted due to cellular organelles makes the interior of cells tightly packed (Fig. 2.1). Nevertheless, it is a highly hydrated environment, where solvation is made by water molecules and voids are filled by water that solubilizes ions and small molecules. Thus, virtually all exposed molecules in a cell are under the chemical and physical influence of water. The interior of a cell is not an aqueous solution, but the chemical reactions of the living cells are typical chemical reactions of aqueous solutions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Notes
- 1.
- 2.
- 3.
Ostrowski A et al (2012) The role of training in the development of adaptive mechanisms in freedivers. J Hum Kinet 32:197–210.
- 4.
Jackson DC (2004) Acid-base balance during hypoxic hypometabolism: selected vertebrate strategies. Respir Physiol Neurobiol 141:273–283.
- 5.
Ilardo MA et al (2018) Physiological and genetic adaptations to diving in Sea Nomads. Cell 173:569–580.
- 6.
- 7.
Hogg C et al (2011) Arctic reindeer extend their visual range into the ultraviolet. J Exp Biol 214:2014–2019.
- 8.
Tyler N et al (2014) Ultraviolet vision and avoidance of power lines in birds and mammals. Conserv Biol 28:630–631.
- 9.
Cressey D (2014) Why reindeer steer clear of power lines, Nature News.
- 10.
EarthSky team (2012) Reindeer see a twilight world in UV light. EarthSky News. http://earthsky.org/earth/reindeer-see-a-twilight-world-in-uv-light.
- 11.
Harris J (2006) The dark side of the moon: the making of the Pink Floyd masterpiece. Harper Perennial, London. ISBN: 9780007232291.
- 12.
Osorio B (1911) Phenomenos de phosphorescencia manifestados n’um liquido extrahido d’um peixe da profundidade do Oceano. In: Memorias do Museu Bocage, vol III, pp 1–10. [In Portuguese; available online at https://archive.org/stream/memoriasdomuseub01muse#page/n147 ]. Translated title: Phosphorescence phenomena exhibited by a liquid extracted from a fish from deep sea. A communication of this work was published at the Comptes rendus des scéances de la Société de Biologie de Paris, vol LXXII, Pg 432, 16th March 1912, Une propriété singulière d’une bactérie phosphorescente (premiére note de B Osorio présentée par H. Coutière).
- 13.
Osorio B (1915) Uma propriedade singular de uma bactéria luminosa. In: Arquivos da Universidade de Lisboa, vol II, pp 67–76. [In Portuguese; available online at https://archive.org/details/arquivodaunivers2191univ] Translated title: A singular property of a glowing bacterium.
- 14.
Valjak D (2018) More than 80 years after she passed away, Marie Curie’s remains and personal items are still dangerously radioactive. The Vintage News. www.thevintagenews.com/2018/02/27/marie-curie.
- 15.
Budanovic N (2018) Radium Girls female factory workers exposed to radium poisoning without their knowledge, were even encouraged to lick radium paintbrushes. The Vintage News. www.thevintagenews.com/2018/01/01/radium-girls-2.
- 16.
Grady D (1998) A glow in the dark, and a lesson in scientific peril. The New York Times. www.nytimes.com/1998/10/06/science/a-glow-in-the-dark-and-a-lesson-in-scientific-peril.html.
Selected Bibliography
Dobson CM, Gerard JA, Pratt AJ (2001) Foundations of chemical biology, Oxford chemistry primers. Oxford University Press, Oxford
Gensler WJ (1970) Physical versus chemical change. J Chem Educ 47:154–155
Hubel DH (1995) Eye, brain, and vision. Scientific American Library Series, New York, NY
Author information
Authors and Affiliations
Appendices
Challenging Case 2.1: Going Deep into Acidosis
1.1 Source
This case is inspired by the experience of freediving, one of the daring forms humans use to push the limits imposed by nature. Umberto Pelizzari, an Italian freediver born in 1965, established world records in all the existing disciplines of freediving, being considered among the best of all time. In the profile section of his website, he describes in a very personal perspective the experience of freediving, summarized as the following: “The scuba diver dives to look around, the freediver dives to look inside.”Footnote 1

Umberto Pelizzari. (Picture reproduced with permission from of his website)
1.2 Case Description
Athletes who practice freediving aim to achieve the longest or the deepest breath-hold dive without the use of any breathing apparatus such as a scuba gear. Currently, there are 11 recognized disciplines of competitive apnea defined by AIDA (International Association for the Development of Apnea) and CMAS (Confédération Mondiale des Activités Subaquatiques). Depending on the discipline, the competitors attempt to attain great depths, times, or distances on a single breath, assisted or not by weight to descend or inflatable bag to ascend. As examples of more recent AIDA recognized world records, we have, for static apnea, 11 min and 35 s, by Stéphane Mifsud (men), in 2009, and 9 min and 2 s, by Natalia Molchanova (women), in 2013; and for constant weight apnea, 130 m in 3 min and 55 s, by Alexey Molchanov (men), and 107 m in 3 min and 44 s, by Alessia Zecchini (women), both in 2018 (see more information in AIDA websiteFootnote 2).
Freedivers face as one of their major challenges the interruption of both pulmonary O2 uptake and CO2 excretion, and the primary consequence of this situation is an inadequate O2 delivery to tissues, a condition known as hypoxia. Although hypoxia is experienced by many vertebrates, especially those living in aquatic environments, it is a condition to which human beings, in principle, are not well adapted. Hypoxia is particularly dangerous to brain functioning. The brain of an adult human corresponds to about 2% of its body weight, but accounts for more than 20% of the organism’s energy consumption, as it is the most sensitive organ to oxygen deficit. Brains cannot work anaerobically; very short periods of oxygen deficiency cause unconsciousness as a protective response against brain damage. After few minutes without oxygen, neuron damage may become irreversible, drastically affecting the nervous system functions.
So, under such threatening condition, why are humans attracted to freediving? Probably inspired by aquatic mammals and birds, such as seals, sea lions, whales, and penguins, humans used freediving as a means to gather food: it was a matter of survival for some ancient cultures. Diving was later used in war defenses, such as underwater barricades, and to harvest resources, such as sponges, used for bathing in ancient Greek, as well as pearls, retrieved by divers more than 2000 years ago, mainly in the Indian Ocean. But the development of freediving as a recreative activity has to be explained by other human necessities. In his website, Umberto Pelizzari makes a reflection about his personal perspective of the experience of freediving: “From the depth of 100 m and more, headlong in the abyss, the heartbeat gets slower, the body disappears, and all the feelings take a new form. The only thing that remains in us is the soul. We take a long jump into the soul, which seems to absorb the universe. Every time I ascend, I am making a choice: it is me who is re-discovering myself in my human dimensions, metre by metre, to come up to see the light again. It often happens that I am asked what is there to see deep down in the sea? Maybe the only possible answer is that one does not descend this way to look around, but to look into himself. In the deep I look for myself. This is a mystical experience bordering on the divine. So deep down, I am immensely alone but inside it feels as if all humanity is with me. It is by being human that I surpass the limits we set for ourselves and diving makes us one with the sea and its surroundings. It is here that I become one with the sea and discover my true self. The scuba diver dives to look around, the freediver dives to look inside.”
Being a matter of survival or a whim of human beings, breath-holding and, consequently, O2 deprivation cause a drastic increase in the anaerobic metabolism , which produces lactate and H3O+. This may significantly decrease blood pH, resulting in a condition known as metabolic, or non-respiratory, acidosis. Additionally, the retention of the CO2 produced in cellular metabolism causes an increase in the partial pressure of CO2 (pCO2) in the blood, which may overcome the buffering capacity of the H2CO3/HCO3− system, contributing to the establishment of the acidosis condition, in this case, a respiratory acidosis.
A number of physiological adaptations allow the organism to deal with the hypoxic stress as well as with the acid–base challenge imposed by diving.Footnote 3 This includes a group of cardiovascular responses collectively known as the “diving response,” first described in the classic works of the physiologist Per Fredrik Scholander. The diving response is caused by simultaneous activation of the sympathetic and parasympathetic components of the nervous system and is characterized by (1) bradycardia, i.e., the deceleration of the heartbeat; (2) a selective peripheral vasoconstriction; (3) an adrenal hormonal reaction; and (4) hypometabolism, a remarkable reduction of body metabolic activities.
Regarding acidosis specifically, peripheral vasoconstriction results in a retention of lactic acid and CO2 in the tissues where they are produced, delaying their release into the circulation and, thus, minimizing the metabolic and respiratory components of acidosis.
1.3 Questions
-
1.
The central chemoreceptors responsible for the increase in the rate and depth of breathing are primarily sensitive to changes in the local concentration of hydrogen ions (H+; protons). Based on this fact, explain why one can affirm that the respiratory rate is mainly controlled by the partial pressure of CO2 (pCO2) in the blood (i.e., blood CO2 concentration).
-
2.
The impressive records reported for all disciplines of competitive apnea imply that training improves the adaptative mechanisms of the cardiorespiratory system that minimize the hypoxia-induced tissue damage. Discuss the adaptations that allow elite freedivers or artistic swimmers, for instance, to increase their tolerance to long periods without breathing.
-
3.
The next figure shows the results of an experiment performed with caimans.Footnote 4 Blood lactate concentration, pCO2, and pH were measured during a cycle of diving and surface breathing. Analyze the results and discuss what happens with the metabolic and respiratory components of acidosis during the diving and when the animal returns to the surface and breathes.
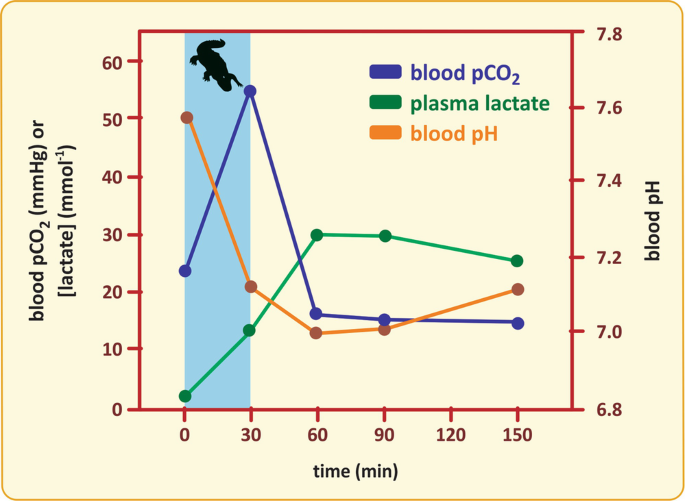
Blood pH variation after a cycle of diving (blue bar—between 0 and 30 min) and surface breathing in caiman (Caiman latirostris). (Figure adapted from the article cited in the footnote 4)
-
4.
Blood pH should be tightly controlled, meaning that the pCO2 vs. HCO3− levels have to be maintained steady. The pH of blood should be around 7.4. According to the American Association for Clinical Chemistry (AACC), acidosis is characterized by a pH of 7.35 or lower. Making use of the Henderson–Hasselbalch equation, evaluate whether the HCO3−/H2CO3− buffer suffices to account for pH regulation within physiological limits.
1.4 Biochemical Insights
-
1.
CO2 dissolved in the blood is converted to di-hydrogencarbonate, or carbonic acid (H2CO3), by carbonase-dehydratase (or carbonic anhydrase), an enzyme present in the erythrocytes. H2CO3 dissociates to bicarbonate (hydrogen carbonate, HCO3−) and hydrogen ions (H+). Therefore, an increase in CO2 concentration in the blood causes an increase in H+ concentration, which, by definition, decreases the pH of the blood. However, it is important to have in mind that the blood–brain barrier (BBB) is not permeable to H+. On the other hand, CO2 easily diffuses across the BBB. Therefore, although changes in plasma pH are not able to stimulate central chemoreceptors, an increase in blood pCO2 directly affects the cerebrospinal fluid (CSF) pH, and is much more effective in triggering respiratory center activity (see below figure).
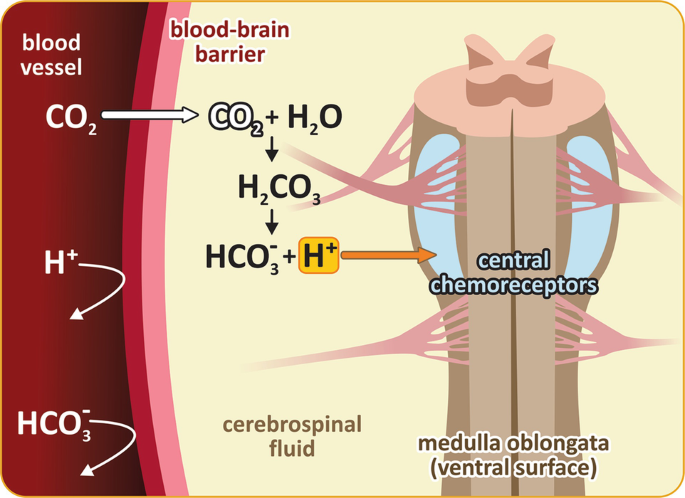
Central chemoreceptors’ stimulation by H+. Since BBB is not permeable to H+, the H+-induced activation of central chemoreceptors is indeed affected by changes in blood pCO2. CO2 easily diffuses across BBB reaching the cerebrospinal fluid (CSF), where it is converted in H2CO3 by carbonic anhydrase, which then dissociates to HCO3− and H+
-
2.
Elite freedivers develop the ability to shift the respiratory stimulus to higher pCO2. However, while this adaptation allows them to hold their breath for longer periods, it may lead to a dangerous decrease in O2 blood saturation during the diving. Thus, freedivers must develop adaptations to increase their tolerance to anoxia as well as to decelerate O2 consumption in the body (see detailed discussion about these adaptations in reference 3).
Different components of the diving response contribute to the adaptation to long-term breath-holding, including bradycardia, hypometabolism, and selective peripheral vasoconstriction. Bradycardia is a diving reflex that generally occurs in sea mammals, being induced by the breath-holding itself but also as a response to the submersion of the face in water, especially when it is cold. The heartbeat may decrease to even 20 beats/min, and this, together with the reduction of body metabolic activities as a whole (hypometabolism), decreases the general cellular demands on O2. Additionally, the selective peripheral vasoconstriction directs the circulating blood to the heart and central nervous system, maintaining O2 delivery to these organs for longer periods without breathing and slowing the rate of O2 depletion by limiting the use of O2 by other tissues. This ensures that the cerebral oxygenation is reduced only slightly until the end of the breath-hold. Another important adaptation is related to the O2 reserve in elite divers’ lungs. It is about threefold higher than in non-divers, allowing that arterial blood O2 saturation goes close to 100% in the well-trained athletes, even after a breath-hold for some minutes.
-
3.
During the diving, the low O2 delivery to the peripheral tissues, especially the muscles, increases the anaerobic metabolism and, consequently, lactic acid production. However, the increase in blood lactic acid concentration is not so prominent because this acid is retained in the tissues due to peripheral vasoconstriction. When the diver returns to the surface and breathes, peripheral circulation is recovered, and lactic acid is flushed into the blood, further increasing acidosis in the first 30 min. However, this condition is ameliorated by pulmonary hyperventilation, which is induced by the high pCO2 reached in the end of the diving period.
-
4.
The Henderson–Hasselbalch equation applied to blood CO2/H2CO3/HCO3− buffering is quite straightforward:
$$ \mathrm{pH}=6.1+\log \frac{\left[{{\mathrm{HCO}}_3}^{-}\right]}{0.03{\mathrm{pCO}}_2} $$This is valid for 1 atm pressure at 37 °C.
The conversion factor has units 0.03 mEq L−1 mmHg−1.
When the ratio [HCO3−]/pCO2 changes, the difference in pH, ΔpH, is:
$$ \varDelta pH=\log \left[{\left(\frac{{\mathrm{pCO}}_2}{\left[{{\mathrm{HCO}}_3}^{-}\right]}\right)}_{\mathrm{initial}}/{\left(\frac{{\mathrm{pCO}}_2}{\left[{{\mathrm{HCO}}_3}^{-}\right]}\right)}_{\mathrm{final}}\right] $$For the sake of simplicity, \( \left(\frac{{\mathrm{pCO}}_2}{\left[{{\mathrm{HCO}}_3}^{-}\right]}\right) \) may by represented by x and so:
$$ \varDelta pH=\log \left[{x}_{\mathrm{initial}}/{x}_{\mathrm{final}}\right]=\log \left[{x}_{\mathrm{initial}}/\left({x}_{\mathrm{initial}}+\Delta x\right)\right] $$Δx is the variation in x: Δx = xfinal − xinitial.
This equation is equivalent to:
$$ \varDelta pH=\log \left[\frac{1}{1+\frac{\Delta x}{x_{\mathrm{initial}}}}\right]=\log \left[\frac{1}{1+{f}_x}\right] $$fx is the increase in x relative to the initial value, xinitial.
This other form of the Henderson–Hasselbalch equation allows for calculating the variation in pH when x varies by a certain percentage (see next figure) in the simplistic scenario of having the buffering system as the only regulator of pH. The onset of acidosis corresponds to a drop in pH of circa 0.05, which is expected for a moderate increase of x of only 12%.
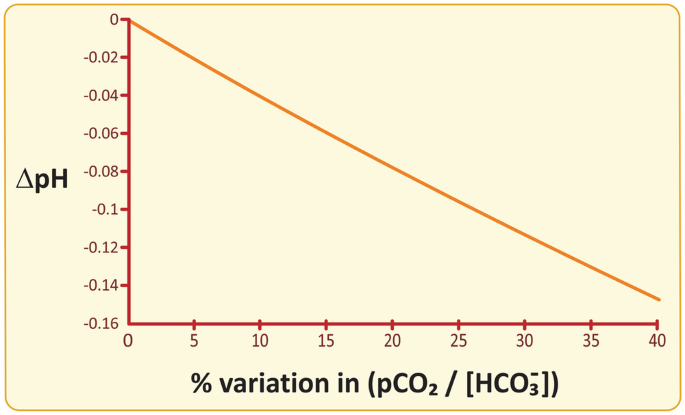
pH dependence on the % variation in pCO2/[HCO3−]. A moderate increase of 12% in pCO2/[HCO3−] would lead to a pH drop of 0.05, i.e., onset of acidosis, if the HCO3−/H2CO3− buffer was the only protective mechanism against respiratory acidosis
Realistically, pH homeostasis in blood depends on three main mechanisms: buffering, respiratory regulation of pCO2, and renal regulation of [H3O+] and [HCO3−]. These factors concur to maintain steady values of \( \frac{{\mathrm{pCO}}_2}{\left[{{\mathrm{HCO}}_3}^{-}\right]} \). Buffering is not verypowerful, but it is certainly very fast as it does not depend on metabolic or physiological processes. The acid–base buffering of the blood is rapidly responsive and therefore is the forefront mechanism against respiratory acidosis.
1.5 Final Discussion
The metabolic adaptations during breath-hold diving also include hormonal responses. As a result of stress caused by breath-holding, an intense production of catecholamines by the adrenal glands occurs. This induces another important adaptation related to the diving response: “the spleen effect ,” which consists of spleen contractions resulting in the injection of an additional supply of oxygenated erythrocytes into the bloodstream (spleen stores erythrocyte-rich blood). This increases the blood oxygen transport capacity enhancing the resistance to the breath-hold diving.
The spleen effect was firstly described for the Ama (means “sea woman”), a traditional group of Japanese pearl divers. They are mainly women and are known to wear white clothes (see below picture).

Ama female divers in Mie Prefecture, in Japan. (Picture credit: Mikimoto Pearl Island)
More recently, a very interesting study published in the scientific periodical CellFootnote 5 revealed a genetic basis for the spleen effect . A research group from the University of Copenhagen performed genomic analyses of the Bajau, a population living in Southeast Asia (see localization in the below map) also known as “Sea Nomads,” whose subsistence lifestyle is entirely based on breath-hold diving. These people are known by their extraordinary breath-holding abilities, spending most of their time underwater and diving over 70 m using only a set of weights and a pair of wooden goggles.

The researchers first compared Bajau’s spleen size with that of another population, the Saluan people, who, although living very close to the Bajau, are not genetically related to them and have a completely different lifestyle, mainly based on agriculture. They found that the Bajau spleen was on average about 50% bigger than the Saluan spleen.
After the comparative genomic analyses, it was possible to find a genetic basis for this difference (see also a comment of the result published in the National Geographic magazineFootnote 6). So, the natural selection of a genetic variant allowed the Bajau to be adapted to their means of survival, explaining, at least in part, their impressive diving abilities.
Challenging Case 2.2: Reindeer Know the Color of UV Light
1.1 Source
Glen Jeffery and colleagues (University College London) discovered that reindeers can see UV (ultraviolet) light and use this perception to find food and stay safe. Sensitivity to UV light was known in insects but is rare in mammals. The study was published in 2011, in the scientific periodical Journal of Experimental Biology .Footnote 7

Cover of the periodical Journal of Experimental Biology, vol. 214, 2011. (Reproduced with permission; image credit: Kia Krarup Hansen)
1.2 Case Description
Living in the Arctic in the winter is challenging for vision : the sun barely rises in the middle of the day, and blue light, the visible light most efficiently scattered, is not abundant. UV light is more efficiently scattered but not perceptible to the vision of almost all animals. However, an amazing exception was found: reindeers can see UV light! Considering snow reflects most UV light that falls on it, this is an enormous advantage. Color patterns not perceived by other species are recognized by reindeers. This is the case of urine waste in the soil, meaning the presence of predators or competitors. Likewise, food such as lichens contrast more with the snow background, making it easier for reindeers to find them. The furs of wolves, for instance, also contrasts more pronouncedly with snow, making camouflage and stealth attacks on reindeers much more difficult. Interestingly, it was known that rodents and some species of bats perceive UV light in other environments, but the advantage of this capability remains elusive in these species.
Jeffery’s team first shone LED light of different wavelengths, including UV, into the eyes of 18 anaesthetized reindeers while recording with an electrode whether nerves in the eye fired, indicating that the light had been seen. The UV light triggered a response in the eyes of all the reindeer. Then they used a UV camera in the Artic to analyze patterns in the environment, as if the camera was an artificial reindeer eye to give a “black and white” idea of the presence of UV stimulus. That was when they noticed the effect caused by urine, lichens, and fur.
Later, Jeffery proposed that sensitivity to UV may be the reason why reindeers, like some other mammals and some birds, avoid power lines.Footnote 8 They see UV discharges as “corona ” of light along power cables and also as intermittent bright flashes from cable insulators. Although this is a plausible explanation for reindeers to avoid power lines during the Arctic dark winter, it is puzzling that the effect during the day, when UV light is everywhere, is the same.Footnote 9
In addition to the interesting facts revealed about reindeer biology, the research raises important perspectives on eye health. In human eyes, UV light causes damages, sometimes irreversible, to the human retina. Reindeers, in contrast, handle UV light without negative consequences. The molecular basis for UV sensitivity and protection are not known. It may be that reindeers have a unique way of protecting themselves. If so, how can we learn from them? “We can learn a lot from studying the fundamental biology of animals and other organisms that live in extreme environments. Understanding their cell and molecular biology, neuroscience, and other aspects of how they work can uncover the biological mechanism that meant they can cope with severe conditions. This knowledge can have an impact on animal welfare and has the potential to be taken forward to new developments that underpin human health and wellbeing,” said rightly by Douglas Kell, Chief Executive of the agency that funded the study of Glen Jeffery.Footnote 10
1.3 Questions
-
1.
Why can humans not see UV light?
-
2.
When using a UV camera to image a person, what differences do you expect to see before and after the person applies a sunscreen cream on his/her face?
-
3.
Plan a simple lab experiment to test the efficacy of sunscreen creams and glass lenses in blocking UV light transmission.
-
4.
Are humans sensitive to IR radiation?
1.4 Biochemical Insight
-
1.
In addition to the wavelength of absorption of visual pigments, human lenses do not let UV radiation through into the eye due to the presence of tryptophan derivatives (see Fig. 2.15).
-
2.
This is the situation presented by the video titled “How the sun sees you” (https://youtu.be/o9BqrSAHbTc), which readers are encouraged to watch. Sun light, UV light included, is reflected by the person in front of the camera. UV light that is reflected or scattered by the skin (or cloths, lenses of glasses, or creams applied on the skin) and impinges on the camera lens is collected by the camera and appears white in the image. On the contrary, UV that is absorbed does not reflect or scatter into the camera and the image is thus black. Therefore, areas of the skin reflecting UV appear white, while areas absorbing UV appear black. When applying sunscreen cream, the areas covered by the cream appear black, as seen in the following figure. Likewise, areas covered by lenses of glasses appear black. This holds even if the glasses are not sunglasses as glass transparent to visible light may absorb UV light .

Sunscreen cream covering part of the cheek contrasts with the unprotected skin. The cream absorbs the UV light with negligible absorption of visible light, so it appears black in the UV camera. (Frame from the video “How sun sees you,” https://youtu.be/o9BqrSAHbTc. Image credit: Thomas Leveritt)
-
3.
The following experiment is very simple, fast, and informative:
-
(a)
Use a quartz glass surface (like the quartz glass used in cuvettes for UV–Vis absorption spectroscopy). Quartz glass is transparent (does not absorb) to UV light. Alternatively, you can use special plastic, transparent to UV light.
-
(b)
Spread a thin layer of sunscreen cream of different protection factors or a regular hydration cream on the cuvette surface.
-
(c)
Place the surface covered with cream layer in the sample compartment of a UV–Vis spectrophotometer, perpendicular to the light beam that impinges on the sample compartment.
-
(d)
Register the UV–Vis spectrum covering the UV and visible region on the spectrum (e.g., 250–500 μm). Do not forget to account for the blank.
-
(e)
Check the cutoff wavelength of light absorption.
Comparing the absorption spectra of the sunscreen creams and the hydration cream allows for studying the reason behind different protective powers. The same experiment can be carried out with glass lenses. In this case, place the lens perpendicular to the light beam directly in the sample compartment. Compare lenses from sunglasses and regular transparent lenses . See typical results for these experiments in the following figure.
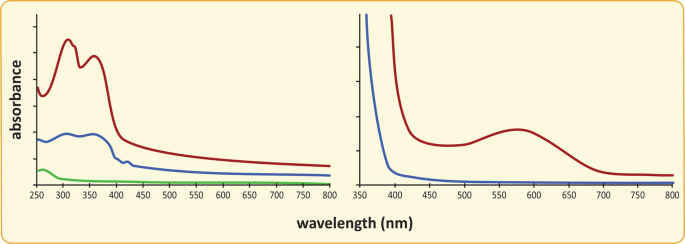
Sunscreen cream UV-Vis absorption spectra. (a) UV–Vis absorption spectra showing the wavelength dependence of the absorbance of sunscreens with factors 50 (red) and 30 (blue), compared to a regular hand cream (green). (b) UV–Vis absorption spectra of lenses of sunglasses (red) and regular transparent glasses (blue). Cutoff absorption wavelengths of 400 and 375 nm are detected for the glasses, and 400 nm for the sunscreen creams, meaning the sunscreen and glasses protect for UV light having wavelength lower than these values. The difference between sunglasses (“dark” lenses) and regular glasses with transparent lenses is the absorption of light in the visible region of the radiation spectrum
-
(a)
-
4.
Yes, but not as visible radiation . Humans perceive IR radiation as heat.
1.5 Final Discussion: Examples of Missing and Misplaced Colors in Literature and Music
Colors no one has seen cannot be named or described. An arbitrary name can be given but arbitrary perceptions are impossible to describe. Unperceived realities are non-existing. They cannot be described, so cannot be communicated, so are no part of the tangible nature. Flicts is a book for children written by a famous Brazilian writer and artist, Ziraldo, about a different color that does not fit in the rainbow, on the flags of the world or anywhere else. The unknown color itself, named Flicts, is the main character of the book. It feels misplaced and sad for not having the strength of color red or the greatness of yellow or the peace that resides in blue. [Bear in mind that blue is the color of the poets, interestingly.] Flicts finally finds its place being the color of the dark side of the moon. The side very few human have seen. Neil Armstrong, the astronaut who first stepped on the moon, confirmed to Ziraldo that the dark side of the moon is flicts after reading the book. Flicts was first published in 1969, the same year Neil Armstrong landed on the moon. The book was a tremendous success, with more than 400,000 copies sold in Brazil and translation to several other languages.
Curiously, “The dark side of the moon ” is the title of one of the bestselling rock albums of modern history, by the band Pink Floyd. It has a song titled “Any colour you like.” It’s instrumental … No lyrics. Further enticing our correlations, the iconic album covers pictures a glass prism causing refraction of light, which divides into different colors as the refraction angle depends on wavelength (violet, having lower wavelength, has the higher refraction angle—see the album cover in the next figure). Should the dependence on wavelength be the reverse, the sky would be red, not blue, and the sunsetting would be blue, not red (as implied from the information in Sect. 2.2). The color of the sky is dominated by the radiation that most scatters in the atmosphere, and the sunset is dominated by the radiation that is less scattered, so more likely to travel directly from the sun into our eyes without being deviated. Strikingly, instead of seven colors, as in the rainbow (a natural refraction of light in raindrops), “The dark side of the moon” covers shows only six: indigo is missing .Footnote 11

Cover and back cover of “The dark side of the moon.” Indigo color is missing. Another curiosity: Imagine many covers and back covers placed side by side and realize they would form a continuum in which a light beam is endlessly decomposed and recombined. Image by Storm Thorgerson/Hipgnosis from cover and back cover of Pink Floyd’s album “The dark side of the moon ,” 1973. (Reproduced with permission of Paul Loasby)
Challenging Case 2.3: Malacocephalus—The Strange Case of the Radioactive Fish from the Deep Sea
1.1 Source
The centennial library of the School of Sciences of the University of Lisbon (Portugal) hides some surprising relics. Among them, a scientific report from 1911, presenting a presumed radioactive fish captured offshore from Sesimbra, Lisbon, in Portugal, caught our attention.Footnote 12

Cover of the fascicle 1 of the “Memórias do Museu Bocage,” 1909. (https://archive.org/details/memoriasdomuseub01muse/page/n2)
1.2 Case Description
Balthazar Osório (1855–1926, Portugal) was a surgeon and a zoologist. He devoted his life to medicine, teaching zoology, and researching on sea fish and crustaceans. Balthazar Osório published a preliminary note in 1911 about a finding in a fish, Malacocephalus laevis, which seems bizarre in present days: a radioactive pocket present in the gut.
Balthazar was puzzled by the ancient practice of the fishermen of Sesimbra in baiting named “candil.” They used to fish “rat fish” (Malacocephalus laevis; see next figure) at 400 m deep, squeeze its belly, and scrub the glowing substance coming out of the belly on the inner side of skin previously removed from shark species such as Scyllium canicula and Pristiurus artedi (Galeus melastomus). The inner part of the skin would still be covered with a muscle tissue layer and would preserve the liquid glowing for more than 20 h. Even if the skin was not hydrated for some days and had lost its brightness, glowing was recovered if the skin was immersed in seawater. The skin would then be cut in pieces and the glowing pieces put above the hooks in the fishing line to attract fish. Candil could also be used as bait.

(Upper panel) Malacocephalus laevis as drawn in the report of the Challenger (UK) expedition (1873–1876) by Albert Günther. The apertures in the belly were noticed and highlighted in the illustration. (Lower panel) Illustration of Balthazar’s paper: The protrusion in the belly is also highlighted. The common name in Sesimbra, Portugal, is “peixe rato” (“rat fish”), but in other parts, it is named softhead grenadier, armed grenadier, or smooth-headed rattail, for instance
According to Balthazar’s description, the substance was collected from a protuberance near the anal orifice of the “rat fish” (see previous figure), was dense/jelly and yellowish in color, and glowed blue very intensively. The biological role of the liquid was elusive and the early twentieth century technology did not allow to observe the fish in its own environment, 400 m deep. He hypothesized that the fish would release the illuminating substance in order to get a better vision around to hunt or attract preys but could not test this hypothesis. Balthazar also noticed that the protuberance formed by the organoid that contained the glowing matter could eventually serve as a lamp. He devoted his efforts to unravel the chemical nature of the glowing substance and its photophysical properties. Two main reasons convinced Balthazar that the glow would emanate from bacteria: (1) the muscle tissue in the inner side of the shark skin is an excellent growing medium for bacteria, and (2) the recovery of glow after immersion of the dried skin in seawater seemed “reviviscence,” an old uncommon name for “life recovery” typical in bacterial populations. In a follow-up work, in 1915, he reported the confirmation of this hypothesis.Footnote 13 The jelly substance indeed contained bacteria. He cultivated and observed the bacteria on a microscope. The bacteria looked like bacillus and would have 1.6–2.6 μm length and half this distance in width.
Another naturalist, named Fisher, had described a smaller (1.15–1.75 μm) glowing bacterium found in the waters of the “Indies” (Caribean): Bacillus phosphorescens . Nevertheless, Balthazar was in the presence of bacteria living enclosed inside fish, probably in symbiosis, while Fisher described free-floating bacteria. More importantly, Fisher’s bacillus would glow white, slightly bluish, while Balthazar’s bacillus would glow pure blue. “Blue is the light from burning carbon fuels”, he described. Based on the observations at the microscope, on the very specific environment of the species (inside a specific closed anatomic structure of a fish), and the unique characteristics of the color of the glowing light, Balthazar was convinced he discovered a new species and named it Bacillus malacocephali to stress the intimate relationship between the hosting fish (Malacocephalus) and the bacterial species he was classifying.
The light emitted by the bacteria was in itself a mystery Balthazar was committed to solve. For this, he used the technology available in the 1910s:
-
1.
Spectral analysis of the light using a glass prism to find what basic colors (wavelengths in modern analysis) it was made of. He was convinced the light was due to “phosphorescence” of chemical elements in bacterial composition and was determined to identify the element(s) through the identity “fingerprint” mixture of lights emitted.
-
2.
Photographic analysis of the glow to study the ability to be registered in different kinds of photographic paper. In the early days of photography technology, it was not obvious that all visible light would interact with light-sensitive doped surfaces the same way.
-
3.
The electroscope assay to check if light emission is associated with radioactivity. Radioactivity was known but the biological effects of radioactive radiation were not. The hypothesis that the bacteria could contain radioactive elements in enough quantity to glow strong seemed plausible at that time. The laws of physics of electricity and electrostatics were not as developed and widely disseminated as today.
Balthazar found that the spectrum of light was made of a continuum of colors (wavelengths) between the “B and F lines of Fraunhofer.” Fraunhofer’s set of “lines” were wavelength/color markers typical of atmospheric gases that were used to establish a standard scale. B and F “lines” are marks that correspond to 686.7 nm and 486.1 nm, respectively, perceived as colors red and blue, respectively (see next figure).

(Left panel) Fraunhofer lines in the visible spectrum of light. When sunlight traverses the atmosphere, its gases absorb light of well-defined wavelengths, so that the sunlight spectrum at the surface of the Earth has decreased intensity at those wavelengths. These sharp wavelengths at which intensity is sharply decreased are named Fraunhofer lines (indicated by the red arrows in the intensity spectrum and the black lines in the visible spectrum in the top). Lines B and F correspond to atmospheric H-containing gases and O2 absorption of sunlight. (Right panel) A normalized bioluminescence spectrum of emission of Photobacterium phosphoreum (“Bacillus malacocephali”) using a modern spectrofluorometer
A continuum spectrum was puzzling as chemical elements and simple diatomic molecules have very well-defined wavelengths of emission. Balthazar was hoping to find a new chemical element. “If the rocks and the atmosphere have revealed most metals and metalloids known, it is reasonable to assume that the sea, given its immense extension, or the land covered by the sea, will also reveal new elements,” he wrote. Nature goes much further than human expectation and Balthazar got a puzzling result. A new element could not be inferred … And it was not a known element either. The answer had to wait for developments in the area of metabolism. Chemistry and physics were developing scientific disciplines in the 1910s but biochemistry was still incipient.
The analyses with the photographic paper were less puzzling. Using paper with silver bromide from the still existing manufacturer Kodak, he found that the light from Bacillus malacocephali would “impress” the paper quickly: 30-s exposure in standard conditions was enough, which was considered relevant given “the small quantity” of bacteria used. Balthazar then used a filter with a photo imprinted between the source of light (glowing tubes) and the photographic paper and obtained a reproduction of the photographs used as filters. Moreover, he used the glow to illuminate a silver coin and photograph it. A clear photo was obtained. With proper exposition time, the glowing light was as competent to generate photos as daylight. Importantly, bacterial suspension and extracts of bacteria had the same result.
The experiment with the electroscope is straightforward but led Balthazar to a misleading conclusion. Strangely, the description of the procedure is detailed and seems to be carried out thoroughly, duly controlled but, even so, not reliable. He “loaded” the electroscope (see below figure), meaning static electricity potential was applied, and the two metallic foils made of aluminum repealed each other. When the fish organoid was placed near the pole, the two foils came together. To make sure the result was due to the light and not the organic tissues of the fish, he emptied the organoid of glowing matter and repeated the procedure. The two foils did not change position. He then repeated the experiment with seawater containing the glowing liquid. The two foils moved closer to each other albeit not as extensively as with the fish organoid. The conclusion was obvious: the glowing extract taken from Malacocephalus laevis was radioactive!

Basic scheme of an electroscope . Two metallic foils are suspended from a metallic wire connected to a metallic surface on top. When the electroscope is loaded, electrons are provided or removed from these metallic systems through electrostatics. The two foils then acquire the same charge and repel each other. When neutrality of the metal parts is recovered, the two foils come together
1.2.1 A Modern Interpretation
In the 1910s, scientific knowledge did not allow Balthazar Osorio to understand that the conclusion on a radioactive anatomic structure in a living being was not plausible. The low availability of radioactive elements in nature and the extreme ionizing effects of this class of radiation make Balthazar’s conclusion barely possible. Failure in finding a chemical element to which to assign light emission and the weak knowledge yet available on radiochemistry pushed him into a wrong conclusion, which was also one that seemed possible at that time. Today’s explanation relays on knowledge in photochemistry, radiochemistry, and biochemistry of metabolism that became available much later than the 1910s. It is now known that more than half of the approximately 300 species of macrourids (the taxonomic family that includes the Malacocephalus genera) have bulbous ventral light organs (named photophores) that are situated anterior to the anus and from which a duct opens into the perianal groove of the rectum. Lenses and reflector systems (see next figure) may be present, and the light may be diffused throughout specialized regions of the ventral musculature. The bulbs harbor symbiont bacteria that can readily be cultured in vitro. Isolates from species from three genera other than Malacocephalus have been identified as Photobacterium phosphoreum , but it is likely that other macrourids harbor the same species. This bacterium is adapted to low temperature and high pressure in contrast to other related species such as P. fischeri or Baneckea harveyi. It is not known for sure if P. phosphoreum is Bacillus malacocephali after being reclassified by modern taxonomy or if, on the contrary, the work of Balthazar was never acknowledged and considered.

Basic structure of a photophore, located in the dermis with an aperture in the epidermis (a). The aperture consists of a lens made of chitin or protein (f). The bioluminescence-producing cells, such as Photobacterium phosphoreum , are generally referred to as photocytes (c). Light emitted by photocytes is reflected in a mirror surface (e) made of guanine crystals, proteins, collagen, or chitin, supported in a pigmented layer of chromatophores (d). Light wavelength/color is then refined by an absorption or interference filter film (b) before being refracted by the lens (f)
In fact, the light emitted by the symbiotic bacteria is not caused by the presence of a chemical element. If this was the case, Balthazar would have observed discrete wavelengths/colors of emission. Moreover, light emission would only be observed after absorption of light, which would make light emission at 400 m deep in the oceans impossible as sunlight reaching this depth is very faint. Instead, light emission is owed to organic molecules, which explains the continuum of wavelengths observed by Balthazar, and is part of a process that depends on the metabolism of bacteria. The process exists in many bacteria and is named bioluminescence (see next figure). An oxidation of an electron donor molecule is catalyzed by the enzyme luciferase, the electrons being transferred to molecular oxygen, O2, while complexed to luciferase. This complex is unstable and highly energetic. “Excess” energy is released as a photon, a “particle” of light (or “quantum”). In some cases, this light is the observed bioluminescence; in other cases, the energy is transferred to fluorescent proteins that will emit light of well-defined wavelength/color. Different species may have their own kinds of proteins, therefore emitting light of species-specific colors. The process of conversion of energy from a chemical source into light in this case is incredibly efficient: nearly one photon emitted per molecule oxidized, i.e., 100% conversion efficacy.

(a) Outline of bioluminescence reactions. (b) Chemical structure of the most common electron donor molecules, generally named luciferins. One species-dependent luciferin combines with an enzyme, luciferase, forming a complex that reacts with oxygen. The redox process has a “highly energetic” intermediate that releases “excess energy” as a photon, a “particle” of light, or resonates with a fluorescent protein that will capture the energy and fluorescent light of a given species-specific color
1.3 Questions
-
1.
Propose advantages that bioluminescence might confer to Malacocephalus laevis.
-
2.
Propose hypotheses to explain why the color of bioluminescence associated with most deep-sea fishes is blue.
-
3.
Bioluminescence-generating metabolism uses molecular oxygen (O2). This means its metabolism is relatively recent and may have started for reasons other than bioluminescence. Propose an explanation.
-
4.
Why is hosting radioactive bacteria not plausible in light of current scientific knowledge?
-
5.
Propose an explanation for the misinterpretation of the electroscope experiment.
1.4 Biochemical Insight
-
1.
Bioluminescence enables animals to communicate through signals encoded in the frequency and duration of light flashes, hunt by luring preys, evade predators creating diversion, or camouflage. Fish living at depths reached by sunlight and having the ability to emit blue light from their ventral surface typically use bioluminescence for camouflage. When seen from below against the water surface background, the fish are easily detected by the shadow they cause. Blue light emission counteracts the shadowing and allows the fish to better escape from predators.
-
2.
Water is not totally transparent. Because of its molecular structure, water absorbs energy that contributes to several vibrating modes. This energy may be light of well-defined wavelengths in the infrared region of the radiated spectrum. A very weak absorption of radiation occurs in the red-infrared transition region. Small volumes of water, such as in a test tube or a domestic drinking glass, do not absorb significant quantities of the sun radiation and appear transparent, yet higher volumes absorb red radiation and the water appears to have the complementary color: green. This is why by the seaside, at shallow depths, the clear water is transparent but, as the depth increases, the perceived water color changes to green. At even higher depths, the volume of water that absorbs light is such that red and green light are absorbed. In other words, only blue light is not yet absorbed. This is the reason why divers at depths 20–500 m see the aquatic environments in shades of blue, the dominant color (see below figure). In fact, blue light is, in practice, the only illumination from sun available below 100 m. Therefore, camouflage demands blue light. At depths below 500 m, even blue light is absorbed due to the cumulative effect of the mass of water gradually absorbing light from the surface to the bottom of the ocean. The end result is total darkness. If water was totally transparent, sunlight would shine even at the deepest abyss trenches, kilometers away from the surface.
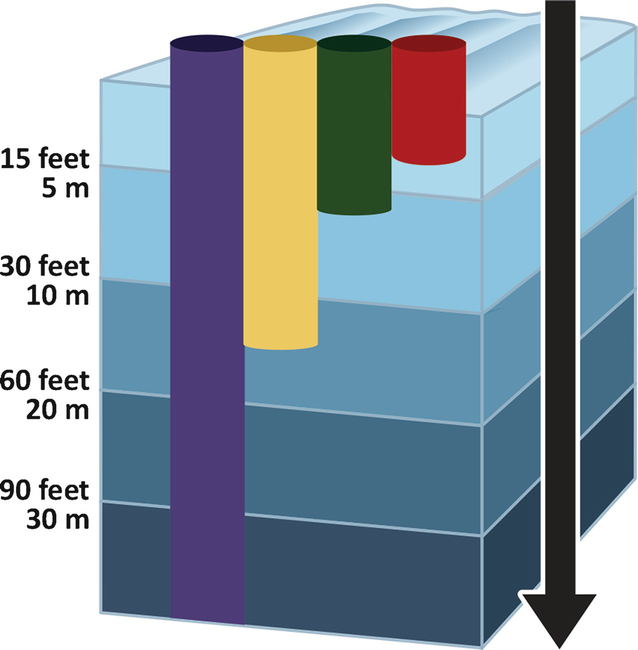
Sunlight penetration in water. Up to circa 3 m depth, all light wavelengths are not significantly absorbed; water is transparent and the sunlight remains “white.” At higher depths, blue light is increasingly dominant because red and yellow radiation are absorbed. At 30 m deep, only blue light illuminates the environment. This situation persists down to 500 m, where illumination is so dim it vanishes
-
3.
The series of chemical reactions that cause bioluminescence differ among different organisms but they all follow common principles (see the last figure in the case description section): a redox reaction brings a molecule to a higher energy state and the “excess energy” is released by means of light emission. Molecular oxygen (O2) is the species that accepts the electrons, water being formed. This rudimentary form of extracting energy from oxidation of organic molecules and having O2 as final electron acceptor encompasses the complex form of oxidative phosphorylation by which many animals obtain energy from nutrients. The main difference being that instead of the energy being released by light emission, energy is stored as ATP (as will be detailed in Sect. 6.2).
O2 released into the atmosphere by primitive photosynthetic processes was extremely oxidant and therefore toxic. O2 can be reduced to form water, which is a form of protection by O2 consumption. Cells able to reduce O2 were the best fitted to survive in the new oxidative atmosphere being formed by algae ancestors (see next figure). Bioluminescence, which is a form of O2 disposal, may have been a form of protection in the early times of massive atmospheric O2 poisoning. In this sense, it may have been the reverse of photosynthesis: photosynthesis consumed light to synthetize organic molecules, O2 being a by-product, whereas bioluminescence used organic molecules to produce light, making use of O2. Later, some cells evolved into not wasting energy as light but rather to synthetize other molecules that could be used in their own chemical reactions. Some cells remained bioluminescent and took advantage of light emission in different situations. In bacteria and fungi, for instance, light emission is not essential to survival. Bioluminescent bacteria and fungi develop even in condition in which bioluminescence is halted.

Sedimentary fossil of cyanobacteria stromatolites (2000 million years) from Pilbara, Western Australia. Stromatolites produced the oxygen that accumulated in the sea and the atmosphere. Many organisms that had lived without oxygen went extinct in what was probably a huge extinction event. (Collection of the Museu Nacional de História Natural e da Ciência, Universidade de Lisboa, Portugal, Inventory reference MUHNAC-MNHN/UL.II.495)
-
4.
Radioactive elements are present in Earth biomes in trace amounts, not enough to accumulate extensively in living cells and emit the light intensity observed in bioluminescent species. Moreover, even if this was the case, the energy of radioactive radiation is such it can break covalent bonds in organic molecules, i.e., it is highly ionizing radiation. Exposure to radioactivity, depending on duration and radiation intensity, is extremely aggressive to cells. Even extremely resistant cells, such as Deinococcus radiodurans (see Box 2.2), cannot survive for long in these conditions.
-
5.
Radioactive materials discharge the electroscope because ionization caused by radioactivity neutralizes the metallic foils, therefore ending repulsion between them. Other materials able to exchange ions with the metal in the electroscope may have the same effect. Likewise, light able to remove electrons from the metal by photoelectric effect (electron release by impinging light) may have the same effect inasmuch the electroscope is charged negatively. In principle, only ultraviolet light can remove electrons from non-alkali metals, but longer wavelengths suffice if they are present. It may be that Balthazar observed the discharge of the electroscope due to redox and other electric phenomena in the sample where bioluminescent processes were active and/or due to the effect of blue light impinging on the impure alkali-oxidized surface of the metal of the electroscope. The first cause seems more plausible as Balthazar made a through and carefully planned set of experiments, with all proper controls; therefore, extensive oxidation of the metal was not likely.
1.5 Final Discussion
1.5.1 Scientific Knowledge Through Time
World War I in Europe did not create a proper environment for sharing knowledge among nations, and the work of Balthazar may have been overlooked. Before him, a famous naturalist, Albert Günther (1830–1914), keeper of the Department of Zoology in the British Museum, who participated in the scientific expedition of the ship “Challenger,” in 1873–1876, had already described one individual specimen of Malacocephalus laevis caught near Pernambuco, in Brazil (shown in the first figure in this case). Before that, the only specimen known was the one classified originally by Richard Thomas Lowe (1802–1874), caught at the Madeira Island (Portugal). The British Museum conserved in its collection another specimen caught also in Madeira Island before 1915.
The glowing bacterial species was classified by Balthazar as Bacillus malacocephali , but it probably is Photobacterium phosphoreum . Because microbial taxonomy was not so well developed and exchange of knowledge and information dissemination was slow, this bacterium was classified as a new species differently by many researchers in several countries: Micrococcus phosphoreus Cohn 1878, Bacillus phosphorescens II Baumgarten 1888, Photobacterium phosphorescens Beijerinck 1889, Bacillus hermesi Trevisan 1889, Bacillus phosphoreus (Cohn) Mace 1901, Photobacter phosphorescens Beijerinck 1901, Bacterium phosphoreum (Cohn) Molisch 1912, Photobacter phosphoreum (Cohn) Beijerinck 1916, Photobacterium phosphoreum (Cohn) Ford 1927, Micrococcus physiculus Kishitani 1930, Coccobacillus acropoma Yasaki and Haneda 1936, and Photobacterium profundum Weisglass and Gavrilovic 1963, for instance .
1.5.2 Luminescent Art
Luminescent bacteria are easy to cultivate in vitro and may glow bright enough to produce a reasonable “bio-lamp.” In the World Exhibition of 1900, in Paris, Dubois had impressed the visitors by illuminating a pavilion, “The optics palace,” with bioluminescent bacteria in suspension in 25 L containers. For the 1900s standards, the light produced was comparable to light from artificial sources in other pavilions. In present days, bioluminescence cannot compete with artificial illumination systems, but bacterial glow has been translated into the world of artistic creation. Bioluminescent art exhibitions are a reality and the works presented are impressive. There are even festivals of bioluminescent art such as the Bioluminescence Festivals in Auckland, New Zealand .
1.5.3 Bioluminescent Bays
There are many species of aquatic microorganisms that respond to mechanic stimuli with light emission. They are the reason behind the luminescence sometimes observed in the revolved water when boats move (named wake). In extreme cases, the density of luminescent microorganism in water may be such that a night swim is a dazzling spectacle. Vieques Bay in Puerto Rico, for instance, is world famous for the glowing water where waves break around moving swimmers . Elucidative photos are available in https://vieques.com/island-bioluminescent-bay/.
1.5.4 Alternative Case Study: Marie Curie and the Radium Girls
Unaware of the biological effects of radioactivity, Marie Curie (Nobel Prizes in Physics and Chemistry in 1903 and 1911, respectively, for the studies on radioactivity) was exposed for decades to materials containing radium and polonium, elements she contributed to discover. Radium’s bluish glow dazzled a lot of people, Marie Curie included, for being fancy. The hidden danger was only realized later. In the 1920s, colleagues of Marie Curie and radiation workers died from leukemia. Marie Curie’s decades of exposure left her chronically ill and nearly blind from cataracts, and ultimately caused her death at 67 years old, from either severe anemia or leukemia. But she never fully acknowledged that her work had ruined her health. Her daughter, Irene Joliot-Curie, and son-in-law, Frederic Joliot-Curie—also Nobel Prize winners—continued her work with radioactive material. Eventually, both also died of diseases induced by radiation. What makes radium so dangerous is that it forms chemical bonds in the same way as calcium, and the human body can absorb it into the bones. Then, it eradiate cells at close range, which may cause bone tumors or bone marrow damage that can give rise to anemia or leukemia.
Marie Curie died in 1934. She was buried at the Sceaux Cemetery, in Paris. However, in 1995, her remains were transferred to the Pantheon. When she was exhumed from the original resting place, she was so radioactive she was interred in the Pantheon in an inch-thick lead coffin that prevent the radiation from crossing. Actually, since the unfortunate Curies were contaminated with radium 226, the most stable isotope of radium that has a half-life of approximately 1500 years, their remains will stay dangerously radioactive for at least that long.Footnote 14 Furthermore, since Marie Curie was not aware that her experiments were harmful, she contaminated her entire household along with many of her personal items. She frequently carried samples of radium and polonium in the pockets of her lab coat and brought them home to analyze in her spare time. She unwittingly contaminated all of her clothes; her books, notebooks, and cookbooks; and her jewelry, furniture around her home, and various other personal items.
In 1938, radioactive consumer products were banned in the USA by an act issued by the Food and Drug Administration. By that time, it was known that radioactive elements can be used as a powerful and efficient energy source, but also that their effects on health can be devastating.
Another case in which fascination for the glow of radioactive materials combined with ignorance of its biological hazards cost human lives was the case of the so-called Radium Girls, factory workers exposed to radium poisoning in the wristwatch industry. In the 1910s, a special paint containing radium was used to illuminate numbers and dials in watches so they could be seen glowing in the dark. The girls working in the factories were even encouraged to lick the tip of their radium-contaminated brushes that were used to paint the numbers and dials, so that the brush would gain a finer point. Completely unaware of the dangers concerning radium, around 4000 women hired by various radium-dial companies were poisoning themselves on a daily basis. The glow-in-the-dark paint was perceived as so harmless that the women would goof around, painting their lips and nails with it, until the first symptoms of poisoning began to surface.Footnote 15 The dial painters’ first health problems turned up in the 1920s, when some of the women began suffering from fatigue, anemia, and trouble with their teeth. When dentists tried to extract the bad teeth, they were horrified to find jawbones so diseased that chunks of bone came out as well.Footnote 16 The extraction sites didn’t heal, and infections set in. In many cases, the women’s bodies were actually radioactive, because radium had been absorbed by their bones. Government researchers studied live and dead dial painters and used the data to calculate safe exposure levels for future generations of workers. Radium Girls have become a notorious, but tragic, chapter in the history of occupational medicine.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Da Poian, A.T., Castanho, M.A.R.B. (2021). The Chemistry and Physics of Life. In: Integrative Human Biochemistry. Springer, Cham. https://doi.org/10.1007/978-3-030-48740-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-48740-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48739-3
Online ISBN: 978-3-030-48740-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)








