Abstract
Laser interstitial thermal therapy (LITT) offers a minimally invasive approach to the treatment of both regrowth of brain metastases and/or radiation necrosis following radiosurgery (known together as metastatic in-field recurrence). Current indications for LITT include patients who have KPS >70, have good expected survival or systemic therapy options, and are deemed suitable surgical candidates. While LITT treatment was previously offered when patients required initiation of steroids for symptom management, LITT results have been shown to be better when the lesion is smaller and therefore there is a current trend toward using LITT earlier in the course of the progression of these lesions. While surgical access is less invasive, adverse neurological outcomes following LITT are similar to those encountered in a craniotomy.
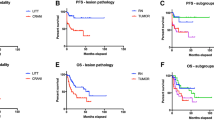
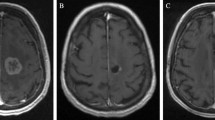
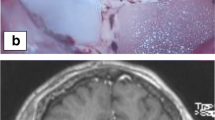
Post-LITT imaging changes can be variable but generally follow a trend of an initial increase in the size of contrast-enhanced volume followed by a steady decrease over the subsequent 3–6 months. Surprisingly, FLAIR volumes often do not increase but rather can decrease rapidly in some cases and is often associated with the ability to wean steroids. With regard to clinical outcomes, recent multicenter prospective series suggest worthwhile short-term progression-free survival rates for LITT, with better outcomes for patients with radiation necrosis as opposed to tumor regrowth. LITT also resulted in an improvement in neurological outcomes and preservation of KPS in many patients. Pilot data suggest that LITT may serve as an alternative to a craniotomy in these patients and additional benefit may be a result of disruption of the blood-brain barrier by the LITT procedure, thus potentially having implications on post-LITT therapeutic management. Larger prospective studies with longer-term follow-up are needed to further refine the indications for LITT and clarify its role in the cancer survivor.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- BBB:
-
blood-brain barrier
- BSE:
-
brain-specific enolase
- HVLT-R:
-
Hopkins Verbal Learning Test-Revised
- ICH:
-
intracerebral hemorrhage
- KPS:
-
Karnofsky Performance Score
- LAASR:
-
Laser Ablation After Stereotactic Radiosurgery study [5]
- LITT:
-
laser interstitial thermal therapy
- MMSE:
-
Mini-Mental State Examination
- MRI:
-
magnetic resonance imaging
- N/A:
-
not available
- PFS:
-
progression-free survival
- POD:
-
progression of disease
- QOL:
-
quality of life
- RN:
-
radiation necrosis
- SF-36:
-
Short-Form Health Survey
- SRS:
-
stereotactic radiosurgery
- TR:
-
tumor regrowth
- WBRT:
-
whole brain radiation therapy
References
Sneed PK, Mendez J, Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123(2):373–86. https://doi.org/10.3171/2014.10.JNS141610.
Miyatake S, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo). 2015;55(Suppl 1):50–9.
Furuse M, Nonoguchi N, Kawabata S, Miyatake S, Kuroiwa T. Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Med Mol Morphol. 2015;48(4):183–90.
Hernandez RN, Carminucci A, Patel P, Hargreaves EL, Danish SF. Magnetic resonance-guided laser-induced thermal therapy for the treatment of progressive enhancing inflammatory reactions following Stereotactic radiosurgery, or PEIRs, for metastatic brain disease. Neurosurgery. 2019;85(1):84–90. https://doi.org/10.1093/neuros/nyy220.
Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–11. https://doi.org/10.3171/2017.11.JNS171273.
Rosomoff HL, Carroll F. Reaction of neoplasm and brain to laser. Arch Neurol. 1966;14(2):143–8.
Carpentier A, McNichols RJ, Stafford RJ, Itzcovitz J, Guichard JP, Reizine D, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(1 Suppl 1):ONS21–9. https://doi.org/10.1227/01.neu.0000335007.07381.df.
Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neuro-Oncol. 2013;113(3):495–503. https://doi.org/10.1007/s11060-013-1142-2.
Rao MS, Hargreaves EL, Khan AJ, Haffty BG, Danish SF. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery. 2014;74(6):658–67. https://doi.org/10.1227/NEU.0000000000000332.
Ali MA, Carroll KT, Rennert RC, Hamelin T, Chang L, Lemkuil BP, et al. Stereotactic laser ablation as treatment for brain metastases that recur after stereotactic radiosurgery: a multiinstitutional experience. Neurosurg Focus. 2016;41(4):E11.
Chaunzwa TL, Deng D, Leuthardt EC, Tatter SB, Mohammadi AM, Barnett GH, et al. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(1):56–63. https://doi.org/10.1093/neuros/nyx142.
Smith CJ, Myers CS, Chapple KM, Smith KA. Long-term follow-up of 25 cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery. 2016;79(Suppl 1):S59–72.
Patel PD, Patel NV, Davidson C, Danish SF. The role of MRgLITT in overcoming the challenges in managing infield recurrence after radiation for brain metastasis. Neurosurgery. 2016;79(Suppl 1):S40–58.
Patel NV, Jethwa PR, Barrese JC, Hargreaves EL, Danish SF. Volumetric trends associated with MRI-guided laser-induced thermal therapy (LITT) for intracranial tumors. Lasers Surg Med. 2013;45(6):362–9. https://doi.org/10.1002/lsm.22151.
Patel PD, Hargreaves EL, Danish AF, Weiner J, Danish SF. Volumetric trends of progressive in-field recurrences after stereotactic radiosurgery of metastatic intracranial tumors. J Radiosurg SBRT. 2018;5(4):293–304.
Nath SK, Sheridan AD, Rauch PJ, Yu JB, Minja FJ, Vortmeyer AO, et al. Significance of histology in determining management of lesions regrowing after radiosurgery. J Neuro-Oncol. 2014;117(2):303–10. https://doi.org/10.1007/s11060-014-1389-2.
Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: single-center experience with the Visualase thermal therapy system. J Neurosurg. 2016;125(4):853–60.
Beechar VB, Prabhu SS, Bastos D, Weinberg JS, Stafford RJ, Fuentes D, et al. Volumetric response of progressing post-SRS lesions treated with laser interstitial thermal therapy. J Neuro-Oncol. 2018;137(1):57–65. https://doi.org/10.1007/s11060-017-2694-3.
Carpentier A, McNichols RJ, Stafford RJ, Guichard JP, Reizine D, Delaloge S, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–50. https://doi.org/10.1002/lsm.21138.
Stewart T, Tsai SC, Grayson H, Henderson R, Opelz G. Incidence of de-novo breast cancer in women chronically immunosuppressed after organ transplantation. Lancet. 1995;346(8978):796–8.
Rammo R, Asmaro K, Schultz L, Scarpace L, Siddiqui S, Walbert T. The safety of magnetic resonance imaging-guided laser interstitial thermal therapy for cerebral radiation necrosis. J Neuro-Oncol. 2018;138(3):609–17. https://doi.org/10.1007/s11060-018-2828-2.
Hong CS, Deng D, Vera A, Chiang VL. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neuro-Oncol. 2019;142(2):309–17. https://doi.org/10.1007/s11060-019-03097-z.
Leuthardt EC, Duan C, Kim MJ, Campian JL, Kim AH, Miller-Thomas MM, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613. https://doi.org/10.1371/journal.pone.0148613.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sujijantarat, N., Danish, S.F., Chiang, V.L. (2020). LITT for Metastatic In-Field Recurrence. In: Chiang, V., Danish, S., Gross, R. (eds) Laser Interstitial Thermal Therapy in Neurosurgery. Springer, Cham. https://doi.org/10.1007/978-3-030-48047-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-48047-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48046-2
Online ISBN: 978-3-030-48047-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)