Abstract
[IX] The most important motive that stimulated the research on the laws that make up the subject of thermodynamics was the wish to find the cheapest way to obtain work from the forces of nature.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Notes
- 1.
P. Epstein has given evidence of interesting data in his Textbook of Thermodynamics, which shows the important contribution to the discovery of the first law by the Medici.
- 2.
The third edition of the textbook by V. D. Waals, edited by prof. Ph. Kohnstamm, does contain a treatment of the “Second Law” (Zw.H) directly related to the receptions of this book. This is also partly the case for the course by L. G. de Haas-Lorenz. Compare also with A. Landé: Axiomatiske Begründung der Thermodynamik von C. Caratheodory. Handb. d. Phys. Bd. IX, p.282, §I. (Herausgeg. C. H. Geiger u. K. Scheel Verl. Julius Springer.)
- 3.
Regarding the true meaning of the H-Function for the “Second Law,” c.f. appendix II, and regarding the reconciliation incompatible things, c.f. appendix III.
- 4.
C.f. citation.
- 5.
Appendix I, Note 2, p. X
- 6.
Our definition of “equilibrium” relies, indeed, on an idealized extrapolation of experience: nowadays one also thinks about the spontaneous departure from a state that remains unchanged for and an extremely long period of time: this in connection with the meaning of the thermodynamics quantities by means of the kinetic theory. The quantum-mechanical interpretation also does not assume any infinitely lasting states of equilibrium.
- 7.
C.f. §25.
- 8.
C. Carathéodory, Untersuchungen über die Grundlagen der Thermodynamik. Math. Ann. 67, 335, 1909.
- 9.
M. Born, Kritische Betrachtungen zur traditionellen Darstellung der Thermodynamik. Phys. ZS. 22, 218 und 282, 1921.
- 10.
The critically examined determination of these concepts can be found in the above-cited work of Carathéodory, and this discussion will be ignored in this paper. See also C. Carathéodory: Über die Bestimmung der Energie usw. Berl. Ber. 1925.
- 11.
This does not imply that the system has to be adiabatically isolated from its environment. Think of a system composed of two gases that are separated from one another by a moving and adiabatically isolating piston. One can let this system undergo a change of state, throughout which dQ = dQ1 + dQ2 = 0 is satisfied constantly, but dQ1 = −dQ2 ≠ 0, and both parts of the system, that are adiabatically isolated from one another, respectively, take this quantity of heat from and transfer this quantity of heat to their environment.
- 12.
See §5 of this paper.
- 13.
One means “unattainable by means of a quasi-static adiabatic process,” as only this is necessary for the definition of entropy.
- 14.
(Annotation made in the correction of this paper): it would have been better to let the axiom of uniqueness precede the coupling axiom.
- 15.
A counterexample is given by the case of the coupling “after the term dW,” e.g., by the coupling of the work done in order to change the volume of a gas (Druckarbeit): two systems can exchange work in such a way that either the pressures are equal, or otherwise only all the forces that they exert on one another are equal without the pressures being equal.
Due to this state of affairs, one can extract work from a reservoir of constant pressure (and consequently transform it into heat):

Let the system has to be a quantity of gas isolated by two movable pistons a and b, which are of the same size. The reservoir consists of an infinitely large quantity of gas that is enclosed by two pistons 1 and 2 of different size. One can, alternatively, let piston 1 press on piston a and piston 2 press on piston b. In the first case, we have \(dW = p_{1} Sdl\) for the System and \(dW^{\prime } = pS_{1} dl^{\prime }\) for the reservoir, where \(S\) and \(S_{1}\) are the cross sections of piston a and 1, respectively, \(p_{1}\) and \(p\) are the pressure values of the system and of the reservoir and \(dl\) and \(dl^{\prime }\) the displacement of both pistons. In the second case, we similarly have \(dW = p_{2} Sdl\) and \(dW^{\prime } = pS_{2} dl^{\prime }\).
Clearly, we will have \(dl = dl^{\prime }\). Since, however, we need to have \(dW = - dW^{\prime }\), then
\(p_{1} S = pS_{1}\) and \(p_{2} S = pS_{2}\).
With the help of this couplings, we can let the system carry out the cycle represented in Fig. 1. The quantity of work obtained in this cycle is
\(- \left\{ {\mathop \int \limits_{A}^{B} p_{1} Sdl + \mathop \int \limits_{C}^{D} p_{2} Sdl} \right\} = - \left\{ {p_{1} \mathop \int \limits_{A}^{B} dv + p_{2} \mathop \int \limits_{C}^{D} dv} \right\} = - \left( {p_{1} - p_{2} } \right)\left( {V_{B} - V_{A} } \right).\)
This quantity of work is positive and is obtained from a single reservoir of constant pressure. P. Ehrenfest has brought the significance of the ambiguity of the types of coupling in this context to my attention.
- 16.
See the analogy in the previous footnote.
- 17.
It is easily accepted that for \(T \ne 0\) the coefficients \(Y_{i}\) do not have any singularities, which could be a reason for the ambiguity of the function S. If the value of the coefficients \(Y_{i}\) vanish when the absolute temperature has the value zero, then the differential \(dS\) can produce an indeterminacy of the type (\(\frac{{Y_{i} }}{T} = \frac{0}{0}\)), for which S would become ambiguous. Moreover, if the absolute temperature has value zero, this would also give a reason for the breakdown of the formulation of Thomson’s formulation of the law despite the validity of Axiom C. In fact, if one could set to zero the temperature of the colder reservoir in a Carnot cycle, then one would have \(Q_{2} = \mathop \int \nolimits dQ = 0\mathop \int \nolimits dS = 0 (S_{B} - S_{A} )\), where the difference of the entropies would be finite.
- 18.
Assume we reach the value zero for absolute temperature. Two types of attitude can be held toward this value for absolute temperature: either one declares this value is unattainable by means of a special axiom, and in this case Thomson’s (and Clausius’) principle holds for “for all attainable states,” or one declares that the principle holds “for all processes in which the value zero for absolute temperature is not attained.”
- 19.
The fact that the existence and the meaning of entropy contained within this principle follows from its derivation from the second law, which Clausius provided himself. Further, it follows from axiom A and C that two adiabatic paths Wa and Wb that follow a part of an isothermal path from the two ends a and b, and cannot have any point in common. If one wants to perform a cycle, that contains the isotherm ab as one of its parts, then the two adiabatic paths Wa and Wb have to be connected by a non-adiabatic path. And although this non-adiabatic path might be an isotherm (cd), then it cannot have the same temperature as ab (otherwise we would again have
\(\int dQ = T_{1} \mathop \int \limits_{a}^{b} dS + T_{1} \mathop \int \limits_{c}^{d} dS = T_{1} (S_{b} - S_{a} ) + T_{1} \left( {S_{d} - S_{c} } \right) = 0\)
because \(S_{b} = S_{c}\) and \(S_{a} - S_{d}\)). In order for the heat to be emitted to a reservoir that has a different temperature \(T_{2} \ne T_{1}\) along the second path—as is required by Clausius’ Principle—it is impossible for this reservoir and the system to have different temperature. Axiom B is therefore necessary also in this case.
- 20.
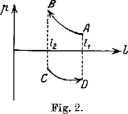
Analogously to how one can extract work from both “work-reservoirs,” as is represented in Fig. 2. Imagine a coil that tends to expand with higher temperatures and to contract with lower temperature within the lengths (l1l2).
- 21.
In the interpretation of absolute temperature in classical statistical mechanics, it naturally follows that this quantity can only be positive, in so far as it is the mean kinetic energy of the molecules. However, whenever one is compelled—e.g., because of quantum theory—to deviate from this interpretation, Axiom D requires another special statistical interpretation.
- 22.
This also explains why the various attempts to construct analogies to the second law could not be carried out completely.
- 23.
The condition for integrability is, in fact
\(Y_{1} \left( {\frac{{\partial Y_{2} }}{{\partial x_{3} }} - \frac{{\partial Y_{3} }}{{\partial x_{2} }}} \right) + Y_{2} \left( {\frac{{\partial Y_{3} }}{{\partial x_{1} }} - \frac{{\partial Y_{1} }}{{\partial x_{3} }}} \right) + Y_{3} \left( {\frac{{\partial Y_{1} }}{{\partial x_{2} }} - \frac{{\partial Y_{2} }}{{\partial x_{1} }}} \right) = 0\)
for the Pfaffian expression for three parameters. In our case, however, this condition is violated when \(c_{1} \ne c_{2} .\)
- 24.
L. c.
- 25.
The note by Arthur E. Ruark, “The Proof of the Corollary of Carnot’s Theorem” (Phil. Mag. 49, 584, 1925) has appeared during the writing of the present work. In that work, Ruark also points at the possibility of the latter two formulations.
- 26.
Because \(Q^{\prime \prime }\) can be equal to or even bigger than \(Q_{2}\); as a consequence, formulations 1 and 2 would not be valid in the “reversed” world.
- 27.
One could think, for the case of slow processes, of expressing their energy balance in terms of an equation with infinitely many terms, which, however, would not be holonomic.
- 28.
And for those changes of state that depart only slightly from quasistaticity.
- 29.
Let us compare the thermally non-homogeneous systems that go through quasi-static changes of state discussed in §5 with thermally non-homogeneous systems that go through non-static changes of state. Let us not be disturbed by the fact that, for the latter processes, the expression for \(dQ\) would contain infinitely many terms. What these two groups of processes have in common is that for both groups, the expression \(dQ\) is not holonomic, i.e., it does not have an integrating denominator. As a consequence, both systems could perform a cyclic process, in which for one part of the path heat is extracted from a single reservoir of constant temperature, and in which for the remaining path the total quantity of heat \(dQ\) obtained by the total system is zero. The fundamental difference, however, is that, on this kind of “adiabatic” path, a system from the first group could not be isolated adiabatically from its environment (otherwise—because of adiabatic isolation with each other—each of its components would not obtain any heat, and this would reduce excessively the variability of the parameters)—therefore, there should be other heat reservoirs in addition to the first one. Among these other reservoirs there also have to be ones (as one can show), that, on the whole, take up heat from the system. On the other hand, a system from the second group can remain adiabatically isolated from its environment during the whole part of the adiabatic section of the path of the cycle, as each of its parts can obtain the required heat from its neighboring parts. This is the reason that, with regard to non-static processes, something from Clausius’ Principle will remain only if the non-static processes are prohibited in at least one direction.
- 30.
Remark d has to be mentioned—despite its complete abstraction from physical aspects—as this remark illuminates the mathematical relations contained in the second law from a new perspective and it shows once more the very different role that the axioms of the second and third group play in thermodynamics.
- 31.
One believed, at the beginning, that the incessant increase of entropy (with which one identified the whole second law) had really been proven through the Boltzmannian H-Theorem on the basis of kinetic interpretations, while in classical thermodynamics the second law appears only as a postulate. On closer inspection, however, one sees that both theories need to be founded on axioms that, each in their own language, actually say the same thing: equalization of temperature, pressure, etc., in classical thermodynamics; the Stoßzahlansatz in kinetic theory. Both theories, therefore start more or less from the same axiom. However, in the kinetic theory—now that it has been put under the magnifying glass—it does not appear to be so unconditionally valid as it is for classical thermodynamics; indeed, what is more: the periods in which it [the axiom] is not satisfied and in which all phenomena show an opposite direction, are equally probable as those that show the “normal” course of direction. A discrepancy between classical thermodynamics and the kinetic theory appears in the moment when one wants the absolute equalization of temperature, pressure, etc.—and, therewith, the first two formulations of the Second Law—to be valid for an infinite amount of time.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Baldissera Pacchetti, M. (2021). Translation from German: Foundations of Thermodynamics 1925 and 1956. In: Uffink, J., Valente, G., Werndl, C., Zuchowski, L. (eds) The Legacy of Tatjana Afanassjewa. Women in the History of Philosophy and Sciences, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-030-47971-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-47971-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47970-1
Online ISBN: 978-3-030-47971-8
eBook Packages: Religion and PhilosophyPhilosophy and Religion (R0)



