Abstract
We present another minimally invasive technology for the treatment of hypertrophic scars and keloids: the pulsed-dye laser. Being first introduced by two groups around Schaefer (Germany) and Sorokin & Lankard (USA) in 1966, the pulsed dye laser is a rather new technology. The first clinical use of pulsed-dye lasers was reported in 1983 for the treatment of naevus flammeus, and was the first laser used for the treatment of keloids in the mid-1990s.
Its efficacy is based on the principle of selective photothermolysis, enabling a selective destruction of defined structures absorbing the respective wavelength used, as compared to other lasers working based on thermal coagulation or ablative tissue interaction. The preferred wavelengths being used are 585 or 595 nm, which makes small cutaneous vessels the main targets. Their destruction leads to a diminished blood supply of the irradiated area, thus reducing symptoms of hypertrophic scars like itching, vascularity, and redness, and secondary – probably by the induced hypoxemia – a reduction in scar height and pliability. This therapeutic approach also implies the use of pulsed-dye laser in the prevention of pathologic scars. While significant side effects are usually rare, slight signs of use like edema or scab formation can pertain for several days. Since the sensory impact of laser pulses are comparable to needle pricks, some form of analgesia during the application is highly recommended. The elusive data and still existing scarcity of high-quality studies on the use of pulsed-dye laser, however, make it hard to develop clear recommendations.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara BackgroundHypertrophic scars and keloids can be a major medical concern with varying impact.
Depending on the literature one chooses, prevalence between 30% and 70% for postsurgical hypertrophic scarring is reported; looking at burn injuries, the numbers even range from 60% to 90%. Keloids on the other hand, show a prevalence of about 4–14% in Afro-Americans. Given the fact, that the number of people developing significant scars after surgery or trauma ranges in the lower hundred millions every year, one can easily imagine, that scars can be more than an unavoidable side effect after trauma and pathologic scars are not rare.
Besides the cosmetic appearance and the psychological impact (especially after burns), those pathologic scars tend to be responsible for physical and functional impairing symptoms like pruritus, erythema, (neuropathic) pain, and in extreme cases even restriction of movement. The therapy has a wide variety from topical applications to complete surgery – all being covered in this book.
Since the management of cutaneous scars has and often still does rely on personal preference and experience, rather than large-scale and high-quality studies, to date no standard-of-care for the optimal therapy could be established, at least not based on solid scientific evidence.
Many treatment modalities are accompanied by high recurrence rates and with variable effect, which emphasizes the value of and need for minimally invasive technologies. This and the following chapters introduce and focus on several laser technologies as minimally invasive technologies in the treatment of hypertrophic scars and keloids.
On the following pages, the pulsed-dye laser (PDL) is delineated and its significance in the treatment of hypertrophic scars and keloids discussed.
1 Historical Development
Among the wide variety of available treatment options, laser applications are considered more recent technologies. Medical use of the physical properties of light dates back to ancient times, when Egyptians, Greeks, and Romans used some form of heliotherapy thousands of years BC. However, none other than Albert Einstein first described the stimulated emission of radiation, the base of laser technology, in 1917 [1]. In 1955, 38 years later, the manuscript of the first functioning precursor of a laser, the MASER was published by Gordon, Zeiger, and Townes [2]. Schawlow and Townes, who filed the first US patent for a laser in 1960, altered the Microwave Amplification by Stimulated Emission of Radiation-device (MASER) to visible Light wavelengths (LASER).
In the same year, Maiman built the first functioning laser, which used a synthetic pink ruby crystal as active medium [3]. In the early 1960s, the laser was first used for medical purposes in humans [4], and Townes along with two others (Prokhorov and Basov) were awarded the Nobel Prize in Physics for their work based on the MASER (1964). In 1981, Schawlow (along with Bloembergen) shared this honor for his contribution in the development of the laser spectroscopy.
After the development of the first laser, different kinds of light amplification devices have been developed using all kinds of active media.
The first dye laser was introduced in 1966 by two teams more or less simultaneously: the Americans Sorokin and Lankard at the Thomas J. Watson Research Center, as well as the German Schaefer at the University of Marburg developed the apparatus. While Sorokin and Lankard published the invention [5], Schaefer’s manuscript was rejected. After resubmission, the journal Applied Physics Letter asked Sorokin to review the manuscript, who finally made a publication possible [6], and also acknowledged Schaefer as the first to describe the dye laser’s advantage of adjustable wavelengths.
In 1983, Anderson and Parrish described the principle of selective photothermolysis, which enabled further research to adapt and better understand the medical possibilities of the laser and its tissue interactions [7]. First results of the use of the pulsed-dye laser (PDL) in Naevi flammei were published in the same year [8]. Also in that year, hypotheses for the application of lasers for the treatment of hypertrophic scars were made public [9].
Alster and colleagues were the first to issue their positive results in the treatment of hypertrophic scars (n = 14; [10]) and keloidal post-sternotomy scars (n = 16; [11]) with the 585 nm PDL.
Of note is that these positive results of the PD laser have so far failed to be reproduced in subsequent studies, and only minimal improvements in scar texture have been reported.
2 Technique of a Laser
The following section outlines the principle of a laser with a focus on the basics and the most relevant properties for a clinical understanding.
The mode of action of a laser is based on the emission of light by active medium molecules in an excited state, as described by Einstein [1]. This requires an energy source that is able to excite these molecules. To enhance this effect of excitation and reach higher energy levels, an additional resonator is used. These three components are the key elements of a laser (◘ Fig. 31.1).
The working principle of a laser is displayed (simplified). An energy source causes excitation in atoms of the active medium that, amplified by the resonator, leads to a chain reaction with the photons ultimately exiting through the subtotal-reflective resonator. The detailed view shows photons interacting with excited electrons of the active medium and emission of additional photons of the same energy
Atoms in the active medium, which can be of solid (e.g., ruby crystal), liquid (dye), or gaseous (CO2) state, are excited by supplying them with energy from the energy source. Depending on the used active medium, the time of decay varies until the atoms return to their ground state. The longer the time of decay, the more time remains for an incoming photon to interact with the excited atom, which in turn makes it return to its ground state and emits an additional photon of the same wavelength and frequency (◘ Fig. 31.1, Detail). This doubling ignites a chain reaction leading to the required high intensity of a laser. The resonator, which can be embodied by (various numbers of) plane or curved mirrors, in between the photons are reflected, enhances this effect additionally. One of these mirrors is subtotal-reflective, creating an exit for a part of the photons. In lasers that use dye as active medium, the resonator holds another function. Being equipped with a dispersive element (grid, filter, prism), the wavelength of the emitted light becomes tunable, as described by Schaefer as adjustable wavelengths (see ► Sect. 31.2).
The main difference between laser and regular light lies in its coherence, which allows a monochromatic (just one specific wavelength), high energetic, low divergent emission of light. It can also be applied as continuous wave or in short pulses of up to a femtosecond (one quadrillionth of a second), which allows for a temporal selective application.
3 Tissue Interaction of Laser
The effect and clinical impact of a laser is determined by its interaction with the irradiated tissue. The interaction can basically be distinguished in reflected, scattered, and absorbed photons. While reflective light is predominantly relevant for diagnostic use of lasers, scattering limits the depth of focusing of the laser beam by attenuation. The therapeutic effort is caused by the absorption of light, which can be used for various therapeutic effects. Due to the relevance for the present topic, we only deal with the photothermal effect; other effects can be comprehensively looked up in laser textbooks (see Further Reading).
Since every tissue component shows a different absorption coefficient, which equals the reciprocal of the distance that a photon can travel in that component until it is absorbed, therefore, every tissue component reacts differently to a specific type of light. While, for example, bilirubin and melanin show good absorption of visible light, water (H2O), the main component of human soft tissue, has its absorptive maximum in infrared wavelengths (◘ Fig. 31.2).
The wavelength-dependent absorption coefficients of H2O, oxygenated hemoglobin (HbO2) and melanin are displayed; the wavelengths of pulsed dye (PDL), Nd:YAG, and CO2 laser are presented exemplary. The wavelength of visible light ranges from about 400 to 700 nm. (Design: Dr. med. univ. Hanna Luze, Graz, Austria. © All rights reserved)
The penetration depth of a laser also depends on the wavelength; the higher the wavelength, the higher the penetration depth in the visible spectrum (blue to red).
The photothermal effect describes the transformation of electromagnetic energy (light) to heat by absorption and thereby photoexcitation of matter. Photothermal therapy is the most common application of laser therapy.
The absorptive properties of the irradiated tissue together with the properties of the used laser determine the exact location and effect of the irradiation. The more energy the irradiated matter absorbs, the more heat is produced, therefore explaining the correlation between the absorption coefficient and the different laser wavelengths.
Depending on the temperature reached, edema and apoptosis induction (≥ 45 °C), coagulation, and denaturation of proteins (≥ 60 °C), and even ablation and cutting by vaporization (≥ 100 °C) can be induced.
Another important aspect determining the amount of damage is the fluence. It is given in J/cm2 and describes the energy deposited on a certain area over a certain amount of time. This makes it an excellent parameter suitable for description of a treatment protocol.
4 Selective Photothermolysis
Selective photothermolysis describes a principle that enables selective damage to wavelength-specific pigments within the skin without harming the surrounding or above lying tissues.
The photothermolysis-induced damage is not only transmitted by direct local interaction, but also by heat diffusion additionally irradiating surrounding tissue layers; this principle is of particular importance for calculating selectivity and precise interaction of the laser with adjacent tissue layers.
A basic condition for selective photothermolysis is for the desired target to have a higher absorption coefficient than the surrounding tissues. By pulse-wise-specific irradiation of the target tissue a higher thermal energy is deposited in the target pigment with the higher absorptive coefficient than the surrounding tissue, and directs damaging temperatures only to the target pigment. Due to the pause in between the pulses, there is enough time for the target tissue to cool down by heat diffusion before applying another pulse. On the one hand, depending on the exposure time of the pulses, the amount of damage is relatively well-adjustable to the target area. On the other hand, the wavelength of the chosen and applied light determines the target area. For instance, light of around 580 nm (red light) is predominantly absorbed by hemoglobin, while water has its absorption maximum in the infrared wavelengths and melanin absorbs light throughout the visible region of the electromagnetic spectrum with a decrease toward higher wavelengths. The selectivity of the physical properties allows for a certain approximation of the beam to the target area including a pulse-dependent diameter of several millimeters around it. The downside of this specificity results in a relatively narrow therapeutic spectrum for each type of laser. Due to the monochromatic property of a given laser (emission of light of just one wavelength) and from a technical point-of-view, this leads to different therapeutic targets requiring individual lasers of a different spectrum. This latter fact has made the PDL with its active medium dye and the tuneability (see ► Sects. 31.2 and 31.3) predestined for exploiting this physical principle generating various targets with the same active medium.
5 The PDL and Its Application on Hypertrophic Scars and Keloids
The PDL is based on the principle of selective photothermolysis and was developed to damage and destroy small cutaneous vessels without harming epidermal structures. Being initially developed with a wavelength of 577 nm, the current models mostly work on a spectrum of 585 or 595 nm. Small cutaneous vessels absorb energy at these specified wavelengths. Consequently, the vessels are destroyed, ultimately leading to hypoxemia by diminution of the vascular supply. Then, by allegedly reported secondary effects, disulfide bonds are dissociated, collagen production is reduced, and expression of enzymes such as matrix metalloproteinases is induced [11, 12], leading to a loosening and restructuring of the fibrous structure in hypertrophic scars and keloids. However, there is no ultimate and scientifically proven consensus about the exact mechanisms promoted by PDL.
Hypertrophic scars and keloids often show significant characteristics like erythema and pruritus, which can be caused by hyperemia. Destruction of small cutaneous vessels can therefore significantly reduce these symptoms.
The mentioned restructuring procedures may be responsible for the observed improvement of pliability and height [13].
The properties of PDL and its modes of action also suggest the application in the prevention of hypertrophic scars, since some authors suggest an early laser application after surgery [14, 15].
In support of this theoretical recommendation (early use after surgery) is that the PDL proved to be less effective in thicker scars (>1 cm) due to its restricted penetration depth.
Side effects are relatively rare and include edema, scab formation, and pigmentary disorders (temporary often, permanent rarely), but also range from hypotrophic to hypertrophic scarring [16], but may be related to the therapeutic context of PDL application.
While most PDL work with 585 nm, more recently the 595 nm long-pulsed dye laser has been introduced. Longer pulses have been promoted on the basis of a more effective destruction of larger vessels by higher deposition of energy, and less posttreatment hyperpigmentation. In contrast to that, 585 nm lasers have reportedly ameliorated the scar texture; it remains to be seen if this also applies to the next generation of 595 lasers.
All in all, no scientifically solid high quality studies exist, and the above-mentioned studies do not support the development of definite recommendations for a PDL protocol on hypertrophic scars or keloids. The only common denominator seems to be the minimum requirement of greater than two PDL applications every 4–12 weeks across the scanned literature.
6 Selected Studies and Evidence
The first publications of hypertrophic scars treated with the PDL date back to 1994. Alster presented 14 cases of patients suffering from hypertrophic and/or erythematous scars after trauma or surgery [10]. After treatment with one or two sessions of 585 nm PDL within a 6-week interval, they showed an improvement in erythema and scar flattening of 57–83% as evaluated by two different and independent observers. The fluence used in this study was 6.5–6.75 J/cm2.
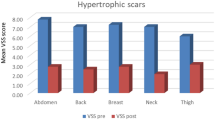
One year later, in 1995, Alster and Williams described another series of patients (n = 16) that had developed keloidal or hypertrophic scars after a median sternotomy [11]. Those patients had half of the scars treated with a similar protocol (mean fluence: 7.00 J/cm2, two sessions, 6–8 weeks apart) and afterwards evaluated by two independent, blinded observers. Significant amelioration of pruritus, tenderness, burning, scar height, pliability, and erythema was observed 6 months after laser treatment, as compared to untreated scars.
Subsequently, no study was able to reproduce these initially promising results. While several studies described changes in scar erythema, pliability, height, and volume, very few demonstrated a statistical significance (also due to the low number of scars treated). Significant improvement in Vancouver Scar Scale scores and pigmentation could be shown by Bowes et al. [17] and Chan et al. [18] for patients treated with PDL compared to no treatment intra-individually, respectively. Alster further showed a significant reduction in pruritus by adding intralesional injection of triamcinolone [19] in contrast to Wittenberg et al. who could not observe a significant reduction of pruritus in hypertrophic scars treated with PDL over patients treated with silicone gel sheeting [20].
Another study by Asilian et al. could reach a significant participant-subjective, inter-individual overall improvement from baseline and a reduction of erythema in patients treated with PDL, intralesional injection of triamcinolone, and 5-fluoruracil compared to the controls treated with the intralesional injection alone [21].
Ouyang et al. (2018) reached significant improvement in height, vascularity, pliability, and Vancouver Scar Scale in 56 patients with fresh (immature), red hypertrophic scars [22], suggesting an early application and possible prevention strategy in conjunction with a recommendation of an international panel of experts [23].
To summarize, data on PDL treatment in hypertrophic scars and keloids is scarce. Studies were conducted with no or insufficient control groups, no standardized treatment protocols, small numbers of participants, or lack of differentiation between the scar types treated. The exact mechanisms of PDL application in hypertrophic scars or keloids remain elusive and precise recommendations cannot be made on the given evidence.
Nonetheless, PDL may serve as effective additional (second-line) therapeutic strategy given the relatively low side-effect spectrum; application in fresh pathologic, erythematous, itching scars and use for preventive strategies can be considered. PDL use is limited by its low penetration depth (thick scars) and higher amount of melanin as concurrent absorber (darker skin types).
7 Clinical Relevance
Since most of the published research demonstrated inconclusive results of the use of PDL for hypertrophic scars and keloids, no solid recommendation can be derived from the given literature. Hypertrophic scars tend to involute over time, and without the evidence served by adequate control groups in published research, it remains difficult to support them. Since medical device regulations will require more published research in the future (notably for device registration in the European Union), future therapeutic registration or re-registration might add in encouraging industry to support more science-driven studies.
Active hypertrophic scars as seen with the clinical sign of erythema or symptom of pruritus can be treated by PDL with a low level of side effects but only if used in conjunction with other more proven methods.
Limiting factors are thick scars (>1 cm) and dark skin types. As a result the PDL can play a role in the prevention of pathologic scars. Future studies should aim toward more standardized research with an adequate selection of control groups (e.g., intra-individual comparison) , and are still necessary to reach the actual potential of the PDL.
8 Conclusion
This chapter dealt with the minimally invasive treatment of hypertrophic scars and keloids with the pulsed-dye laser. Being the first chapter about laser technology, an introduction on lasers, their functioning, and an outline of the temporal development were given. We then explained the principle behind the PDL, called selective photothermolysis, and clarified its relevance in the treatment of pathologic scars. Lastly, selected studies in terms of clinically applied PDL were presented, the evidence was analyzed, and the clinical relevance delineated.
The reader should now be able to sort the relevance of PDL for treatment of hypertrophic scars and keloids in the whole arsenal of treatment methods for pathologic scars.
Summarizing, we want to emphasize one more time that laser technology itself is a rather new technology, which certainly holds many advantages – not least its minimal invasiveness and low side-effect profile. With only few studies available, we refrain from giving absolute recommendations. We rather want to encourage the reader, if interested in this topic, to help creating more evidence and perform further research on this specific topic. PDL holds potential, that still is to be grasped comprehensively.
Take-Home Messages
-
Pulsed-dye laser is based on a principle called selective photothermolysis.
-
Its wavelength ranges from 585 to 595 nm.
-
PDL’s main targets are cutaneous vessels that are coagulated by the irradiation.
-
PDL should be applied a minimum of two times every 4–12 weeks.
-
PDL application can reduce symptoms like pruritus or erythema.
-
PDL should NOT be used for thick scars (>1 cm) and/or in dark skin types.
-
PDL should only be used in combination with other treatment modalities to achieve the most effective treatment.
-
The low risk and side-effect profile makes the PDL a valuable alternative for the prevention of pathologic scars.
-
Long-term outcome and high-quality intervention studies are necessary to provide proper evidence for PDL application in hypertrophic scars and keloids.
References
Einstein A. Zur Quantentheorie der Strahlung. Phys Zeitschrift. 1917;18:121–8.
Gordon JP, Zeiger HJ, Townes CH. The maser – new type of microwave amplifier, frequency standard, and spectrometer. Phys Rev. 1955;99(4):1264–74.
Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187:493–4.
Goldman L, Igelman JM, Richfield DF. Impact of the LASER on nevi and melanomas. Arch Dermatol. 1964;90:71–5.
Sorokin PP, Lankard JR. Stimulated emission observed from an organic dye, chloro-aluminum phthalocyanine. IBM J Res Dev. 1966;10:162–3.
Schäfer FP, Schmidt W, Volze J. Organic dye solution laser. Appl Phys Lett. 1966;9:306–9.
Anderson R, Parrish J. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220(4596):524–7.
Strempel H, Klein G. New approach in the laser therapy of nevus flammeus. Z Hautkr. 1983;58(13):967–74.
Castro DJ, Abergel RP, Meeker C, Dwyer RM, Lesavoy MA, Uitto J. Effects of the Nd:YAG laser on DNA synthesis and collagen production in human skin fibroblast cultures. Ann Plast Surg. 1983;11(3):214–22.
Alster TS. Improvement of erythematous and hypertrophic scars by the 585-nm flashlamp-pumped pulsed dye laser. Ann Plast Surg. 1994;32(2):186–90.
Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet. 1995;345(8959):1198–200.
Dierickx C, Goldman MP, Fitzpatrick RE. Laser treatment of erythematous/hypertrophic and pigmented scars in 26 patients. Plast Reconstr Surg [Internet]. 1995;95(1):84–90; discussion 91–2. Available from: http://europepmc.org/abstract/MED/7809272.
Gauglitz GG, Potschke J, Clementoni MT. Therapy of scars with lasers. Hautarzt. 2018;69(1):17–26.
Karmisholt KE, Haerskjold A, Karlsmark T, Waibel J, Paasch U, Haedersdal M. Early laser intervention to reduce scar formation - a systematic review. J Eur Acad Dermatol Venereol. 2018;32(7):1099–110.
Brewin MP, Lister TS. Prevention or treatment of hypertrophic burn scarring: a review of when and how to treat with the pulsed dye laser. Burns. 2014;40(5):797–804.
Raulin C, Kimmig W, Werner S. Laser therapy in dermatology and esthetic medicine. Side effects, complications and treatment errors. Hautarzt. 2000;51(7):463–73.
Bowes LE, Nouri K, Berman B, Jimenez G, Pardo R, Rodriguez L, et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled Nd:YAG laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg. 2002;28(8):714–9.
Chan HHL, Wong DSY, Ho WS, Lam LK, Wei W. The use of pulsed dye laser for the prevention and treatment of hypertrophic scars in Chinese persons. Dermatol Surg. 2004;30(7):987–94.
Alster T. Laser scar revision: comparison study of 585-nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003;29(1):25–9.
Wittenberg GP, Fabian BG, Bogomilsky JL, Schultz LR, Rudner EJ, Chaffins ML, et al. Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment. Arch Dermatol. 1999;135(9):1049–55.
Asilian A, Darougheh A, Shariat F. New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for the treatment of keloid and hypertrophic scars. J Isfahan Med Sch. 2012;32(7):907–15.
Ouyang HW, Li GF, Lei Y, Gold MH, Tan J. Comparison of the effectiveness of pulsed dye laser vs pulsed dye laser combined with ultrapulse fractional CO2 laser in the treatment of immature red hypertrophic scars. J Cosmet Dermatol. 2018;17(1):54–60.
Mustoe TA, Cooter RD, Gold MH, Hobbs FDR, Ramelet A-A, Shakespeare PG, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110(2):560–71.
Further Reading
Berlien H-P, Müller GJ. Applied laser medicine. 1. Auflage. Berlin Heidelberg: Springer; 2012.
Landthaler M, Hohenleutner U. Lasertherapie in der Dermatologie: Atlas und Lehrbuch, 2. Auflage. Berlin, Heidelberg: Springer; 2006.
Raulin C, Greve B. Laser und IPL-Technologie in der Dermatologie und Ästhetischen Medizin. 2. Auflage. Stuttgart: Schattauer-Verlag; 2003.
Raulin C, Karsai S. Lasertherapie der Haut. 1. Auflage. Berlin, Heidelberg: Springer; 2013.
Svelto O. Principles of lasers. 5th ed. Berlin, Heidelberg: Springer; 2010.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Nischwitz, S.P., Lumenta, D.B., Spendel, S., Kamolz, LP. (2020). Minimally Invasive Technologies for Treatment of HTS and Keloids: Pulsed-Dye Laser. In: Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G. (eds) Textbook on Scar Management. Springer, Cham. https://doi.org/10.1007/978-3-030-44766-3_31
Download citation
DOI: https://doi.org/10.1007/978-3-030-44766-3_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44765-6
Online ISBN: 978-3-030-44766-3
eBook Packages: MedicineMedicine (R0)