Abstract
Using Botulinum neurotoxin type A (BoNTA) has yielded promising results in the treatment of immature scars. The biological effects of the toxin on tissue healing appear to be complex and multidimensional and still require additional research. Nevertheless, it is clear that not only does BoNTA reduce muscle tension at the edges of wounds, but it also provides anti-inflammatory effects, promotes angiogenesis and healing, and exerts mediatory or inhibitory effects on a variety of cells. In clinical practice, this pluripotency of BoNTA has been recognized as a therapeutic choice for both prophylaxis and treatment of excessive scarring.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Background
Botulinum toxin type A (BoNTA) and occasionally of serotype B has been used for treatment of conditions associated with muscle spasm, hyperhidrosis and improving appearance of static rhytides.
The same neuronal effects and a muscle tension reduction at the site of injury were initially attributed to the benefits of BoNTA in a scar formation. However, a more detailed research into effects of BoNTA on wound healing suggested a variety of biologic effects of the toxin. New possibilities emerged for using BoNTA to modify early tissue repair mechanisms to promote more favourable outcomes and less noticeable scars.
2 Chapter Introduction
The Botulinum toxin A (BoNTA), and to a lesser degree type B (BoNTB), is used in clinical practice for the treatment of conditions associated with muscle spasms. The therapeutic indications include cervical dystonia, blepharospasm, strabismus, chronic migraine, and severe axillary hyperhidrosis. BoNTA is also approved for esthetic use in the treatment of facial wrinkles such as “crow’s feet” and frown lines. In addition, there are many unlicensed off-label uses of BoNTA for the treatment of various other conditions.
The demand for BoNTA products is rapidly expanding due to ever-increasing esthetic applications, and there is a plethora of BoNTA formulations available across global markets. This can make it difficult to identify an adequate product for any specific application.
The most popular and well-tested products are Botox (onabotulinumtoxinA) and Vistabel (specifically for esthetic applications) that are manufactured by Allergan (USA). Dysport (abobotulinumtoxinA), marketed as Azzalure for cosmetic applications, is made by Ipsen Ltd. (UK) and Galderma Laboratories (USA). Xeomin (incobotulinumtoxinA) and its esthetic brand, Bocouture, are made by Merz Pharmaceutical (Germany).
Less known preparations include Evosyal made by Puretox (USA) and Linurase made by Phiderma (Canada). BoNTB preparations such as Myobloc (rimabotulinumtoxinB) are marketed by Solstice Neurosciences (USA). Other BoNTA products are available in Asian and Russian markets.
Although normally injected into the skin layer, several products for the topical delivery of BoNTA have become available recently. Examples include ANT-1207 (Anterios/Allergan), CosmeTox (USA), and RT001 (Revance, USA).
Each BoNTA product has a Botulinum toxin combined with a variety of other complex proteins, and each manufacturer uses a different assay to determine the potency of their batches. These differences make it difficult to compare clinical responses across products.
Recent studies have shown that BoNTA improves the appearance of established scars and can also positively affect the early stages of tissue healing for a more favorable mature scar. This chapter examines evidence of the BoNTA effects on early stages of scar formation and describes how this can be employed for controlling scar development as it occurs. Practical guidance on optimal doses of injections and on treatment protocols is described and evaluated. Choices of the BoNTA preparations used for immature scar treatment are also discussed.
3 Botulinum Exotoxin, Structure, and Mechanism of Action
Botulinum neurotoxin (BoNT) is an exotoxin of Clostridium Botulinum bacteria , which has several serotypes. In clinical practice, the serotypes A (BoNTA) and B (BoNTB) are most used for therapeutic, experimental, and esthetic purposes; however, due to their superior longevity of action, preparations of BoNTA have become the most popular.
3.1 Structure of BoNTA
The BoNTA molecule is made of a heavy (100 kDa) and a light (50kDA) polypeptide chain, linked together by a disulfide bond. The neurotoxin complex also includes associated nontoxic proteins: three hemagglutinin (HA) proteins and one non-HA protein. These play a role in transporting and protecting the toxin core.
3.2 Neuronal Mechanism of Action of BoNTA and Effects on Immature Scars
BoNTA impairs release of acetylcholine by disabling a soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) protein. The latter facilitates acetylcholine release by mediating docking and fusion of synaptic vesicles with the inner surface of the axonal membrane at the sites of release.
BoNTA adheres by its heavy chain to cholinergic cell membrane surface structures such as ganglioside moieties, a vesicular protein (SV2) and synaptotagmin. Its light chain enters the neuronal terminal and reaches the cytosol (vesicles). It cleaves SNARE proteins: synaptosomal-associated protein (SNAP)-25, syntaxin, and vesicle-associated membrane protein (VAMP ) also known as synaptobrevin II. The acetylcholine release into the synaptic cleft is impaired and the cholinergic mediation of neurons is blocked. Furthermore, BoNTA has also been shown to impair release of other neurotransmitters, such as glutamate, substance P (SP), and calcitonin gene-related peptide (CGRP).
The absence of the necessary mediator leads to a “chemical denervation” and temporary atrophy of the neuromuscular junction with a subsequent block of muscle contraction or reduction of exocrine glandular secretion.
Several studies involving animal and human subjects have tried to determine how reducing muscle contractility in the immediate vicinity to a fresh wound improves the esthetic result in scars. To date, findings have been inconclusive.
Immobilization of underlying musculature, reducing tension of the wound and skin (similar to the use of a surgical Z-plasty), has resulted in more favorable scarring according to Ziade M et al. (2013). Patients in this research were injected within 3 days following facial surgery, and results were assessed using a visual analogue scale. However, no statistically significant differences were found between the two groups in this study when using other assessment methods [1].
In another example of effects of BoNTA on the early stages of wound healing, researchers Lee B-J et al. (2009) [2] used a rat wound model. In this study, each of 15 animals was its own control. The results showed significant differences in wound size between BoNT-treated and untreated control wounds. Also observed were less infiltration of inflammatory cells, fewer fibroblasts, and a lower expression of transforming growth factor (TGF)-β1 (as compared to the control wounds).
TGF-β1 is a cytokine that has multiple mediatory actions in tissue healing, and it is involved in the formation of fibrotic tissue and hypertrophic scars. The finding that BoNT-treated wounds show a lower amount of TGF-β1 may be the result of the chemoimmobilization of the muscle as well as of a direct effect of BoNTA on the expression of TGF-β1 in fibroblasts and fibroblast proliferation.
It is known that a muscle paralysis due to the inhibition of acetylcholine exocytosis is reversible by natural SNARE protein recovery. After application of BoNTA, it takes 2 weeks to 4 months for neurotransmission to recover. Therefore, its period of action is definitely within the timeframe of early scar formation. Nevertheless, the effects of BoNTA on tissues are more complex than just reducing tension and decreasing TGF-β1.
3.3 Nonneuronal Mechanisms of Action of BoNTA and Effects on Immature Scars
More research into Botulinum toxin demonstrates that its biological effects on various cells and tissues are much more complex than previously understood [3]. The BoNT receptors and intracellular targets are not unique for neurotransmission, as several of these receptors and targets have been found in neuronal and nonneuronal cells.
BoNTA, for example, can attach to some other cell-surface proteins and, through them, modulate the function of a variety of human cell types [3]. These proteins include E-cadherin, fibroblast growth factor receptor 3 (FGFR3), and vanilloid receptors. Indeed, epidermal keratinocytes, dermal fibroblasts, sebocytes and vascular endothelial cells, adipocyte-derived mesenchymal stem cells, and many other cells including macrophages and neutrophils have receptors capable of binding and cleaving the BoNTA molecule. Therefore, the effects of BoNTA at the nonneuronal level have an impact on inflammatory and immunological cascades, neurosensory signaling, cellular inhibition and proliferation, vascular and tissue differentiation, and growth (or atrophy). Familiarity with these effects is useful in understanding how BoNTA is helpful for tissue healing and scar development.
3.3.1 Effects of BoNTA on the Inflammatory Cascade
Both pro- and anti-inflammatory effects by BoNTA have been demonstrated in animal models and cell cultures. Those effects are expressed on additional binding sites for BoNTA and its carrier proteins. These have been identified at the RMS 13 receptor sites of skeletal muscle cells, TIB-152 of lymphoblasts, Detroit 551 of fibroblasts, and SH-SY5Y of neuronal cells. BoNTA alone does not induce inflammatory responses; it only does so in a complex with the Neurotoxin Associated Protein (NAP). The latter is responsible for the release of pro-inflammatory cytokines such as IL-6, IL-8, MCP-1, and TNF- α at these sites [4]. This last reference suggests that it is NAP that determines the pro-inflammatory effects of BoNTA at the time of the toxin application.
The inflammatory phase of a wound healing lasts approximately 2–4 days. It is marked by an abundant release of cytokines, growth factors (such as platelet-derived growth factor (PDGF)), interleukin-1 and interleukin-8 (IL-1 and IL-8), chemokines, and hormones. These all work to sustain activation of the target cells and promote migration of the inflammatory cells.
PDGF and transforming growth factor-β (TGF-β) released by Alpha granules of platelets attract neutrophils and macrophages. The latter scavenges the wound site, and the former produces transforming growth factor (TGF), tissue growth factor-α (TGF-α), and epidermal growth factor (EGF). These give rise to fibroblast and keratinocyte migration, signaling the start of the proliferative phase of healing.
Once the heavy and light chains of BoNTA have reached their targets, inflammation starts downscaling. They reduce lymphocyte proliferation and migration and decrease cytokine expression. This is seen a few days after wounds have been treated with BoNTA [2, 3].
BoNTA has also demonstrated the ability to decrease inflammatory enzyme cyclooxigenase-2 (COX-2) and prostaglandin E2 receptors [5] in animal models and cell cultures. It also decreases the infiltration of monocytes and macrophages while blocking expression of interleukins-1B (IL-1β). Moreover, it is able to suppress nitric oxide and tumor necrosis factor-α (TNF-α) via inhibition of specialist receptors on macrophages [6].
Inflammation in tissues is often associated with pain and itching. BoNTA reduces pain by local muscle spasm relief and by blocking cholinergic and other neuropeptide sensory transmission. However, it is now known that BoNTA has effects at sites distant to the injection location, as well as at a central level. In addition to the local uptake of BoNTA in the synaptic terminal, a distinct retrograde transport pathway results in accumulation of BoNT toward the neuronal soma. This retrograde channeling facilitates remote action of BoNTA at the dorsal root ganglion and the spinal cord, and it is believed to explain the efficacy of BoNTA used for the control of pain [7]. In addition to neurons, the glial cells such as Swann’s and astrocytes are also receptive to BoNTA, which suggests yet another pain modulation mechanism.
BoNTA also has been shown to reduce infiltration by cutaneous lymphocytes and decrease acanthosis, processes associated with intense itching (most commonly mediated by histamine). BoNTA affects chemotaxis of mast cells, affecting their migration and histamine release and IL-4 expression. The histamine acts as the main mediator of H1-H4 receptors, responsible for activation of the target molecules within the sensory neurons that code pruritic signals. BoNTA also downregulates transient receptor potential channel type V1 (TRPV1) and type A (TRPA1), responsible for histamine-mediated and non-histamine-dependent itch, respectively [8].
It is believed that the ability of BoNTA to moderate a florid inflammatory response and control itch and pain may explain its off-label use in treating scar hypertrophy and keloid formation. A recent double-blind randomized study concluded that BoNTA was as effective as steroids when injected into keloid scars, but patients additionally reported the alleviation of pain and itching after use of the former [9].
In experiments, BoNTA has demonstrated properties inhibiting the overgrowth of a variety of cells, including malignant proliferation in breast, prostate, and colon cancers. It has been theorized that this is the same mechanism as the one that provides its inhibitory effects on fibroblast proliferation. This has motivated the use of BoNTA on the unchecked growth of the cells leading to keloid deposition.
3.3.2 Effects of BoNTA on Fibroblasts and Keratinocytes
BoNTA has a direct effect on dermal cells such as fibroblasts and keratinocytes and through them can positively mediate dermal tissue remodeling. This is an important characteristic for reversing the effects of skin aging, assisting in wound epithelization, and reducing scar formations.
Fibroblasts and their differentiated subset, myofibroblasts, are the main components of the proliferation phase in wound healing. The transforming growth factor-beta 1(TGF-β1) prompts some of the fibroblasts to differentiate into myofibroblasts, possessing a retractile protein alpha-SM (α-SM), similar to those in smooth muscle cells. Myofibroblasts create bridging connections and promote the approximation and retraction of wounds. They do this by creating a matrix that further promotes the migration of additional fibroblasts and keratinocytes [10]. BoNTA seems to interrupt the differentiation of fibroblasts to myofibroblasts by blocking TGF-β1 signaling and in so doing reduces excessive wound retraction and scar thickening [11].
Fibroblasts are also responsible for angiogenesis in a healing wound. This is mediated by fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF), both of which also promote epithelization and collagen synthesis. Epithelial growth will happen either from the basement membrane or from the edges of the wound by migrating keratinocytes . BoNTA acts to increase migration, proliferation, and differentiation of keratinocytes and the expression of epidermal growth factor (EGF). Collagen type III is mainly produced in this stage, actively promoted by IL4 mediators. Subsequently collagen type III degrades, and it is replaced by collagen type I with reorganized orientation of the fibers. This adds strength to the resulting scar.
BoNTA influences the ratio of collagen I to collagen III by increasing or inhibiting its degradation by matrix metalloproteinases (MMPs) [12]. Excess of collagen synthesis by fibroblasts can determine scar hypertrophy and keloid formation, whereas lack of collagen matrix can predispose to a weak scar with atrophy [13].
3.3.3 Effects of BoNTA on Vascular Endothelium
BoNTA has been shown to have protective effects on dermal flap survival in animal models even with adverse conditions such as nicotine exposure, oxidative stress, or preexisting diabetes. BoNTA reduced accumulation of reactive oxygen species and prevented oxidative damage to endothelial cells. It increased the blood flow in dermal vasculature by dilating lumen of the blood vessels. The model skin flaps also had increased production of vascular endothelial growth factor (VEG) and expression of platelet endothelial cell adhesion molecules 1, CD31 and CD34 lymphocyte subsets, interleukin (IL)-1, and tumor necrosis factor (TNF) [14].
4 Clinical Application of BoNTA for Treatment of Scars
The time frames of the various stages of wound healing are not precise and can be overlapping. The vascular stage lasts for roughly 24 to 48 hours after trauma, and the inflammatory phase runs 2 to 4 days. The proliferative phase starts from roughly day 3, peaks around day 7, and finishes after 2 to 4 weeks. Finally, scar maturation (often referred to as remodeling) can take up to 12 months.
The usefulness of BoNTA in the management of immature scars (by definition, less than a year old) can be summarized as follows:
-
Reduces local muscle contractility, allowing better approximation of the edges of healing wounds
-
Moderates the inflammatory response at the site of the injury via suppression of inflammatory cytokines and neuropeptides
-
Reduces pain and itching in healing wounds (these effects can be both peripheral at the site of the scar and central through a retrograde uptake of the toxin)
-
Inhibits excessive proliferation of fibroblasts and their increased differentiation under TGF-β1 into myofibroblasts and so downgrades the over-proliferative scarring and retraction of the wound bed
-
Mediates MMP enzymatic activity in controlling expression of collagen fibers, optimizing collagen I to III ratios, and preventing fibrosis and scar thickening
4.1 Timing of BoNTA Injections
The most efficacious timing of BoNTA application remains to be determined. For example, some researchers advocate injecting the toxin before surgery [15, 16] for better titration of BoNTA dose and improving local circulation via inhibition of norepinephrine. In other studies, BoNTA have been applied intraoperatively [17] at the time of wound closure or within the first 24 hours [2, 18,19,20]. Several clinicians have reported using the toxin after 72 hours [1], while others injected BoNTA at the time of suture removal (7 to10 days) [21]. In another study, BoNTA was only used at the time of a scar revision [22].
As can be seen, there are a wide variety of protocols proposed in the literature for the timing of BoNTA injections. That said, in the context of the discussion in Sect. 2, there are good reasons to prefer a single, early application of BoNTA from the onset of an injury. This timing is the one that has the best potential of benefitting from all the additional effects of BoNTA.
4.2 Choosing BoNTA Preparation
The majority of published studies have used BoNTA preparations , including ona-, abo-, and incobotulinumtoxinA. However, BoNTB had been reported to also have beneficial results in wounds healing in a study involving facial reconstruction surgery [17]. Most other published studies use BoNTA, namely, the brand preparations such as Botox, Dysport, and Xeomin. Quantitative reporting on conversions of BoNTA unit rate and levels of toxin spreading is mainly based on these three products. Given the availability of the data, it would seem prudent to continue using the well-tested products when planning a scar treatment.
4.3 Reconstituting BoNTA
Currently BoNTA preparations are manufactured in lyophilized powder form. There have been some attempts at creating liquid preparations; however, these are not widely available.
Normal saline is most commonly used for the reconstitution of the toxin. Gassner et al. (2000) [18] have recommended mixing BoNTA with lidocaine and epinephrine, the former for immediate efferent block and the latter for the reduction of toxin diffusion.
Preparing BoNTA with bacteriostatic normal saline (BNS) reduces the discomfort of injections. Various dilution proportions have been tried, but the most common volume of saline used per 100 U of BoNTA was 1.5 to 2.5 ml. For keloid and large surface scars, the volume of BNS can be doubled.
4.4 Dose of BoNTA for Scar Treatment
A dose titration for a novel application of any medicine is always a challenge, and the amount of BoNTA required for the treatment of scars seems to be much lower than traditional doses used for immobilizing muscles and treating wrinkles. The most popular BoNTA doses tried in various studies have been 5 U [21], 7 U [18, 19], and 10 U [2]. In the main, however, these studies do not provide a rationale for their choices.
One reference, however, performs a preliminary systematic study of BoNTA dosages to determine optimum outcomes. Quantities of 5 U, 15 U, and 25 U of BoNTA were used on the postoperative wounds of rhytidectomy patients [20]. For the specific approach of this study, the best results were obtained with the use of about 15 units of BoNTA. That said results might vary significantly if other parameters are adopted. Facets that could be investigated going forward could include modifying the injection protocol. For example, these results suggest that increasing the number of injection points might achieve a more uniform reduction in scar width.
This last study seems to correlate to in vitro experiments demonstrating the dose dependencies of keratinocytes and endothelial cell function when subjected to the effects of BoNTA [25]. Doses lower than 20 units were found to support proliferation of endothelial cells but higher than 20 units impaired keratinocyte and endothelial migration and growth, these being responsible for epithelization and angiogenesis, respectively.
4.5 BoNTA for Treatment of Keloid and Hypertrophic Scars
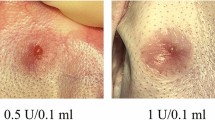
The treatment of pathologically healed wounds such as keloid and hypertrophic scars is a difficult and often fruitless task. BoNTA has demonstrated some promising results for reducing stiffness, hardness, and pain characteristic for these types of scars; see ◘ Figs. 26.1 and 26.2.
Studies based on repeated monthly application of BoNTA, involving multiple injections of the toxin covering the entire surface of a scar, have shown some effectiveness [23, 24]. In these cases, the maximum concentration of 35 U was diluted to enable full coverage of the scar area. The injection volume was reduced into a microdroplet to ensure distribution was mainly in the skin and the superficial muscle layer [24].
4.6 Summary of Practical Guidelines for the Application of BoNTA in the Treatment of Scars
In summary, a practical clinical approach for the treatment of scars is to administer BoNTA reconstituted in 1.5 to 2.5 ml of bacteriostatic normal saline (BNS). Injections should be made using a superficial intradermal technique. The expectation is that the toxin will travel in the vertical and horizontal plane, reaching superficial muscles and even distant central locations by retrograde uptake. Leaving a 1 cm margin from the freshly sutured wound (see ◘ Fig. 26.3) and 1 cm between injection points (see ◘ Fig. 26.4) would seem to be adequate based on reported BoNTA diffusion distances. The total dose of BoNTA should remain below 20 U. A one-off treatment is likely sufficient to promote better healing of the wound and to achieve more favorable scarring.
For scars already displaying features of keloid or hypertrophic change , reconstituting 20 U of BoNTA in 3 to 5 ml of BNS is recommended. An example of this is shown in ◘ Fig. 26.5. Injections, delivered in microdroplets, should be applied to cover the whole surface of a pathological scar (similar to a mesotherapy technique). Monthly repeated treatments, consisting of three to eight sessions, are advised.
5 Conclusions
The scope of using of Botulinum toxin for various conditions is ever expanding, and the benefits it provides for tissue healing and immature scar management are gaining recognition and acceptance. Clearly, the complex effects of BoNTA on various cells and tissues are still not fully understood, and we continue to discover more about its full range of biological effects and useful applications. This chapter concludes with a description of practical approaches for the use of BoNTA in managing scarring in a clinical environment.
Take Home Message
-
Effects of BoNTA on wound healing are multifaceted and not restricted just to the underlying muscles relaxation and a wound tension reduction. The toxin exerts influence on various cells and tissues involved in tissue repair following an injury. BoNTA can alter inflammatory reactions, cellular proliferation, mediation and inhibition
-
Early application of BoNTA, within 24–72 hours of initial injury seems to ensure a less conspicuous scar
-
All well known commercially available BoNTA preparations seem to be effective for that purpose
-
A dose of 15 -20 units of BoNTA demonstrated to be sufficient to benefit for a wound of a traditional rhytidectomy length (15–20 cm). The preparation injected in equal amounts of 1–5 U spaced evenly along the length of the wound 1cm from the border. Injection points can be spaced either along the both sides of the wound or a just unilaterally.
-
Dilution of the BoNTA for wound treatment was 1.5 -2 ml of Bacteriostatic Normal Saline for 100 u of the Botox (or equivalent in case of other products). For larger surface wounds BoNTA could be diluted in a double amount of saline to provide a sufficient volume for coverage, as the dose of up to 20 u is still applicable in these cases
References
Ziade M, Domergue S, Batifol D, Jreige R, Sebbane M, Goudot P, Yachouh J. Use of botulinum toxin type a to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg. 2013;66(2):209–14.
Lee BJ, Jeong JH, Wang SG, Lee JC, Goh EK, Kim HW. Effect of botulinum toxin type a on a rat surgical wound model. Clin Experiment Otorhinolaryngol. 2009;2(1):20.
Grando SA, Zachary CB. The non-neuronal and nonmuscular effects of botulinum toxin: an opportunity for a deadly molecule to treat disease in the skin and beyond. Br J Dermatol. 2018;178(5):1011–9.
Wang L, Sun Y, Yang W, Lindo P, Singh BR. Type a botulinum neurotoxin complex proteins differentially modulate host response of neuronal cells. Toxicon. 2014;82:52–60.
Chuang YC, Yoshimura N, Huang CC, Wu M, Chiang PH, Chancellor MB. Intravesical botulinum toxin a administration inhibits COX-2 and EP4 expression and suppresses bladder hyperactivity in cyclophosphamide-induced cystitis in rats. Eur Urol. 2009;56(1):159–67.
Yoo KY, Lee HS, Cho YK, Lim YS, Kim YS, Koo JH, Yoon SJ, Lee JH, Jang KH, Song SH. Anti-inflammatory effects of botulinum toxin type a in a complete Freund’s adjuvant-induced arthritic knee joint of hind leg on rat model. Neurotox Res. 2014;26(1):32–9.
Fonfria E, Maignel J, Lezmi S, Martin V, Splevins A, Shubber S, Kalinichev M, Foster K, Picaut P, Krupp J. The expanding therapeutic utility of botulinum neurotoxins. Toxins. 2018;10(5):208.
Gazerani P. Antipruritic effects of botulinum neurotoxins. Toxins. 2018;10(4):143.
Shaarawy E, Hegazy RA, Abdel Hay RM. Intralesional botulinum toxin type a equally effective and better tolerated than intralesional steroid in the treatment of keloids: a randomized controlled trial. J Cosmet Dermatol. 2015;14(2):161–6.
Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability. 2011;20(4):108–20.
Lee SD, Yi MH, Kim DW, Lee Y, Choi Y, Oh SH. The effect of botulinum neurotoxin type a on capsule formation around silicone implants: the in vivo and in vitro study. Int Wound J. 2016;13(1):65–71.
Oh SH, Lee Y, Seo YJ, Lee JH, Yang JD, Chung HY, Cho BC. The potential effect of botulinum toxin type a on human dermal fibroblasts: an in vitro study. Dermatol Surg. 2012;38(10):1689–94.
Chambers A. Unified approach to the treatment of hypertrophic and atrophic scars: a pilot study. Am J Cosmet Surg. 2016;33(4):176–83.
Kim TK, Oh EJ, Chung JY, Park JW, Cho BC, Chung HY. The effects of botulinum toxin a on the survival of a random cutaneous flap. J Plast Reconstr Aesthet Surg. 2009;62(7):906–13.
Mahboub T, Ahmed Sobhi MD, Habashi H. Optomization of presurgical treatment with botulinum toxin in facial scar management. Egypt J Plast Reconstr Surg. 2006;30:81–6.
Lebeda FJ, Dembek ZF, Adler M. Kinetic and reaction pathway analysis in the application of botulinum toxin a for wound healing. Journal of toxicology. 2012;2012.
Flynn TC. Use of intraoperative botulinum toxin in facial reconstruction. Dermatol Surg. 2009;35(2):182–8.
Gassner HG, Sherris DA, Otley CC. Treatment of facial wounds with botulinum toxin a improves cosmetic outcome in primates. Plast Reconstr Surg. 2000;105(6):1948–53.
Gassner HG, Brissett AE, Otley CC, Boahene DK, Boggust AJ, Weaver AL, Sherris DA. Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study. In: Mayo clinic proceedings, vol. 81(8): Elsevier; 2006. p. 1023–8.
Chambers A. Effects of botulinum toxin a observed during early scar formation following Rhytidectomy: controlled, Double-Blinded Pilot Study. Am J Cosmet Surg. 2018;18:0748806818794528.
Goodman GJ. The use of botulinum toxin as primary or adjunctive treatment for post acne and traumatic scarring. J Cutan Aesthet Surg. 2010;3(2):90.
Wilson AM. Use of botulinum toxin type a to prevent widening of facial scars. Plast Reconstr Surg. 2006;117(6):1758–66.
Xiao Z, Zhang F, Cui Z. Treatment of hypertrophic scars with intralesional botulinum toxin type a injections: a preliminary report. Aesthet Plast Surg. 2009;33(3):409–12.
Wu WT. Skin resurfacing with Microbotox and the treatment of keloids. In: Botulinum toxins in clinical aesthetic practice: CRC Press; 2011. p. 204–19.
Gugerell A, Kober J, Schmid M, Buchberger E, Kamolz LP, Keck M. Botulinum toxin a: dose-dependent effect on reepithelialization and angiogenesis. Plast Reconstr Surg Glob Open. 2016;4(8).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Chambers, A. (2020). Treatment of Immature Scars with Botulinum Toxin. In: Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G. (eds) Textbook on Scar Management. Springer, Cham. https://doi.org/10.1007/978-3-030-44766-3_26
Download citation
DOI: https://doi.org/10.1007/978-3-030-44766-3_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44765-6
Online ISBN: 978-3-030-44766-3
eBook Packages: MedicineMedicine (R0)