Abstract
South Africa has a long history of managing the establishment and spread of invasive floating macrophytes. The past thirty years of research and the implementation of nation-wide biological and integrated control programmes has led to widespread control of these species in many degraded freshwater ecosystems. Such initiatives are aimed at restoring access to potable freshwater and maintaining native biodiversity. However, in recent years, there has been a decline in populations of floating invasive plants, and an increase in the establishment and spread of submerged and emergent invasive plant species, which poses significant threats to aquatic ecosystems. This chapter highlights the vulnerability of South Africa’s eutrophic systems to successful colonisation by this suite of new macrophytes following the successful biological control of floating invasive macrophytes, and explores a new regime shift in invasive populations partly driven by biological control. We suggest that a more holistic approach to the control of invasive plants would be required to ensure long-term ecosystem recovery and sustainability.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Aquatic ecosystems in South Africa have been prone to invasion by alien macrophytes, since the first introductions in the early 1900s. These alien freshwater plant species have become invasive in many rivers, man-made impoundments, lakes and wetlands in South Africa (Hill 2003), due to anthropogenic dissemination, combined with increasing urbanisation, industry and agriculture, which have resulted in nutrient enrichment and ultimately eutrophication. Aquatic macrophytes have a number of key traits that increase their invasiveness , such as rapid vegetative and sexual reproduction leading to fast population build-up, the ability to regenerate from fragments, high phenotypic plasticity and efficient dispersal mechanisms (Hill and Coetzee 2017). If the impacts of these invasive macrophytes are to be alleviated, then reductions in agricultural, industrial and urban runoff that are high in nitrates, ammonium, and phosphates will be needed (Cook 2004; Chambers et al. 2008).
This chapter reviews the factors that contribute to the invasiveness of alien freshwater macrophytes in South Africa, discusses their impacts, and assesses the control programmes implemented against these aquatic invaders.
2 Invasive Macrophytes
The most important invasive freshwater macrophyte in South Africa remains Water Hyacinth, which was first recorded as naturalised in KwaZulu-Natal in 1910. Four other species have also been extremely problematic, but are currently under successful biological control and together with Water Hyacinth, were referred to as the ‘Big Bad Five’ (Table 4.1). The presence of new invasive aquatic plant species, which are still in their early stages of invasion but targets for biological control, have been recorded recently in South Africa. These include submerged, rooted emergent, free-floating and rooted floating macrophyte species (Table 4.1). Additional species that are widespread invaders elsewhere in the world, but are not yet present in South Africa, pose a major threat should they be introduced (Table 4.1).
3 Pathways of Introduction
Invasive macrophyte species have been introduced and spread by means of numerous pathways, including the horticultural and aquarium trade , unintentional movement of propagules (i.e., hitchhikers) via boating enthusiasts and anglers, and, increasingly, via the unregulated internet trade that supplies aquatic plant enthusiasts (Cohen et al. 2007; Maki and Galatowitsch 2004; Padilla and Williams 2004; Martin and Coetzee 2011). For example, the horticultural and aquarium trade is the primary introduction pathway of submerged plants, such as E. densa and H. verticillata into new areas, including South Africa (Brunel 2009; Maki and Galatowitsch 2004). Alien submerged plants are traded either under their correct names, their synonyms, or common names (Hussner et al. 2014). Unfortunately, the general public and plant dealers are often unaware of the ecological repercussions of the species they trade. These species are released intentionally or unintentionally into water bodies and subsequently spread via plant fragments, with water flow and water sport equipment having been identified as the major vectors (Coetzee et al. 2009; Heidbüchel et al. 2016). This lack of knowledge regarding invasive aquatic species results in less care being given to the overflow of ponds or the disposal of plants, which are often discarded into ponds, ditches, streams and rivers (Duggan 2010). Invasive submerged plants in particular, most likely originating from aquarium releases, pose a significant negative environmental and economic threat to South Africa. They have been allowed to escape and spread with few or no control measures, as most attention has been paid to controlling the more obvious floating aquatic plant invasions. Awareness and publicity programmes on potential new threats could go a long way towards preventing their introduction and trade, as well as improved phytosanitary efforts and border control (Hill and Coetzee 2017).
4 Drivers of Invasion
The biology of freshwater macrophytes contributes to their invasiveness as they are capable of rapid asexual reproduction, and the most damaging species (e.g. Water Hyacinth and Water Lettuce) produce long-lived seeds. Once established, four factors contribute significantly to the invasiveness of these macrophytes: the lack of competition due to the paucity of native floating macrophytes (Cook 2004); the lack of co-evolved natural enemies in their adventive range (McFadyen 1998); disturbance , which includes eutrophication (Coetzee and Hill 2012); and the alteration of hydrological flows through the impoundment of streams and rivers, creating permanent waterbodies that are no longer prone to flooding or drought (Hill and Olckers 2001). Thus, aquatic plant invasions in South Africa are examples of ‘back-seat drivers’ (sensu Bauer 2012) in that they rely on the broad ecosystem disturbance (MacDougall and Turkington 2005) of slow-flowing permanent waters caused by impoundments, and eutrophication, which facilitates their establishment. This, linked with a lack of natural enemies, allows them to proliferate, thereby gaining a competitive advantage over native aquatic plants (Coetzee and Hill 2012).
5 Impacts
The negative socio-economic and environmental impacts of invasive aquatic plants have been well documented globally (e.g. Cilliers et al. 2003; Coetzee et al. 2018). Invasive floating plants and dense populations of submerged invasive plants form large continuous mats that significantly diminish the potential to utilise waterbodies, and reduce aquatic biodiversity and ecosystem functioning (Hill 2003). In large river systems in South Africa, such as the Vaal River and several inland impoundments (e.g. the Hartebeespoort and Roodeplaat dams ), invasive populations block access to sporting and recreational areas and decrease waterfront property values (McConnachie et al. 2003). Such impacts harm the economies of communities that depend upon fishing, tourism and water sports for revenue. Losses to the agricultural community involve the replacement costs of irrigation pumps that block and burnt out, the drowning of livestock (McConnachie et al. 2003) and water loss (Fraser et al. 2016; Arp et al. 2017).
Dense mats of floating invasive plants reduce light to submerged plants, thus depleting dissolved oxygen in aquatic communities. The consequent reduction in phytoplankton alters the composition of invertebrate communities, with knock-on effects at lower and higher trophic levels. For example, Midgley et al. (2006) and Coetzee et al. (2014) showed that Water Hyacinth mats significantly reduced the diversity and abundance of benthic invertebrates in impoundments in a temperate and subtropical region of South Africa, respectively.
The cost to control freshwater invasive macrophytes is also significant. The Department of Environmental Affairs spent some ZAR 42 million (approx. US$3 million) between 2010 and 2018, mainly on herbicide control of Water Hyacinth at a cost of ZAR 1800 per hectare (approx. US$130) (A. Wannenburgh, pers. comm.). However, the cost of control varies depending on the locality and application required. For example, van Wyk and van Wilgen (2002) compared the costs of controlling Water Hyacinth under herbicide application, biological control, and integrated control. The most expensive method was herbicidal control (US$250 per ha), while a biological control approach was much less expensive (US$44 per ha), but the best return of investment was provided by integrated methods (US$39 per ha). McConnachie et al. (2003) showed that Nett Present Value (NPV) of avoided impacts arising from the biological control of Red Water Fern in South Africa between 1995 and 2000 amounted to US$206 million, which converted to a benefit–cost ratio of 2.5:1 for the year 2000, increasing to 13:1 in 2005, and 15:1 in 2010, and although not calculated is still accruing as the weed remains under complete control. While these examples show the economic benefit of an intervention such as biological control, it is in contrast to manual removal, where for example, some EUR 14,680,000 was spent between 2005 and 2008 to remove nearly 200,000 tons of Water Hyacinth from the Guadiana River, Spain (75 km of river) (Ruiz Téllez et al. 2008). However, in this example, Water Hyacinth re-invaded the river, most likely from seed, or scattered plants that the mechanical harvesting had missed, and in 2010, an additional 5 tons of the weed was removed, followed by >51,000 tons, and then 170,000 tons in 2012 and 2016 respectively. In 10 years of control (2005–2015), up to EUR 26,000,000 was spent (Duarte 2017). Despite this effort, scattered populations of Water Hyacinth has spread along 150 km of the river, almost reaching Portugal and Alqueva, the largest Reservoir in Europe, and this management option has thus failed.
Impacts associated with the new suite of aquatic invasive species are yet to be manifest themselves, particularly those of wetland invaders such as S. platyphylla and I. pseudacorus whose distributions are increasing exponentially across South Africa (Box 4.1). Reductions in wetland floral and faunal biodiversity are expected. The extent of the alteration to sedimentation processes, hydrology and subsequent wetland ecosystem service provisioning are not known, but are likely to be significant.
Box 4.1 Spread of Delta Arrowhead in South Africa
Sagittaria platyphylla Engelm. (Alismataceae; Delta Arrowhead) is a freshwater aquatic macrophyte that has become an important invasive species in freshwater ecosystems in South Africa. The plant was first discovered in the Kranzskloof Nature Reserve, KwaZulu-Natal, in 2008, followed by identification of invasions in the Eastern Cape in Makhanda (Grahamstown) Botanical Gardens and Maden Dam near Stutterheim, and Jonkershoek trout hatchery near Stellenbosch in the Western Cape, in 2009. These invasions are assumed to be the result of unintentional introductions via dumping of fish tank contents, and intentional planting for trout fry.
Sagittaria platyphylla is now regarded as one of the fastest-spreading invasive species in the country (Henderson and Wilson 2017). It is also invasive in Australia where its invasion biology and spread has been studied extensively. The plant’s ability to reproduce sexually and asexually contributes to its rapid ability to spread. Each S. platyphylla plant produces numerous inflorescences every few weeks, with approximately 70,000 achenes produced per inflorescence (Adair et al. 2012; Broadhurst and Chong 2011). Therefore, even a small population of S. platyphylla could produce hundreds of thousands of viable achenes every few weeks. Achenes are able to disperse to new sites via wind and water dispersal, and attachment to recreational equipment and water birds (Adair et al. 2012). Asexual reproduction occurs via vegetative propagules, such as underground stem fragments, daughter plants (runners), stolons and tubers (Broadhurst and Chong 2011). The underground tubers allow the plant to survive through drought, water drawdown, frost and chemical and mechanical management (Adair et al. 2012).
Annual surveys conducted to monitor the spread, density and distribution of the plant in South Africa, showed an increase in the number of invaded sites from a single site in 2008, to 16 sites by 2009, 19 sites in 2013, and over 33 sites in 2017 (first figure below). Sagittaria platyphylla has been successfully eradicated from two sites in South Africa through the South African National Biodiversity Institute’s Biological Invasions Directorate, but it has spread from a number of sites. Six populations have been monitored since 2008, and results show that the plant has spread on average 11.4 ± 4.6 km from each site (second figure below), at an average of 1.4 km per year (MPH, unpublished data). The furthest the species has spread from a single location is 27 km in the uMngeni River system in KwaZulu-Natal.
Integrated chemical and mechanical control of S. platyphylla has not succeeded in slowing its spread in South Africa, as it continues to invade new sites. Options for biological control using host specific weevils in the genus Listronotus (Coleoptera: Curculionidae) are currently under investigation in quarantine at Rhodes University’s Centre for Biological Control.

Increase in the number of sites invaded with Sagittaria platyphylla (Delta Arrowhead) in South Africa since its first identification in 2008

Spread (in km) of Sagittaria platyphylla (Delta Arrowhead) from key invasion sites in South Africa
6 Control
A number of management options are available for the control of invasive macrophytes, but their success often depends on the use of integrated strategies. Here we review briefly the various options available.
Small invasions of aquatic macrophytes may be removed manually by hand, or mechanically using specialised harvesters, but this is labour-intensive and requires frequent follow-up treatments because not all plants are removed, allowing the regeneration of the population via vegetative reproduction. In South Africa, mechanical control of aquatic plants is not promoted, but there are some examples, particularly in the City of Cape Town where managers have adopted a ‘zero tolerance’ approach to aquatic invasive plants, and deploy mechanical harvesters to remove invasive vegetation, particularly from canals in the city (Fig. 4.1). These efforts have largely been unsuccessful due to rapid increase in biomass and because the high costs to not justify continuous removal (L. Stafford, pers. comm.).
Herbicidal control using glyphosate is most widely used to control Water Hyacinth in South Africa, but is limited in its success as it is temporary (Hill 2003). New invasions invariably regenerate from untreated plants, and seeds germinate from the hydrosoil following clearing, therefore requiring repeated applications. Integrated control, combining biological control with limited herbicide applications can reduce plant coverage and collateral damage to native vegetation (e.g. Jadhav et al. 2008 ). Herbicidal control is not recommended for the floating species under effective or complete biological control (i.e., P. stratiotes, S. molesta, M. aquaticum and A. filiculoides). Newly-identified Category 1a aquatic invaders (see Box 1.1 in van Wilgen et al. 2020, Chap. 1, for a definition of categories), such as I. pseudacorus and S. platyphylla, are targeted for eradication by the South African National Biodiversity Institute’s Biological Invasions Directorate (SANBI’s BID), and these species require both mechanical and herbicidal control. Herbicides are registered for use against some of these new invaders, but should be seen as short-term solutions because their distribution has developed beyond the lag phase of invasion, and eradication is no longer possible.
Large populations of floating macrophytes can be controlled effectively through biological control, which is both economically and environmentally sustainable (Hill et al. 2020). Floating macrophytes are particularly susceptible to biological control with a number of successful cases throughout the world, and in South Africa. For example, P. stratiotes, S. molesta, M. aquaticum and A. filiculoides have all been brought under complete biological control by a single agent in as little as 2 years, to a point where they no longer threaten aquatic ecosystems (Hill 2003). In contrast, biological control of Water Hyacinth has been variable, depending on water nutrient quality, cold winter temperatures and interference from herbicide operations (Coetzee et al. 2011a). In systems such as New Year’s Dam near Alicedale in the Eastern Cape , where the water is oligotrophic, the biological control of Water Hyacinth has been highly successful (Hill and Coetzee 2017). Ultimately, the long-term success of floating macrophyte control requires the integration of a variety of methods, with the most emphasis on reducing nitrate and phosphate pollution into aquatic environments (Hill 2003).
Utilisation of the excessive biomass of floating aquatic plant invasions, particularly in poorer rural areas, is often encouraged as a management option, where local communities are perceived to benefit from their use (Coetzee et al. 2009). Unfortunately, this is rarely effective due to the effort required to remove significant amounts of high water content biomass, and may even promote their spread. Water Hyacinth, for example, is nearly 95% water, and to gain 1 tons of dry material, 9 tons of fresh material is required, decreasing the commercial viability of such harvesting operations (Julien et al. 1999).
While South Africa has decades of experience in controlling floating aquatic plants, the initiation of biological control programmes against new aquatic invaders is in its early stages. The most recent release of an aquatic plant biological control agent was made in early October, 2018, when a leaf-mining fly, Hydrellia egeriae Rodrigues (Diptera: Ephydridae), was released on the Nahoon River, East London , Eastern Cape , for the control of the submerged Brazilian Waterweed, E. densa (Box 4.2).
Box 4.2 Release of the First Biological Control Agent Against Egeria densa
Egeria densa (Brazilian Waterweed), first recorded in South Africa in 1963 from the Durban area, is currently regarded as the most widely distributed submerged invasive aquatic plant species in South Africa. It forms dense populations in slow-moving rivers, and impoundments. The species is native to South America, and was most likely introduced to South Africa via the aquarium and ornamental plant trade. It is still traded in South Africa, despite its status as a Category 1b invasive (see Box 1.1 in van Wilgen et al. 2020, Chap. 1, for a definition of categories), increasing the propagule pressure on South African waterbodies.
A biological control programme was initiated against E. densa in 2014, following the identification of the leaf-mining fly H. egeriae as a potential agent by Cabrera-Walsh et al. (2013) (figure below). The initial research into the biology and host specificity of the fly was followed by its importation into the USA as a candidate control agent, after which it was imported into

First release of the leaf mining fly, Hydrellia egeriae (Diptera: Ephydridae), against Egeria densa (Brazilian Waterweed) on the Nahoon River in East London. (Photo: J.A. Coetzee). Inset A: adult fly, inset B: fly larva in a leaf mine. (Photographs courtesy of R. Smith)
quarantine in South Africa by the Centre for Biological Control at Rhodes University. Permission for the fly’s release was granted in June 2018, following the results of no-choice and paired choice tests which indicated that the physiological host range of the fly is limited to species within the Hydrocharitaceae, with a significantly higher preference and performance on its host plant. Additionally, continuation tests showed that none of the non-target species was able to sustain H. egeriae populations for more than three generations (Smith et al. 2019).
Mass rearing of the fly commenced at the Waainek Mass Rearing Facility at Rhodes University, shortly after permission for its release was granted. The Nahoon River in East London was chosen as the first release site for the fly largely due to the size of invasive populations of E. densa, and because it was the first population identified in South Africa during annual countrywide surveys, in 2008. It is also a site that has undergone a regime shift driven by biological control, from a floating plant dominated state of Water Hyacinth to a submerged stable state of E. densa. The fly was released on 12 October 2018, and the first post-release survey a month later confirmed its establishment in the system (RS, pers. obs.). Further releases will be made at invaded sites around the country.
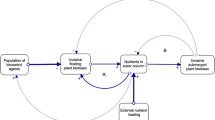
7 Regime Shifts and Alternate Stable States
The integrated control programme against invasive macrophytes in South Africa has been highly successful, as measured by an increase in the number of sites under biological control, coupled with a significant reduction in the cover of these invasive plants and a degree of recovery of ecosystem services (Hill and Coetzee 2017; Zachariades et al. 2017). However, unless the primary driver of invasions (i.e., eutrophication by nitrates and phosphates) in aquatic ecosystems is addressed, we anticipate a succession of invasions by a new suite of emergent and submerged invasive aquatic plant species (Coetzee et al. 2011a, b).
Ecosystems that are successfully colonised by non-native species often remain in long-term stable degraded states (Scheffer et al. 2003). However, there is evidence that the successful control of floating invasive plants can facilitate the proliferation of a new suite of invaders, inducing a secondary degraded stable state (Strange et al. 2018). As a result of successful biological control and the subsequent decomposition of floating plant biomass, there is an increase in available nutrients, light and space within the water column. Invasive submerged plants can successfully capitalise on this new abundance of resources and proliferate (Chimney and Pietro 2006; James et al. 2006; Longhi et al. 2008). This is confounded by high levels of external nutrients that facilitate plant growth and help to sustain a new stable regime of submerged invasive plant dominance (Duarte 1995). The systems thus have two alternate stable states, one dominated by floating invasive plants and the other by submerged invasive plants, with biological control triggering the shift between these stable states (Strange et al. 2018).
8 Discussion
We have shown that biological control has played a significant role in the recovery of aquatic biodiversity (Midgley et al. 2006; Coetzee et al. 2014), but such biodiversity benefits will be short-lived in impacted ecosystems unless integrated catchment management addresses eutrophication. If not, new invasions will replace the plants that have been cleared. To minimise the impacts of invasive submerged plants, research in South Africa must now focus on understanding the mechanisms facilitating these new invasions, and on devising successful management strategies . Such strategies must also address ecosystem-level responses to control to improve the chances of long-term success. Traditionally, intervention has been aimed at restoring ecosystems dominated by an invasive species by removing the invader (Dobson et al. 1997; Prach et al. 2001; Young 2000). However, when we consider such restoration in the context of regime shifts between degraded stable states, there is a clear need to adopt a more holistic approach. It is important to consider the effect that invasive species have upon the multitrophic interactions that define ecosystem structure and functioning. Further multitrophic studies could also help to elucidate the drivers that determine levels of success and failure in the establishment of both invasive species, and their biological control agents (Harvey et al. 2010).
Identifying management interventions that will be both successful and economically justifiable will require a thorough understanding of the affected ecosystem as a whole. The most efficient management can be obtained by prioritising those systems where management interventions would be most likely to succeed. South Africa is in the relatively early stages of research into the control of submerged invasive macrophytes. Experience gained in South Africa in the successful biological control of floating invasive plants may well be the route to follow. It can be a lengthy process, but could well deliver excellent results.
The single most important mitigation measure to reduce further impacts of invasive macrophytes is prevention of invasions at the outset (Tamayo and Olden 2014). Although legislation to prevent introduction and enforce management of invasive alien species does exist, the lack of financial resources and manpower to implement these legal requirements remains a challenge. Furthermore, it is important to coordinate actions against invasive macrophytes in neighbouring countries, otherwise a species that is being controlled or eradicated in one country might simply reinvade from an invaded neighbouring country through shared watersheds, rendering all efforts futile (Faulkner et al. 2017). This would require an effective biosecurity approach that builds on knowledge of potential invaders and invadable systems, and pathways of introduction and spread, incorporated into early detection and rapid response programmes (Hussner et al. 2017). Recent improvements in South Africa’s biosecurity and risk assessment processes of the Department of Environmental Affairs and SANBI’s BID are positive steps towards reducing risk from new introductions (Kumschick et al. 2018, 2020, Chap. 20).
References
Adair RA, Keener BR, Kwong RM et al (2012) The biology of Australian weeds, 60. Sagittaria platyphylla (Engelmann) J.G. Smith and Sagittaria calycina Engelmann. Plant Prot Quart 27:47–58
Arp RS, Fraser GC, Hill MP (2017) Quantifying the economic water savings benefit of water hyacinth (Eichhornia crassipes) control in the Vaalharts irrigation scheme. Water SA 43(1):58–66. https://doi.org/10.4314/wsa.v43i1.09
Bauer J (2012) Invasive species: “back-seat drivers” of ecosystem change? Biol Invasions 14:1295–1304. https://doi.org/10.1007/s10530-011-0165-x
Bownes A (2010) Asian aquatic moth Parapoynx diminutalis, accidentally introduced earlier, contributes to control of an aquatic weed Hydrilla verticillata in South Africa. Afr J Aquat Sci 35:307–311. https://doi.org/10.2989/16085914.2010.490988
Broadhurst L, Chong C (2011) Examining clonal propagation of the aquatic weed Sagittaria platyphylla. RIRDC Publication No. 11/020, Project No. AWRC 08-65, RIRDC
Brunel S (2009) Pathway analysis: aquatic plants imported in 10 EPPO countries. EPPO Bull 39:201–213. https://doi.org/10.1111/j.1365-2338.2009.02291.x
Cabrera-Walsh G, Magal Dalto Y, Mattioli FM et al (2013) Biology and ecology of Brazilian elodea (Egeria densa) and its specific herbivore, Hydrellia sp., in Argentina. BioControl 58:133–147. https://doi.org/10.1007/s10526-012-9475-x
Chambers PA, Lacoul P, Murphy KJ, Thomaz SM (2008) Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595:9–26. https://doi.org/10.1007/s10750-007-9154-6
Cheek MD (2018) First confirmed record of Nymphoides peltata (SG Gmel.) Kuntze (Menyanthaceae) naturalised in southern Africa. Bothalia 48:1–4. https://doi.org/10.4102/abc.v48i1.2258
Chimney MJ, Pietro KC (2006) Decomposition of macrophyte litter in a subtropical constructed wetland in south Florida (USA). Ecol Eng 27:301–321. https://doi.org/10.1016/j.ecoleng.2006.05.016
Cilliers CJ, Hill MP, Ogwang JA, Ajuonu O (2003) Aquatic weeds in Africa and their control. In: Neuenschwander P, Borgemeister C, Langewald J (eds) Biological control in IPM Systems in Africa. CAB International, Wallingford, pp 161–178. https://doi.org/10.1079/9780851996394.0161
Coetzee JA, Hill MP (2012) The role of eutrophication in the biological control of water hyacinth, Eichhornia crassipes, in South Africa. BioControl 57:247–261. https://doi.org/10.1007/s10526-011-9426-y
Coetzee JA, Hill MP, Schlange D (2009) Potential spread of the invasive plant Hydrilla verticillata in South Africa based on anthropogenic spread and climate suitability. Biol Invasions 11:801–812. https://doi.org/10.1007/s10530-008-9294-2
Coetzee JA, Hill MP, Byrne MJ, Bownes A (2011a) A review of the biological programmes on Eichhornia crassipes (C.Mart.) Solms (Pontederiaceae), Salvinia molesta D.S.Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragaceae) and Azolla filiculoides (Lam.) (Azollaceae) in South Africa. Afr Entomol 19:451–468. https://doi.org/10.4001/003.019.0202
Coetzee JA, Bownes A, Martin GD (2011b) Prospects for the biological control of submerged macrophytes in South Africa. Afr Entomol 19:469–487. https://doi.org/10.4001/003.019.0203
Coetzee JA, Jones RW, Hill MP (2014) Water hyacinth, Eichhornia crassipes (Mart.) Solms-Laub. (Pontederiaceae), reduces benthic macroinvertebrate diversity in a protected subtropical lake in South Africa. Biodivers Conserv 23:1319–1330. https://doi.org/10.1007/s10531-014-0667-9
Coetzee JA, Hill MP, Hussner A et al (2018) Invasive aquatic species. In: Hughes J (ed) Freshwater ecology and conservation, approaches and techniques. Oxford University Press, Oxford, pp 338–358. https://doi.org/10.1093/oso/9780198766384.003.0016
Cohen J, Mirotchnick N, Leung B (2007) Thousands introduced annually: the aquarium pathway for non-indigenous plants to the St Lawrence Seaway. Front Ecol Environ 5:528–532. https://doi.org/10.1890/060137
Cook CDK (2004) Aquatic and wetland plants of Southern Africa. Backhuys Publishers, Leiden, pp 140–148
Dobson A, Bradshaw AD, Baker AJM (1997) Hopes for the future: restoration ecology and conservation biology. Science 227:515–522. https://doi.org/10.1126/science.277.5325.515
Duarte CM (1995) Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41:87–112. https://doi.org/10.1080/00785236.1995.10422039
Duarte L (2017) Water hyacinth invasion on Guadiana River. Some number, facts and thoughts. Steemit. Biology-Blogs https://steemit.com/biology/@liliana.duarte/water-hyacinth-invasion-on-guadiana-river-some-numbers-facts-and-thoughts. Accessed 15 Jun 2017
Duggan IC (2010) The freshwater aquarium trade as a vector for incidental invertebrate fauna. Biol Invasions 12:3757–3770. https://doi.org/10.1007/s10530-010-9768-x
Faulkner KT, Hurley BP, Robertson MP et al (2017) The balance of trade in alien species between South Africa and the rest of Africa. Bothalia 47(2):a2157. https://doi.org/10.4102/abc.v47i2.2157
Fraser G, Martin J, Hill MP (2016) Economic evaluation of water loss saving due to the biological control of water hyacinth at New Year’s Dam, Eastern Cape Province, South Africa. Afr J Aquat Sci 41:227–234. https://doi.org/10.2989/16085914.2016.1151765
Harvey JA, Bukovinszky T, van der Putten WH (2010) Interactions between invasive plants and insect herbivores: a plea for a multitrophic perspective. Biol Conserv 143:2251–2259. https://doi.org/10.1016/j.biocon.2010.03.004
Heidbüchel P, Kuntz K, Hussner A (2016) Alien aquatic plants do not have higher fragmentation rates than native species: a field study from the River Erft. Aquat Sci 78(4):767–777. https://doi.org/10.1007/s00027-016-0468-1
Henderson L, Wilson JRU (2017) Changes in the composition and distribution of alien plants in South Africa: An update from the Southern African Plant Invaders Atlas. Bothalia 47(2):a2172. https://doi.org/10.4102/abc.v47i2.2172
Hill MP (2003) The impact and control of alien aquatic vegetation in South African aquatic ecosystems. Afr J Aquat Sci 28:19–24. https://doi.org/10.2989/16085914.2003.9626595
Hill MP, Coetzee JA (2017) The biological control of aquatic weeds in South Africa: current status and future challenges. Bothalia 47(2):a2152. https://doi.org/10.4102/abc.v47i2.2152
Hill MP, Olckers T (2001) Biological control initiatives against water hyacinth in South Africa: Constraining factors, success and new courses of action. In: Julien MH, Center TD, Hill MP (eds) Proceedings of the Second Global Working Group Meeting for the Biological and Integrated Control of Water Hyacinth, Beijing, China, 9–12 October 2000, pp 33–38
Hill MP, Moran VC, Hoffmann JH et al (2020) More than a century of biological control against invasive alien plants in South Africa: a synoptic view of what has been accomplished. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, pp 549–568. https://doi.org/10.1007/978-3-030-32394-3_19
Hussner A, Nehring S, Hilt S (2014) From first reports to successful control: a plea for improved management of alien aquatic plant species in Germany. Hydrobiologia 737:321–331. https://doi.org/10.1007/s10750-013-1757-5
Hussner A, Stiers I, Verhofstad MJJM et al (2017) Management and control methods of invasive alien freshwater aquatic plants: a review. Aquat Bot 136:112–137. https://doi.org/10.1016/j.aquabot.2016.08.002
Jaca T, Mkhize V (2015) Distribution of Iris pseudacorus (Linnaeus, 1753) in South Africa. BioInvasions Rec 4:249–253. https://doi.org/10.3391/bir.2015.4.4.03
Jadhav A, Hill MP, Byrne M (2008) Identification of a retardant dose of glyphosate with potential for integrated control of water hyacinth, Eichhornia crassipes (Mart.) Solms-Laubach. Biol Control 47:154–158. https://doi.org/10.1016/j.biocontrol.2008.08.005
James CS, Eaton JW, Hardwick K (2006) Responses of three invasive aquatic macrophytes to nutrient enrichment do not explain their observed field displacements. Aquat Bot 84:347–353. https://doi.org/10.1016/j.aquabot.2006.01.002
Julien MH, Griffiths MW, Wright AD (1999) Biological Control of Water Hyacinth. The Weevils Neochetina bruchi and N. eichhorniae: Biologies, Host Ranges, and Rearing, Releasing and Monitoring Techniques for Biological Control of Eichhornia crassipes. Monograph 60. Australian Centre for International Agricultural Research (ACIAR), Canberra, Australia, 87 pp
Kumschick S, Wilson JR, Foxcroft LC (2018) Framework and guidelines for conducting risk analyses for alien species. Preprints 2018110551. https://doi.org/10.20944/preprints201811.0551.v1
Kumschick S, Foxcroft LC, Wilson JR (2020) Analysing the risks posed by biological invasions to South Africa. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, pp 569–592. https://doi.org/10.1007/978-3-030-32394-3_20
Longhi D, Bartoli M, Viaroli P (2008) Decomposition of four macrophytes in wetland sediments: Organic matter and nutrient decay and associated benthic processes. Aquat Bot 89:303–310. https://doi.org/10.1016/j.aquabot.2008.03.004
MacDougall AS, Turkington R (2005) Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86:42–55. https://doi.org/10.1890/04-0669
Madeira PT, Hill MP, Dray FA Jr et al (2016) Molecular identification of Azolla invasions in Africa: The Azolla specialist, Stenopelmus rufinasus proves to be an excellent taxonomist. S Afr J Bot 105:299–305. https://doi.org/10.1016/j.sajb.2016.03.007
Maki K, Galatowitsch S (2004) Movement of invasive aquatic plants into Minnesota (USA) through horticultural trade. Biol Conserv 118:389–396. https://doi.org/10.1016/j.biocon.2003.09.015
Martin GD, Coetzee JA (2011) Fresh water aquatic plant invasion risks posed by the aquarium trade, aquarists and the internet trade in South Africa. Water SA 37:371–380. https://doi.org/10.4314/wsa.v37i3.68488
Martin GD, Coetzee JA, Lloyd M et al (2018) Invaded habitat incompatibility affects the suitability of the potential biological control agent Listronotus sordidus for Sagittaria platyphylla in South Africa. Biocontrol Sci Tech 28:468–485. https://doi.org/10.1080/09583157.2018.1460314
McConnachie AJ, de Wit MP, Hill MP et al (2003) Economic evaluation of the successful biological control of Azolla filiculoides in South Africa. Biol Control 28:25–32. https://doi.org/10.1016/S1049-9644(03)00056-2
McFadyen REC (1998) Biological control of weeds. Annu Rev Entomol 43:369–393. https://doi.org/10.1146/annurev.ento.43.1.369
Midgley JM, Hill MP, Villet MH (2006) The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. Afr J Aquat Sci 31:25–30. https://doi.org/10.2989/16085910609503868
Nxumalo MM, Lalla R, Renteria JL, Martin G (2016) Hydrocleys nymphoides (Humb. & Bonpl. ex Willd.) Buchenau: first record of naturalization in South Africa. BioInvasions Rec 5:1–6. https://doi.org/10.3391/bir.2016.5.1.01
Padilla DK, Williams SL (2004) Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front Ecol Environ 2:131–138. https://doi.org/10.1890/1540-9295(2004)002[0131:BBWAAO]2.0.CO;2
Prach K, Bartha S, Joyce CB et al (2001) The role of spontaneous vegetation succession in ecosystem restoration: a perspective. Appl Veg Sci 4:111–114. https://doi.org/10.1111/j.1654-109X.2001.tb00241.x
Ruiz Téllez T, Martín de Rodrigo López E, Lorenzo Granado G, Albano Pérez E, Morán López R, Sánchez Guzmán J (2008) The water hyacinth, Eichhornia crassipes: an invasive plant in the Guadiana River Basin (Spain). Aquat Invasions 3:42–53. https://doi.org/10.3391/ai.2008.3.1.8
Scheffer M, Szabó S, Gragnani A et al (2003) Floating plant dominance as a stable state. Proc Natl Acad Sci U S A 100:4040–4045. https://doi.org/10.1073/pnas.0737918100
Schooler S, Cabrera-Walsh W, Julien M (2009) Cabomba caroliniana Gray (Cabombaceae). In: Muniappan R, Reddy GVP, Raman A (eds) Biological control of tropical weeds using arthropods. Cambridge University Press, Cambridge, pp 88–107. https://doi.org/10.1017/CBO9780511576348.006
Smith R, Mangan R, Coetzee JA (2019) Risk assessment to interpret the physiological host range of Hydrellia egeriae, a biocontrol agent for Egeria densa. BioControl 64(4):447–456. https://doi.org/10.1007/s10526-019-09942-4
Strange EF, Hill JM, Coetzee JA (2018) Evidence for a new regime shift between floating and submerged invasive plant dominance in South Africa. Hydrobiologia 817:349–362. https://doi.org/10.1007/s10750-018-3506-2
Tamayo M, Olden JD (2014) Forecasting the vulnerability of lakes to aquatic plant invasions. Invas Plant Sci Manage 7:32–45. https://doi.org/10.1614/IPSM-D-13-00036.1
van Wilgen BW, Wilson JR (2018) The status of biological invasions and their management in South Africa 2017. South African National Biodiversity Institute, Kirstenbosch and DST-NRF Centre of Excellence for Invasion Biology, Stellenbosch
van Wilgen BW, Measey J, Richardson DM et al (2020) Biological invasions in South Africa: an overview. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, pp 3–30. https://doi.org/10.1007/978-3-030-32394-3_29
van Wyk E, van Wilgen B (2002) The cost of water hyacinth control in South Africa: a case study of three options. Afr J Aquat Sci 27:141–149. https://doi.org/10.2989/16085914.2002.9626585
Young TP (2000) Restoration ecology and conservation biology. Biol Conserv 92:73–83. https://doi.org/10.1016/S0006-3207(99)00057-9
Zachariades C, Paterson ID, Strathie LW, Hill MP, van Wilgen BW (2017) Biological control as a management tool for suppression of invasive alien plants in South Africa. Bothalia 47(2). https://doi.org/10.4102/abc.v47i2.2142
Acknowledgements
This research was funded through the Department of Environmental Affairs, Natural Resource Management Programme, (previously the Working for Water Programme). Additional funding was provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa. Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors, and the National Research Foundation accepts no liability in this regard.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Hill, M.P., Coetzee, J.A., Martin, G.D., Smith, R., Strange, E.F. (2020). Invasive Alien Aquatic Plants in South African Freshwater Ecosystems. In: van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T. (eds) Biological Invasions in South Africa. Invading Nature - Springer Series in Invasion Ecology, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-030-32394-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-32394-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32393-6
Online ISBN: 978-3-030-32394-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)