Abstract
The paper shows feasibility and efficacy of thermal modification of basalt fiber to increase its corrosion resistance and durability in a cement matrix. The authors justify the mechanism of phase and structure transformation of the fiber subsurface layer providing its increased physicochemical properties.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Under the conditions of real operation, the elements of concrete structures suffer cyclic, fatigue, impact, stretching and twisting loads, which causes uncontrolled cracking and consequent destruction of the cement matrix (Lesovik et al. 2018).
This problem was solved by using fibers to reinforce concrete matrix, which prevents brittle fracture of concrete and enables control of cracking (Klyuyev et al. 2013).
The analysis of approaches to enhancing the corrosion resistance of glass fiber in alkaline medium demonstrates that the thermal treatment of basalt fiber without its crystallization with initiation of a number of processes that increase alkali resistance and strength of the fibers should be considered as the most advanced and economically feasible method for increasing the stability of basalt fiber for its consequent application for concrete production on the basis of cement binders. (Knot’ko et al. 2007; Knotko et al. 2011)
According to the accepted working hypothesis, the increase of alkali resistance of fiber surface should be achieved by thermal treatment. This method is considered to be the simplest, affordable and economically reasonable. Its technical performance is predetermined by a range of physicochemical processes taking place in glass fibers during thermal treatment: oxidation, structure and diffusive rearrangement of ions in the material, annealing and densification of the structure, pre-crystallization and crystallization, etc.
2 Methods and Approaches
The main component in the work was basalt fiber produced at Novgorod glass fiber plant using machine factory BASK by blowing the melt by vertical air stream.
The method for investigation of thermal treatment impact on the fiber properties included step-wise heating of the fiber from 300 to 700 °C (700 °C is the working temperature of the basalt fiber, Twork) with the step of 100°. The isothermal holding time was 30 min, which considered the microscopic dimensions of fibers and striving to provide high productivity of the thermal treatment process. The fiber was cooled down in air by convective heat exchange. The fiber specimens then were tested for alkali resistance in model solutions represented by cement water extract.
3 Results and Discussion
According to preliminary data on the resistance of the fiber from different producers in alkaline and acidic medium, the chosen basalt fiber stands out for its high alkali and acid resistance. This is explained by the wash-out of cations persisting in vitrified phase sue to alkaline hydrolysis into the silicon dioxide solution with formation of aluminates and zincates with consequent liberation of anions SiO44−, Si2O52− and SiO32−. Insoluble complex aluminate and zincate salts accumulate on the chemically active fiber surface, which inhibits further leaching of silicon. In the absence of further introduction of alkaline component for supporting necessary pH, the decomposition retards.
The corrosion of basalt fiber and its consequent disruption is caused by both interaction with hydroxyl groups of silicon-oxygen radicals and capability of cations dissolved in water alkaline medium to exchange with cations comprising the basalt fiber.
Noteworthy, during the utilization of basalt fiber in real conditions in concrete, the fiber dissolution degree will be not that significant, since the solution processes will die down along with curing and solidification of the cement. Nevertheless, the chemical processes involving the fiber that take place in concrete during its life should not be underestimated. This necessitates the development of the fiber modification method to improve its resistance to aggressive alkali medium.
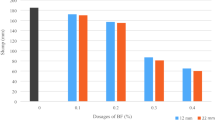
The analysis of obtained data (Fig. 1) confirmed that thermal treatment of fiber at 500 °C was the most effective, as it promoted its alkali resistance as compared to non-treated fiber by 35.3% (after 28 days). Further increase of the treatment temperature was useless because, firstly, the fiber alkali resistance reduced; secondly, it was not economically reasonable. The effect of increased alkali resistance at 300 and 400 °C amounted to 26.7 and 30%, respectively.
Heating of basalt fibers leads to negligible reduction of their mass due to loss of adsorbed and chemically bound water, while further heating increases the mass by binding of air oxygen during divalent iron FeO oxidation into trivalent Fe2O3. The increased oxidation degree of iron cations leads to their decreased ion radius and reduction of the coordination number with formation of groups of iron-oxygen tetrahedrons [FeO4]−Na+ that are embedded into the glass structure forming complex iron-aluminum-silicon-oxygen framework and increasing the degree of association, stability and density of the structure. Similar structural restructuring triggers the diffusion redistribution of modifying cations (Na+, Ca2+) in the fiber.
The thermal treatment is accompanied by microdefect healing, structure densification and its approaching to stable equilibrium state.
The processes described above are most active at 500 °C—the temperature comparable with the glass transition temperature Tg (corresponds to the viscosity of 1012,3 Pa s and transition of glass from solid into plastic state)—which conditions the highest alkali resistance of fibers and stability of their work in cement concretes.
At higher temperatures (600–700 °C) and decreased viscosity (1010–107 Pa s), the surface of basalt fibers suffers the manifestation of structural pre-crystallization and crystallization processes accompanied by the formation of diverse defects, which increase the free energy of the material and makes it more chemically active. This results in accelerated interaction with alkali and increased fiber mass losses. Besides, the thermal treatment at high temperature leads to the strength loss of basalt fibers: at 600 °C to negligible strength decrease; at 700 °C to the loss of 50% of initial strength.
Thermal treatment of basalt fibers at 500 °C initiated a number of important physicochemical and structural processes leading to the modification of glass fiber surface with acquisition of higher chemical resistance.
The holding close to the glass transition temperature Tg leads to gradual densification of the structure and its transition into more stable equilibrium state with higher chemical resistance. During the structure stabilization, numerous microdefects heal inside and on the surface of the fiber, which results in its decreased free energy and chemical activity. The structure densification also decreases the rate of numerous diffusion processes, which are the basis of any chemical reactions with solid phases.
Activation of iron ion oxidation during thermal treatment leads to substantial restructuring of the surface layer of basalt fiber: modifying ions of Fe2+ after oxidation to Fe3+ embed as iron-oxygen tetrahedrons [FeO4]5− into aluminum-silicon-oxygen lattice of the glass, thus increasing the degree of its association and chemical resistance.
Preliminary 30-min processing of the basalt fiber at 500 °C by 35% increases its resistance in alkaline medium of curing cement and can be recommended as effective, simple and economically reasonable method for modifying basalt fiber for its effective implementation as a micro-reinforcing component of fibrous concretes.
4 Conclusions
Thus, the processes initiated by thermal treatment promote the corrosion resistance of fibers in alkaline medium, which prolongs the corrosion resistance and increases the efficacy of fiber application as a micro-reinforcing component for cement composites.
References
Klyuyev SV, Klyuyev AV, Sopin DM, Netrebenko AV, Kazlitin SA (2013) Heavy loaded floors based on fine-grained fiber concrete. Mag Civil Eng 38(3):7–14
Knot’ko AV, Garshev AV, Davydova IV, Putlyaev VI, Ivanov VK, Tret’yakov YuD (2007) Chemical processes during the heat treatment of basalt fibers. Prot Metals 43(7):694–700
Knotko AV, Pustovgar EA, Garshev AV, Putlyaev VI, Tret’yakov YD (2011) A protective diffusion layer formed on surface of basaltic fiberglass during oxidizing. Prot Metals Phys Chem Surf 47(5):658–661
Lesovik VS, Glagolev ES, Popov DY, Lesovik GA, Ageeva MS (2018) Textile-reinforced concrete using composite binder based on new types of mineral raw materials. In: IOP conference series: materials science and engineering, vol 327, no 3
Acknowledgements
The work has been fulfilled within the project Federal Target Program of Research and Development on “Priority Development Fields of science and technology sector in Russia for 2014–2020”, the unique project number is RFMEFI58317X0063.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this paper
Cite this paper
Strokova, V., Nelyubova, V., Zhernovsky, I., Masanin, O., Usikov, S., Babaev, V. (2019). Impact of Thermal Modification on Properties of Basalt Fiber for Concrete Reinforcement. In: Glagolev, S. (eds) 14th International Congress for Applied Mineralogy (ICAM2019). ICAM 2019. Springer Proceedings in Earth and Environmental Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-22974-0_94
Download citation
DOI: https://doi.org/10.1007/978-3-030-22974-0_94
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22973-3
Online ISBN: 978-3-030-22974-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)