Abstract
The authors have developed a method for producing highly dispersed sillenite bismuth silicate in the system Na2O-Bi2O3-SiO2 (NBS) from water solutions of organosilicon monomers (sodium methylsiliconate) and bismuth nitrate. The paper studies the phase composition and microstructure of the synthesized NBS material at different temperatures. The morphology of crystals in the NBS material and the peculiarities of its thermal-oxidative breakdown are investigated. X-ray diffraction spectra obtained using a cuКα-source are used to evaluate the crystal lattice spacing and to analyze the broadening of the maximum-intensity diffraction line for this crystal with due consideration of crystal indices h, k, l by the approximation method to determine the dimensions of the coherent scattering region and microdistortions of the crystal lattice Δa/a. The authors established that the silicate shell on Bi12SiO20 particles is close to the silicates with continuous chain radicals [SiO3] 2−∞ , and a part of them are bridges between the bismuth silicate particles.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Sillenite
- Phase composition
- Microstructure
- Crystal morphology
- Thermal treatment
- Crystal lattice microdeformation
1 Introduction
The development of highly dispersed metal-organosiloxane fillers with modified surface allows solving a multitude of important problems in the field of radiation materials science (Pasechnik 2006). The promising approach is to use water-soluble chemically active organosiloxanes as the basis for production of metal oligomers. A new technological approach to the solution of the stated complex problem is required.
As of today, the chemistry of organosiloxane compounds of bismuth attract particular attention. This is conditioned by multiple valuable properties of organosilicon compounds (high thermal stability, hydrophobicity, dielectric characteristics and resistance to a range of aggressive media). Besides, bismuth atoms have large capture cross-section of gamma-radiation, which is almost the same as for lead atoms in a wide energy spectrum. The presence of vacant 3d-orbitals in silicon atoms conditions high reactivity of bond ≡Si-OH in silicate minerals.
2 Materials and Methods
The authors have developed a method for producing highly dispersed sillenite bismuth silicate in system Na2O-Bi2O3-SiO2 from water solutions of organosilicon monomers (sodium methylsiliconate) and bismuth nitrate (Yastrebinskii et al. 2018).
The amounts of the components were calculated with the aim of producing stable bismuth silicate Bi12SiO20 \( (6\text{Bi}_{2}\text{O}_{3} \cdot \text{SiO}_{2}) \).
At 100 ℃ we have obtained highly dispersed (0.2–0.3 μm) hydrophobic NBS material that is insoluble in water (NBS wetting angle is 122°). The density is 3780 kg/m3.
According to mass-spectroscopy, NBS material had the following composition (expressed as oxides), wt%: Na2O - 23.83; Bi2O3 - 59.70; SiO2 - 16.47.
Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) of specimens were performed on a STA-449 F1 Jupiter derivatograph (Germany). X-ray diffraction (XRD) analysis of phases and structure was performed on a ARL™ X’TRA Powder Diffractometer (Switzerland) with Cukx source (λkx = 1.542 Å) using a nickel filter. The infrared spectra were obtained on a Specord-75IR spectrometer (Germany). The material microstructure was studied by raster electron microscopy (REM) in the modes of reflected (back-scattered) and secondary electrons.
3 Results and Discussion
Phase Composition and Microstructure of Mineral Phases in NBS Material Synthesized at 100 ℃.
XRD phase analysis using the Powder Diffraction File (PDF) and literature data (Gorshkov and Timashev 1981; Gorelik et al. 2002) allowed detecting the formation of three amorphous-crystalline mineral phases:
-
1.
Metastable bismuth silicate Bi2SiO5 (d = 3.0379 Å/I = 100%; 3.7169 Å; 2.7223 Å) with tetragonal crystal system (a = 3.802; c = 15.134 Å), with the amorphous ring of about 3 Å.
-
2.
Bismuth oxide α-Bi2O3 (d = 3.2596 Å/I = 100%; 3.2596 Å; 1.9625 Å) with monoclinic crystal system (a = 5.8499; b = 8.1698; c = 7.5123 Å) with the amorphous ring of about 3 Å.
-
3.
Bismuth organosilicate H3C(SixBiyOz)Na with the amorphous ring of 10–12 10–12 Å and clear X-ray reflection at d = 11.4513 and 5.7090 Å. However, the precise determination of this composition using PDF failed.
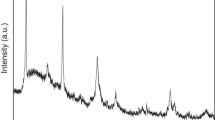
The results of IR-spectroscopy, the silicate phases in NBS powder synthesized at 100 ℃ have linear structure. The splitting of the absorption bands in the range of 1000–1100 cm−1 that is typical for the siloxane bond indicates the presence of several types of siloxane phases (Fig. 1).
Morphology of Crystals in Synthesized NBS Material.
According to REM, NBS material synthesized at 100 ℃ contained particle agglomerations of irregular shape with the size of 0.8–2.5 μm (Fig. 2).
Defectiveness of Crystals in NBS Material Subjected to Thermal Treatment.
X-ray diffraction spectra obtained using a CuКα-source were used to evaluate the crystal lattice spacing and to analyze the broadening of the maximum-intensity diffraction line for this crystal with due consideration of crystal indices h, k, l by the approximation method to determine the dimensions of the coherent scattering region and microdistortions of the crystal lattice Δa/a.
At 100–300 °C XRD analysis detected amorphous-crystalline bismuth organosiliconate H3C(SixBiyOz)Na with the amorphous ring of 10–12 Å and clear X-ray reflection at d = 11.4810 Å and 5.7020 Å.
At the temperature of 200 °C, bismuth silicate Bi2SiO5 in the mixture of minerals in terms of X-ray parameters approaches to the benchmark silicate of this composition (card no. 36-288 PDF: d = 3.0400 Å (I = 100%, hkl = 103).
In the temperature interval of 300–400 °C, metastable bismuth silicate Bi12Si0,87O20 with cubic crystal lattice in the synthesized dry mix transforms into stable bismuth silicate Bi12SiO20 also with cubic lattice.
In the temperature interval of 300–500 °C, the density of dislocations in the structure of bismuth silicate Bi12SiO20 crystals was lowering, while at the temperature higher than 650 °C, it was conversely rising up. The increase of the temperature from 300 to 500 °C improves the parameters of elementary crystal lattice of bismuth silicate Bi12SiO20 by 0.0357 Å and 0.0268 Å, as compared to the benchmark crystal. The volume of elementary crystal cell in this temperature interval grows by 2% and amounts to 1040.5870 Å3.
4 Conclusion
In the study, a method for producing highly dispersed sillenite bismuth silicate in the system Na2O-Bi2O3-SiO2 (NBS) from water solutions of organosilicon monomers (sodium methylsiliconate) and bismuth nitrate was developed. The paper studied the phase composition and microstructure of the synthesized NBS material at different temperatures. The crystalline structure of the substance and the presence of silicate amorphous phase in it were discovered. The paper revealed the morphology of crystals in the synthesized NBS material and the peculiarities of its thermal-oxidative breakdown; the silicate shell on the particles of Bi12SiO20 was close to continuous chain radicals \( \left[ {{\text{SiO}}_{3} } \right]_{\infty }^{2 - } \) and a part of them played the role of bridges between bismuth silicate particles. The determination of physicochemical properties of modified Bi12SiO20 sillenite crystals was of appreciable significance.
References
Gorelik SA, Skakov YuA, Rastorguev LN (2002) X-radiography and electron-optical analysis (in Russian). MISIS, Moscow, 360 p
Gorshkov VS, Timashev VV (1981) Methods of physicochemical analysis of silicates (in Russian). Higher School, Moscow, 335 p
Pasechnik OF (2006) Study of properties and structure of polyamide films after interaction of low-orbit space factors. Obninsk, p 113. (in Russian)
Yastrebinskii RN, Bondarenko GG, Pavlenko VI (2018) Synthesis of stable bismuth silicate with sillenite structure in the Na2O–Bi2O3–SiO2 system. Inorg Mater Appl Res 9(2):221–226
Acknowledgements
The work is realized in the framework of the Program of flagship university development on the base of Belgorod State Technological University named after V.G. Shukhov, using equipment of High Technology Center at BSTU named after V.G. Shukhov, the project within strategic development program No. a-51/17.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this paper
Cite this paper
Pavlenko, A., Yastrebinskiy, R. (2019). Topochemical Transformations in Sodium-Bismuth-Silicate System at 100–900 ℃. In: Glagolev, S. (eds) 14th International Congress for Applied Mineralogy (ICAM2019). ICAM 2019. Springer Proceedings in Earth and Environmental Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-22974-0_28
Download citation
DOI: https://doi.org/10.1007/978-3-030-22974-0_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22973-3
Online ISBN: 978-3-030-22974-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)