Abstract
This introductory chapter begins with the fundamentals of multiphase flow regimes as well as the classification of multiphase systems with phase change. The processes of phase change between solid, liquid, and vapor are discussed. A review of heat and mass transfer with emphasis in multicomponent systems and scaling are presented in this chapter including multiphase notations and concepts.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
References
ANSYS Fluent Theory Guide. (2017). ANSYS, Inc.
Bejan, A. (2013). Convection heat transfer (4th ed.). New York: Wiley.
Bergman, T. L., & Lavine, A. S. (2017). Fundamentals of heat and mass transfer (8th ed.). New York: Wiley.
Bird, R. B., Stewart, W. E., & Lightfoot, E. N. (2006). Transport phenomena (Revised 2nd ed.). New York: Wiley.
Buckingham, E. (1914). On physically similar systems: Illustrations of the use of dimensional equations. Physical Review, 4, 345–376.
Eijkel, J. C. T., & van den Berg, A. (2005). Nanofluidics: What is it and what can we expect from it? Microfluidics and Nanofluidics, 1(3), 249–267.
Faghri, A. (2016). Heat pipe science and technology (2nd ed.). Columbia, MO: Global Digital Press.
Faghri, A., Zhang, Y., & Howell, J. R. (2010). Advanced heat and mass transfer. Columbia, MO: Global Digital Press.
Howell, J., Menguc, M. P., & Siegel, R. (2015). Thermal radiation heat transfer (6th ed.). Boca Raton, FL: CRC Press.
Kays, W. M., Crawford, M. E., & Weigand, B. (2004). Convective heat transfer (4th ed.). New York, NY: McGraw-Hill.
Kleijn, C. R., van Der Meer, H., & Hoogendoorn, C. J. (1989). A mathematical model for LPCVD in a single wafer reactor. Journal of the Electrochemical Society, 136, 3423–3432.
Kulacki, F. A. (2018). Handbook of thermal science and engineering. New York, NY: Springer International Publishing.
Michaelides, E., Crowe, C. T., & Schwarzkopf, J. D. (2016). Multiphase flow handbook (2nd ed.). Boca Raton: CRC Press.
Poling, B. E., Prausnitz, J. M., & O’Connell, J. P. (2000). The properties of gases and liquids (5th ed.). New York, NY: McGraw-Hill.
Serizawa, A. (2010). Dispersed flow. In Thermopedia, Begell House, http://www.thermopedia.com/content/5/. Accessed May 15, 2018.
Welty, J. R., Rorrer, G. L., & Foster, D. G. (2014). Fundamentals of momentum, heat and mass transfer (6th ed.). New York, NY: Wiley.
White, F. M. (2005). Viscous fluid flow (3rd ed.). New York, NY: McGraw-Hill.
Author information
Authors and Affiliations
Corresponding author
Problems
Problems
-
1.1.
Why can severe skin burns be caused by hot steam?
-
1.2.
When 0.01 kg of ice at 0 °C is mixed with 0.1 kg of steam at 100 °C and 1 atm, what is the phase of the final mixture? What is the final temperature of the final mixture?
-
1.3.
An ice skater moving at 8 m/s glides to a stop. The ice in immediate contact with the skates absorbs the heat generated by friction and melts. If the temperature of the ice is 0 °C and the weight of the ice skater is 60 kg, how much ice melts?
-
1.4.
A lead bullet with 30-g mass traveling at 600 m/s hits a thin iron wall and emerges at a speed of 300 m/s. Suppose 50% of the heat generated is absorbed by the bullet, and the initial temperature of the bullet is 20 °C. Find the final phase and temperature of the lead bullet after the impact. The specific heat of the liquid lead can be assumed to be the same as that of the solid lead.
-
1.5.
A rigid tank filled with a mixture of liquid and vapor in equilibrium (Fig. P1.5) is heated at a rate of q (kW) and evaporation takes place in the rigid tank. To maintain a constant pressure during evaporation, a relief valve at the top of the tank is opened and the mass flow rate of vapor through the valve is \(\dot{m}\) (kg/s). Show that the heating rate q is related to the mass flow rate \(\dot{m}\) and properties of liquid and vapor as follows:
$$q = \dot{m}\left[ {h_{\ell \text{v}} + \left( {h_{\ell } - \frac{{v_{\text{v}} e_{\ell } - v_{\ell } e_{\text{v}} }}{{v_{\text{v}} - v_{\ell } }}} \right)} \right]$$(1.106)where \(v_{\text{v}}, v_{\ell }, e_{\text{v}} \, {\text{and}} \, e_{\ell }\) are specific volumes and internal energies of vapor and liquid, respectively.
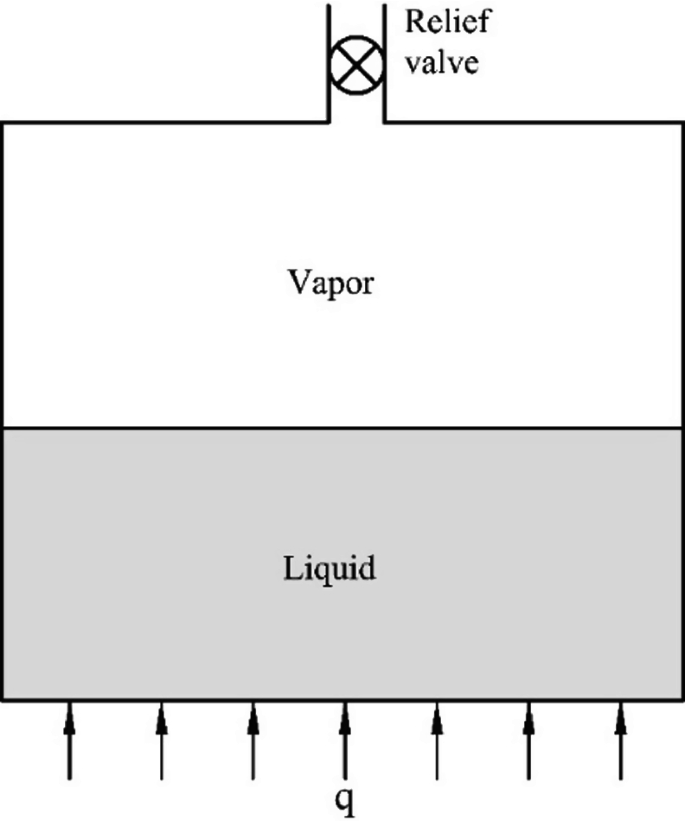
Fig. P 1.5
-
1.6.
Assume an ideal two-component gas mixture A and B, and develop a relation for mole and mass fraction for A and B in terms of partial pressure and total pressure for the mixture.
-
1.7.
Verify that the following equation is valid for a binary system:
$$\frac{{{\mathbf{J}}_{1} }}{{\rho \omega_{1} \omega_{2} }} = \frac{{{\mathbf{J}}_{1}^{*} }}{{cx_{1} x_{2} }}$$ -
1.8.
Prove that the sum of mass fluxes by diffusion for a multicomponent mixture is zero.
$$\sum\limits_{i = 1}^{N} {{\mathbf{J}}_{i} = 0}$$Show that the sum of mass flux relative to stationary coordinate axes for multicomponent mixtures is different from zero.
-
1.9.
Show that the Maxwell–Stefan equation (1.62) for multicomponent system can be reduced to Fick’s law, Eq. (1.58), for a binary system.
-
1.10.
Develop the mass flux relationship for component A, leaving from a solid or liquid wall made of component A only to a binary (Fig. P1.10) mixture of gases A and B in terms of (a) mass fractions and mass density of component A, and (b) molar fractions and molar concentration of component A. Describe the assumptions in arriving at your final result for each case.
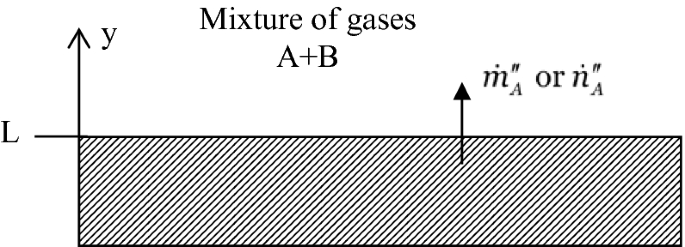
Fig. P1.10
-
1.11.
Air with a velocity of 10 m/s, temperature 22 °C at 50% relative humidity, flows over a swimming pool (40 m by 20 m) along its length direction. The water temperature in the pool is 32 °C. Calculate the average heat loss due to (a) sensible heat using Appendix D and (b) latent heat of vaporization.
-
1.12.
A full water container with a 1 m2 surface area is maintained at 60 °C with surrounding air at 30 °C and 20% relative humidity. Calculate the convection and evaporation heat losses.
-
1.13.
Determine the thickness of an ice layer on a large rectangular swimming pool of 7 × 10 m2 in winter when air blows along the length direction over the pool at 16 km/h and −23 °C. Assume the pool temperature is 5 °C and the inner ice surface temperature is at 0 °C. The convection heat transfer coefficient at the bottom of the ice is 50 W/m2 K. Use Appendix D to calculate the convective heat transfer coefficient on the top of the ice layer. What is the surface temperature on the outer layer of ice?
-
1.14.
A water droplet is placed in dry stagnant air of uniform temperature, \(T_{\infty }\). Show that
-
(a)
\(\frac{{k_{\text{air}} }}{{h_{\ell \text{v}} \rho_{\text{air}} }}{Nu}\left( {T_{\infty } - T_{\text{sat}} } \right) = D_{12} {Sh}\frac{{\omega_{ 1\delta } - \omega_{1\infty } }}{{1 - \omega_{1\delta } }}\)
-
(b)
\(c_{{\text{p}, \text{air}}} \frac{{\left( {T_{\infty } - T_{\text{sat}} } \right)}}{{h_{\ell \text{v}} }} = {Le}\frac{{\omega_{ 1\delta } - \omega_{1\infty } }}{{1 - \omega_{1\delta } }}\)
What are the assumptions made to produce the results found in (a) and (b)?
-
(a)
-
1.15.
Compare the orders of magnitude of the Prandtl, Schmidt, and Lewis numbers for various gases and liquids using data from Appendix B. Can any conclusions be made?
-
1.16.
The convective heat transfer coefficient is a function of the thermal properties of the fluid, the geometric configuration, flow velocities, and driving forces. As a simple example, let us consider forced convection in a circular tube with a length L and a diameter D. The flow is assumed to be incompressible and natural convection is negligible compared with forced convection. The heat transfer coefficient can be expressed as
$$h = h(k,\mu ,c_{\text{p}} ,\rho ,U,\Delta T,D,L)$$(1.107)where k is the thermal conductivity of the fluid, \(\mu\) is viscosity, \(c_{\text{p}}\) is specific heat, \(\rho\) is density, U is velocity, and \(\Delta T\) is the temperature difference between the fluid and tube wall. Equation (1.107) can also be rewritten as
$$F(h,k,\mu ,c_{\text{p}} ,\rho ,U,\Delta T,D,L) = 0$$(1.108)Using Buckingham’s Π theorem show that the number of nondimensional variables is 4, as opposed to the 9 dimensional variables in Eq. (1.108). Furthermore, use dimensional analysis to show that
$$Nu = f(Re, Pr , L/D)$$(1.109) -
1.17.
In Problem 1.16, if the flow is assumed to be compressible and natural convection is not negligible compared with forced convection, the heat transfer coefficient can be expressed as
$$h = h(k,\mu ,c_{\text{p}} ,\rho ,U , g, \beta , \Delta T, D, L, c)$$(1.110)where \(\beta\) is thermal expansion coefficient, and c is local speed of sound. Equation (1.110) can also be rewritten as
$$F(h, k,\mu ,c_{\text{p}} ,\rho ,U,g,\beta ,\Delta T,D,L,c) = 0$$(1.111)Using Buckingham’s Π theorem and dimensional analysis to show that
$$Nu = f( Re , Gr, Pr ,L/D, Ma, Fr)$$(1.112)where Gr, Ma, and Fr are the Grashof number, Mach number, and Froude number, respectively, i.e.,
$$Gr = \frac{{\rho^{2} g\beta \Delta TD^{3} }}{{\mu^{2} }}, \quad Ma = \frac{U}{c},\quad Fr = \frac{{U^{2} }}{gL}$$(1.113) -
1.18.
Estimate the order of magnitude of the thickness of the momentum boundary layer for forced convection flow over a flat plate as shown in Fig. P1.18 using scale analysis.
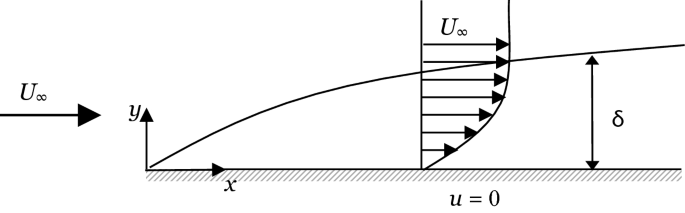
Fig. P1.18
-
1.19.
The skin friction coefficient of Problem 1.18 defined as \(C_{\text{f}} = \tau /\left( {\rho U_{\infty }^{2} /2} \right)\), where \(\tau\) is the shear stress at the surface of the flat plate. Estimate the order of magnitude of the friction coefficient using scale analysis.
-
1.20.
A solid PCM with a uniform initial temperature at the melting point, \(T_{\text{m}} ,\) is in a half space, \(x > 0.\) At time \(t = 0,\) the temperature at the boundary \(x = 0\) is suddenly increased to a temperature, \(T_{0} ,\) which is above the melting point of the PCM. Perform a scale analysis to obtain the order of magnitude of the location of the melting front.
-
1.21.
Perform an Internet search with the phrases “multiphase heat transfer” as well as “multiphase flow” and write a brief summary of what you find and the differences.
-
1.22.
Plasma is regarded as a state of matter in addition to gas, liquid, and solid. Use the Internet to find information about plasmas. How do you compare the properties of plasma with those of a gas, liquid and solid?
-
1.23.
The temperature and dew point of a city in summer are 80 and 40 °F, respectively. What is the relative humidity?
-
1.24.
A two-phase heat sink is a very effective device for electronic cooling. What are the advantages and disadvantages of a two-phase heat sink in comparison with a single-phase heat sink?
-
1.25.
Give two examples of two-phase flow from everyday life and industrial applications, and briefly discuss the physical phenomena involved in your examples.
-
1.26.
Laser welding is a process that uses a laser beam to join two metal workpieces together. Analyze the phase changes involved in the laser welding process. You can use the internet to find related information.
-
1.27.
The working fluid for a high-temperature heat pipe is liquid metal, which is solid at room temperature. Qualitatively analyze the phase-change phenomena involved during the start-up and shutdown of the high-temperature heat pipes.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Faghri, A., Zhang, Y. (2020). Introduction. In: Fundamentals of Multiphase Heat Transfer and Flow. Springer, Cham. https://doi.org/10.1007/978-3-030-22137-9_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-22137-9_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22136-2
Online ISBN: 978-3-030-22137-9
eBook Packages: EngineeringEngineering (R0)




