Abstract
Our goal is to uncover the mechanism underlying tumour-treating fields’ efficacy in killing cancer cells. Modelling the effects of these 200 kHz alternating current electric fields on tumour cell sub-structures has led us to focus on the microtubules (MTs), C-termini and the motor protein kinesin, which are integral to the critical functions of MT transport of proteins during the delicate orchestration of cell division (mitosis). Leading hypotheses of the TTFields’ mechanism that we are modelling include disruption of mitosis functions (such as the ‘kinesin walk’ along MTs), C-termini state transitions and MT polymerization.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Tumor-treating fields (TTFields) are 100–500 kHz electric fields with intensities of about 1–4 V/cm, which are known to exert an anti-mitotic effect on cancer cells. TTFields have been observed to kill virtually all tumour cells in vitro and in some animal preparations [1, 2]. They have minor side effects, are increasingly prescribed for brain cancer and are in development or clinical trials for a number of other aggressive malignant cell types in humans [3, 4]. It was initially believed that TTFields act on highly polar sub-cellular structures, such as tubulin dimers, septin, actin etc., thereby disrupting spindle formation [1]. However, calculations show that TTFields-interaction energy is several orders of magnitude too low to directly disrupt the functionality of these structures, while disruption of other structures such as microtubules (MTs) and motor proteins is possible [6]. We are analyzing various hypotheses for TTFields’ mechanism of action toward optimizing its clinical efficacy using numerical analysis, such as finite element modeling (FEM).
2 Overview of the Models
2.1 Why Computer Modelling?
Cell studies, such as Kirson et al. [1, 2], Gela et al. [7] and Giladi et al. [8], measure empirical outcomes. Unlike modeling, cell studies generally do not reveal low-level intra-cellular mechanisms although they may engender mechanism hypotheses that can be tested by modeling and further cell studies. But cell studies are also expensive and generally take many months, whereas computer simulations are relatively cheap and can provide results in much shorter time frames. Models can also be parameterized to analyze many scenarios with batch parameter sweeps (e.g. Wenger et al. [9]).
2.2 Axiomatizing the Underlying Systems Level
Computer models can be constructed at any biological systems level. Fundamental results at the underlying systems level are taken as assumptions or ‘axioms’ at the level being modelled [10]. For example, molecular dynamics (MD) simulations are currently conducted on a smaller microcosmic scale than FEM and for nanosecond simulated durations. Our FEM simulations can incorporate the output of MD. Figure 6.1 shows a map of electric potential on the surface of a microtubule (MT) predicted by an MD simulation, and Fig. 6.2 shows the map as imported into COMSOL FEM (Burlington, MA) using an interpolation function. The MT potential map is taken as axiomatic at the higher systems level of the cell and used in turn by FEM to predict responses of polarized intracellular structures to the MT surface.
Top: A 2D map of electric potential on a microtubule (MT) surface created in a molecular dynamics simulation of the MT’s underlying tubulin dimers and then used as input to a finite element analysis of the interaction of the MT surface potential with charged C-termini tails and the counter-ion layer they attract [11]. (VMD (National Institutes of Health, Bethesda MD USA) courtesy Josh Timmons, Wong lab, Harvard Medical School). Bottom: The same map imported into a COMSOL finite element model, where the z-axis represents potential in volts and x and y are surface coordinates on the MT
3 Clues to the Mechanisms Are Constraints on the Models
Over the past 15 years, researchers have produced valuable empirical results about TTFields including those summarized in Table 6.1 [1, 2, 8, 9, 12, 13].
While each cell study result constitutes an important constraint on the model, the question remains: which observed effects are causative versus downstream or epiphenomenal?
Cells follow multiple pathways when exposed to TTFields, including apoptosis during interphase, mitotic arrest and death and normal progression into the next cell cycle, possibly indicating multiple mechanisms of action [8]. It also appears TTFields have an effect on the immune system, which may be involved in TTFields’ mechanism [14].
4 Candidates for TTFields Mechanisms
We have collected a prioritized list of hypothesized TTFields mechanisms of action to model and test. These include:
-
4.1.
TTFields disrupt the ‘walk’ of the motor protein kinesin, which carries cargo throughout the cell.
-
4.2.
TTFields disrupt the metastable state transitions of MTs’ Carboxyl-termini (C-termini), which signal protein cargo transport ‘on’ and ‘off’, i.e. enabling or disabling protein transport along the MT.
Both 4.1 and 4.2 are examples of the general hypothesis that energy imparted by TTFields to sub-cellular structures, in these cases, the counter-ion layer surrounding the C-termini, exceeds a disruption metric, such as the energy contributed by ATP to release the kinesin foot (4.1) and one or two multiples of cellular free energy (4.2) [6]. These possibilities are further explored below.
-
4.3.
The electric field or current density edge effects disrupt MT length dynamics.
-
4.4.
TTFields cause torsion or rotation of septin fibers around their longitudinal axis, which interferes with septin assembly.
-
4.5.
Structural deformation of MTs disrupt signaling.
-
4.6.
DEP forces accelerate organelles to furrow, causing cell blebbing.
5 Disruption Metrics Derived from Signal-to-Noise Ratio
Cellular processes constitute signaling that evolved to exceed several background noise levels. Processes that use the least energy are the ‘low-hanging fruit’ of possible TTFields mechanisms, since the least amount of externally imposed energy may disrupt them. Thus, two important disruption metrics are low multiples of these parameters (‘low’ since higher multiples pose higher energy barriers):
-
5.1.
Cellular background thermal energy, given by kT = 4.2 x 10−21 Joule-nanometer
-
5.2.
Cellular free energy, given by −54–101 × 10−19 Joule-nanometer = ~25 kT
where k is Boltzmann’s constant, 1.38 × 10−23 m2 kg s−2 T−1 and T is the absolute temperature in Kelvin [15]. Note that the second metric requires more disruption energy than the first.
-
5.3.
There are conditions under which energy could accumulate or be amplified, exceeding the cellular disruption metrics. Examples include: (1) the amplification of electric field strength at the cellular furrow during late mitosis [9], which we have ruled out for the moment since its timing conflicts with Kirson et al. finding that TTFields’ strongest effect occurs during early mitosis (i.e. prophase) [2], (2) hypotheses that incorporate a resonance effect generated by the unique efficacious frequency range of TTFields, 100–300 kHz [16] (Sect. 6.1), (3) a hypothesis that regions of higher conductivity would shunt higher currents through the cell (Section “Electromechanical Model”) and (4) that edge effects produced when the field is orthogonal to the MT result in current density being amplified at the ends of the MT, which we have found using FEM.
6 Models and Results
6.1 MT Resonance
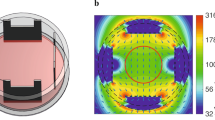
The hypothesis that the ~200 kHz frequency of TTFields may produce a resonance in MTs is in accordance with the empirical finding that TTFields’ second-strongest effect occurs when the field is applied orthogonally to the cell axis, to which many MTs are aligned during mitosis. To test this hypothesis, we performed numerical simulations evaluating the magnitude of the electric current along MTs exposed to TTFields at 200 kHz.
6.1.1 Electromechanical Model
An electromechanical model coupling constitutive relations in structural mechanics and electromagnetics was developed in COMSOL. Structural parameters such as several measures of elasticity were calibrated according to Tuszynski et al. [17], and sinusoidal electric fields of 1 V/cm were applied transversely to model MTs 20–960 nm in length and at frequencies ranging from kHz to THz. Both free and one end fixed boundary conditions were examined at both ends of the MT ‘beam’. Two disruption metrics were examined: (1) maximum deformation and (2) maximum kinetic energy (KE) along the MT.
The net result of these studies was that, without including a viscosity factor for the ambient medium in which the MT was embedded (e.g. cytosol, admittedly the apparent viscosity on a nanometer scale is unclear), we found significant (in terms of KE) resonance peaks in the GHz but not kHz range (Fig. 6.2), supporting others’ studies [18]. Furthermore, we found that incorporating virtually any viscosity factor damped the resonant peaks to a level below the potential for significant disruptive KE.
6.2 MT Conductivity
Santelices et al. recently found that MT conductivity considerably exceeds that of the ambient cytosol by as much as two orders of magnitude [19]. This result suggested that MTs could act as electrical cables shunting relatively high currents through the cell. A theoretical basis for assigning conductivities to the various components of a MT is, however, still unknown.
6.2.1 MT as a Multi-Layered Cable
Accordingly, we modeled the MT as a layered cylinder with an inner lumen radius of 15 nm, helix and component protofilament thicknesses of 4.5 nm, C-termini thickness of 3.5 nm, counter-ions of 2 nm thickness and a 3-nm-thick outer Bjerrum (insulative) layer. The counter-ion layer conductivity was significantly higher than that of cytosol [5, 6, 19].
Not surprisingly, the highest current was found to flow through the counter-ion layer surrounding the C-termini. The current density in this layer may exceed the level required to disrupt intra-cellular processes, such as the motor protein kinesin ‘walk’ along the MT, MT’s C-termini state transitions, or MT polymerization. Current density is highest when the field is aligned with the MT, which is in accordance with in vitro experiments showing that the TTFields’ effect is strongest when aligned with the cell axis, and that overall MT alignment with the cell axis increases during mitosis [1].
6.3 C-Termini State Disruption
If C-termini state transitions were disrupted by TTFields, critical motor protein transport along MTs, and perhaps other critical functions, would be crippled, likely delaying the silencing of a mitotic cell cycle checkpoint that allows cancer cell division to proceed [8].
The energy required for C-termini state transitions was computed in a series of MD simulations by Priel et al. [5], where the ‘up’ or ‘on’ state was lowest energy, and at which background thermal energy of ~25 meV would buffet the C-termini within a 40° cone at physiological temperatures (e.g. 300 K) (Fig. 6.3a, b), while 50 meV would push it beyond 40° (Fig. 6.3c), and 160 meV was required to push a C-termini over a saddle point into its ‘off’ state along the MT surface with energy of 100 meV. We translated these deformations into forces acting on the C-terminus surface to calibrate the model according to the Priel results (Fig. 6.3).
Calibration of C-terminus state transitions according to Priel et al. [5]. Top: Background thermal energy displaces the C-terminus minimally. Bottom: Applied boundary loads of 4 pN and 8 pN displace the C-terminus up to 40°
6.3.1 Model Calibration
The Young’s modulus of the MT was initally set to 2 GPa [15] and adjusted until the following constraints were met, assuming a C-terminus length of 3.5 nm [5]:
-
kT (25 meV) calibration: a force of 4 pN acting through ~1 nm should induce thermal energy like motions of the C-terminus tip.
-
50 meV calibration: a force of 8 pN should displace the C-terminus tip by ~40° (2.4 nm).
-
120 meV calibration: a force of 16 pN should displace the C-terminus tip by ~80° (4.9 nm).
6.4 Kinesin Walk Diffusion Hypothesis
Recent studies hypothesize a highly sensitive phase in the motor protein, kinesin, walk along MTs [20]. The back ‘foot’ of kinesin is released from its MT bond by ATP (10−19 Joules). The kinesin molecule’s neck then lurches forward over a 10 ms period, skipping over where the forward foot is attached to the MT, and placing the new forward foot two tubulin dimers ahead of its previous position (~16 nm total). The final phase of the walk takes place when thermal buffeting randomly positions the forward foot near enough to the dimer for electrostatic forces to bind it. We plan to use modelling to further examine this diffusion phase, wherein a stall force 10−19 N ≤ F ≤ 10−16 N from TTFields would prevent diffusion and disrupt the kinesin walk.
Note that the duration of the diffusion phase is estimated at 4 μs [20] and therefore corresponds to a frequency of 250 kHz, which may indicate a connection to the TTFields’ maximum efficacy frequency of 200 kHz.
7 Conclusion
Numerical modeling is a necessary complement to cell studies since it can examine underlying mechanisms of action relatively quickly and inexpensively. We are systematically using models to analyze hypothesized mechanisms responsible for TTFields efficacy in killing tumour cells. Such an understanding will facilitate moving TTFields’ clinical efficacy toward the 100% ideal achieved in vitro.
References
Kirson, E. D., et al. (2004). Disruption of cancer cell replication by alternating electric fields. Cancer Research, 64, 3288–3295.
Kirson, E. D., et al. (2007). Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proceedings of the National Academy of Sciences of the United States of America, 104, 10152–10157.
Mun, E.J., et al. (2018) Tumor-treating fields: A fourth modality in cancer treatment. Clin Cancer Research, 24(2), 266–275.
Novocure Ltd. (2018). https://www.novocure.com/our-pipeline/. Accessed 23 Oct 2018.
Priel, A., et al. (2005). Transitions in microtubule C-termini conformations as a possible dendritic signaling phenomenon. European Biophysics Journal, 35, 40–52.
Tuszynski, J. A., et al. (2016). An overview of sub-cellular mechanisms involved in the action of TTFields. International Journal of Environmental Research and Public Health, 13, 1128.
Coque, L., et al. (2011). Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related Behavior in the ClockΔ19 mouse model of mania. Neuropsychopharmacology, 36, 1478–1488.
Giladi, M., et al. (2015). Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Scientific Reports, 5, 18046.
Wenger, C., et al. (2015) Modeling Tumor Treating Fields (TTFields) application in single cells during metaphase and telophase. Conference Proceedings: IEEE Engineering in Medicine and Biology Society. New York: IEEE, (pp. 6892–6895).
Newman, J. R. (1956) The world of mathematics; a small library of the literature of mathematics from A’h-mosé the Scribe to Albert Einstein. New York: Simon & Schuster, (Vol. 4, p. xviii, 2535).
Tuszyński, J. A., et al. (2005). Molecular dynamics simulations of tubulin structure and calculations of electrostatic properties of microtubules. Mathematical and Computer Modelling, 41, 1055–1070.
Gera, N., et al. (2015). Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One, 10, e0125269.
Giladi, M., et al. (2014). Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology, 14, 54–63.
Wong, E. T., et al. (2015). Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. British Journal of Cancer, 113, 232–241.
Howard, J. (2001). Mechanics of motor proteins and the cytoskeleton (p. 367, xvi). Sunderland: Sinauer Associates, Publishers.
Porat, Y., et al. (2017) Determining the optimal inhibitory frequency for cancerous cells using Tumor Treating Fields (TTFields). J Vis Exp, 123, 1–8.
Tuszynski, J. A., et al. (2005). Anisotropic elastic properties of microtubules. The European Physical Journal. E, Soft Matter, 17, 29–35.
Fels, D., et al. (2015). Fields of the cell. Kerala: Research Signpost.
Santelices, I. B., et al. (2017). Response to alternating electric fields of tubulin dimers and microtubule ensembles in electrolytic solutions. Scientific Reports, 7, 9594.
Sozanski, K., et al. (2015). Small crowders slow down kinesin-1 stepping by hindering motor domain diffusion. Physical Review Letters, 115, 218102.
Acknowledgements
Thanks to Cornelia Wenger, Eric Wong, Ken Swanson, Josh Timmons, Jeffrey E. Arle and Rohit Ketkar, and the editors for valuable insights and feedback. Funding was provided by Novocure Ltd.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Carlson, K.W., Tuszynski, J.A., Dokos, S., Paudel, N., Bomzon, Z. (2019). Simulating the Effect of 200 kHz AC Electric Fields on Tumour Cell Structures to Uncover the Mechanism of a Cancer Therapy. In: Makarov, S., Horner, M., Noetscher, G. (eds) Brain and Human Body Modeling. Springer, Cham. https://doi.org/10.1007/978-3-030-21293-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-21293-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21292-6
Online ISBN: 978-3-030-21293-3
eBook Packages: EngineeringEngineering (R0)