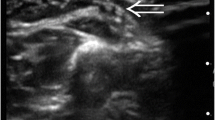
Abstract
Right assessment and selection of the best vein and location is the first stage of the vascular access cycle within quadrant 1 of VHP. This stage begins at the time of admission and continues as diagnosis is established and treatment initiated. Most patients receive their first intravenous device during the assessment in the emergency department, typically a PIVC is inserted in a hurried fashion, and location and method of insertion are often not optimal. Once the patient has stabilized, consideration can be given to the most appropriate vascular access device, one that will provide the administration of the prescribed therapy. Assessments of patient history, comorbidities, contraindications, available veins, diagnosis, and duration of therapy are factors that determine level of risk, the appropriate device, and most qualified inserter. Individuals with ultrasound training can apply their skills for assessment and selection of the right location and vein for device insertion.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Within the first 48 h of admission to an acute care facility, a patient receives a diagnosis, PIVC placement, and initiation of treatment (Santolucito 2001). Since most PIVCs fail within the first 48 h, the optimal window for patient vein assessment and device selection is within this time frame (Hallam et al. 2016; Jackson et al. 2013). A patient-centered approach is focused on the vascular assessment of the patient, recent and past history with access, critical or chronic nature of their illness, comorbidities that may affect the risk of infection or other complications, types of medications to be administered, duration of treatment, future needs, specific access needs, and risk assessment of all factors, followed by vein and device choice (Hallam et al. 2016; Jackson et al. 2013; Moureau 2017).
2 Patient and Vein Assessment
Gaining a patient history and clinical assessment assists in determining device selection. Clinical histories such as past surgery, comorbid conditions, hematological or oncological history, as well as past vascular access-related complications that include difficult venous access or thrombosis are important factors that influence device choice (Sou et al. 2017; Woller et al. 2016). Physical assessment of the patient should include neurological, cardiovascular, respiratory, and gastrointestinal assessment. The assessment of coagulation profile, electrolyte, and full blood count review informs the inserter of areas of potential risk (Lonsway 2010).
Assessment for either peripheral or central access is enhanced with the use of ultrasound (Moureau 2014; Sharp et al. 2015a, b). Determination of the need for central venous access device (CVAD) requires a risk benefit assessment to avoid unnecessary placement of these higher risk devices (Chopra et al. 2015). Risk ratios with CVADs are higher than with peripheral devices both with insertion and post insertion-related complications (Maki et al. 2006; Mcgee and Gould 2003; Sou et al. 2017). The goal with assessment and selection is to choose the lowest risk VAD, which will complete the prescribed treatment plan while minimizing both insertion and post insertion-related complications (Chopra et al. 2013, 2015). The vessel health and preservation model incorporates a planned vascular access assessment that evaluates the patient factors correlated with the treatment plan to select the most suitable device and inserter (Hallam et al. 2016; Hanchett and Poole 2001; Moureau et al. 2012; Rotter et al. 2010).
3 Vessel Assessment
Clinical evaluation of the blood vessels and the pathway determines anatomical placement and ultimately the most appropriate device. Evaluation through observation and palpation of vessels or visualization with ultrasound can facilitate successful insertion and longer dwell times (De La Torre-Montero et al. 2014). Vessels that are tortuous in nature, have bifurcations, or thrombosis make placement of the device difficult and should be avoided (Moureau 2014). Approaches developed by the Italian Group for Venous Access Devices (GAVeCeLT) that include the Rapid Assessment of the Central Veins (RaCeVA) and the Rapid Assessment of the Peripheral Veins (RaPeVA) are protocols that can be used to thoroughly evaluate vessels and surrounding structures (Pittiruti 2012).
Thorough ultrasound assessment for vein selection reduces insertion-related complications (Flood and Bodenham 2013; Pirotte 2008). When urgency of placement is not necessary, time taken with selection of a location for cannula insertion in a stable area, away from joints and movement, results in lower rates of failure (Marsh et al. 2017). Vein selection for PIVC placement must be balanced with consideration for speed versus longer dwell time and reduced complications. Placement of a device in the hand or antecubital fossa is initially easier in most respects due to identification of veins visually and through palpation; however, these devices become dislodged, are uncomfortable for patients, and often fail in less than 72 h (Alexandrou et al. 2018). Vein selection with CVAD is dictated by insertion risk related to site, vein size, depth, and surrounding structures that may impact risk of complications (i.e., nerves, lymphatic tissue, artery) (Moureau 2017).
4 Ultrasound Assessment of the Patient
Ultrasound for vascular access site selection is used to identify and map structures within the arm, chest, neck, and leg that may be most suitable for device insertion and treatment. Ultrasound is used widely for central venous access, PICCs, midlines, and in more recent times for PIVCs too. The patient safety benefits of using ultrasound are undisputed (Bodenham et al. 2016; Gorski et al. 2016; Lamperti et al. 2012; Loveday et al. 2014). Gorski et al., in INS Standards (2016), recommend using visualization technologies like infrared or ultrasound to increase insertion success for patients with difficult access.
With ultrasound imaging and the use of a conductive medium, such as gel, the probe or transducer transmits sound waves that are interpreted on a viewing screen. Scanning for peripheral veins and structures within the arms begins at the level of the forearm working toward the body. Optimal peripheral cannula site selection is one that allows ultrasound-guided needle access in a vein 2–4 mm in diameter or larger and 0.3–1.5 cm in depth (Witting et al. 2010).
It is essential to assess the veins with ultrasound prior to the procedure to select the vein with optimal characteristics including the size, depth, and pathway with minimal risk to arterial/nerve injury, check the vein for patency, ensure thrombosis is not present, and identify any anatomical variations. Ultrasound assessment is undertaken without using a tourniquet to assess the vein in its normal state. Assess the depth, patency, and respiratory collapse; for instance, a critically ill patient may present in a cardiovascular hyperdynamic state with the artery pounding and encroaching the vein, which may itself be collapsing with each respiratory cycle. Application of assessment methods, such as RACEVA and RAPEVA, covered later in this chapter, guides the inserter to select the best vein and identifies any venous abnormalities prior to insertion (Pittiruti and Scoppettuolo 2017).
Anatomy is not exact, and variations exist. For example, the basilic veins are only present in the text book or traditional form of anatomy in 66%, as discovered in a study by Anaya-Ayala et al. (2011), who mapped veins of 290 patients including 426 arms (221 right, 205 left arm). From the mapped veins, the authors identified that the basilic vein joins the axillary vein around the same area that the brachial veins do. In the remaining 34% of patients, either the basilic joins the paired brachial veins in the mid to lower arm or the basilic joins an unpaired brachial in the mid to lower section of the arm (Anaya-Ayala et al. 2011).
During scanning and vein selection, identify arteries and nerves based on anatomical knowledge for the area (e.g., median nerve for PICC, carotid artery for jugular CVAD, pleura for subclavian CVAD). As scanning of the patient continues, look for normal and abnormal features of the vessels: shape, size, path, patency, and flow (Moureau and King 2007).
-
Shape: Observe for irregularities in lumen size and vessel wall thickness. These types of abnormalities are usually best visualized in the sagittal plane.
-
Size: Measure basilic, brachial, and cephalic vein diameter in their native state without a tourniquet. Vein size determines suitability of desired catheter size and number of lumens (i.e., caliber of peripheral vein must at least equal diameter of midline or PICC in French size). Diameter may be measured in the transverse or sagittal plane. The occupation of more than one third of the diameter of a blood vessel with a catheter reduces blood flow within the region and increase thrombosis risk. Generally, the scale in Fig. 2.1 provides a guide for determining the most appropriate catheter for vein size (Sharp et al. 2015a, b, 2016).
-
Path: Note any aberrancy along the course of the vessel (tortuosity), areas of dilation, or stenosis. Also observe for symmetry of the vessel wall looking for irregularities rather than the normal round vessel shape. Uniformity of the path and vein wall is viewed specifically in sagittal, longitudinal view providing a more detailed view of vein path or vein wall abnormalities.
-
Patency: Compress veins. Look for echogenic material within non-compressible veins that may indicate thrombosis or other structures such as nerves or arteries (use pulse wave Doppler if indicated). Look for collaterals around areas of non-compressible or retracted veins.
-
Flow: Observe arterial and venous flow. Flow in the arterial system pulsatile and rhythmical if the patient has normal heart function. Flow in the venous system is typically slower without pulsatility. It is important to note that the “red” and “blue” colors typically used do not demonstrate arterial or venous flow but rather movement toward and away from the probe.
-
Pre-insertion Ultrasound Assessment of the Central and Peripheral Veins (RACEVA & RAPEVA (Emoli et al. 2014; Pittiruti 2012; Pittiruti and Scoppettuolo 2017)): Pre-insertion scanning of the major blood vessels prior to catheter insertion and mapping pathway of the catheter improves success rates and minimize complications. The RACEVA protocol is a six-step assessment of the major central vessels and important associated structures.
5 Rapid Vein Assessment RAPEVA and RACEVA Protocols (Pittiruti and Scoppettuolo 2017)
Rapid assessment peripheral vein assessment (RAPEVA) and rapid assessment central vein assessment (RACEVA) are performed with ultrasound to determine the most appropriate location for catheter insertion. Scanning of the peripheral vasculature includes visualization by starting at the antecubital region of the arm, moving up medially, and toward the chest. More information on ultrasound scanning and the RAPEVA methods are found in the references (Pittiruti 2012).
5.1 RAPEVA Position 1
Position 1—This position identifies vessels of the antecubital fossa, sometimes visible without ultrasound. Probe position should begin at the lateral side of the arm at the cubital crease in transverse position. Assess the smaller cephalic vein for compressibility and thrombosis (Fig. 2.2).
5.2 RAPEVA Position 2
Position 2—Moving from lateral to medial along the antecubital fossa, visualize larger veins with position variation from person to person. Probe position should move to the medial side of the arm at cubital crease in transverse position. Assess the basilic vein in relation to the median cubital vein as well as brachial artery and median and ulnar nerve. Assess basilic vein for compressibility, thrombosis, diameter, and distance from the skin (Fig. 2.3).
5.3 RAPEVA Position 3
Position 3—This position follows the basilic vein with the probe position on the medial side of arm in the bicipital humeral groove. Assess the basilic vein in relation to the ulnar nerve and brachial bundle with the brachial veins, artery, and median nerve. Assess basilic vein along the bicep groove for compressibility, thrombosis, diameter, and distance from the skin (Figs. 2.4, 2.5. This mid-upper arm position is a common location for PICC insertion stabilized by the surrounding bicep, brachialis, and coracobrachialis muscle group.
5.4 RAPEVA Position 4
Position 4—Probe position should be mid arm over bicep region. At this position the basilic vein is likely joined with the brachial veins but may vary in the exact location from person to person. Assess the brachial vein in relation to the brachial artery and median nerve. Brachial veins are paired veins, known as the venae comitantes situated on either side of the brachial artery with pulsations of the artery aiding in venous return. This bundle representing the brachial veins and artery includes the median nerve, one of the largest in the upper extremity. Assess brachial vein(s) for compressibility, thrombosis, diameter, distance from the skin, and optimal position to facilitate needle access while avoiding the artery and nerve (Fig. 2.6).
5.5 RAPEVA Position 5
Position 5—This position assesses the upper arm portion of the cephalic vein which can be difficult to locate. Probe position should be lateral side of the arm below the acromion in transverse mode. Assess the cephalic vein for compressibility and thrombosis (Fig. 2.7).
5.6 RAPEVA Position 6
Position 6—Following the cephalic vein from positions 5 to 6, identifies the intersection with the axillary vein. Probe position should be perpendicular and move to the pectoral groove in transverse mode, below the clavicle (lateral third of clavicle—as with Position 5 of RACEVA in the section that follows). This position assesses the axillary vein (AV) in short axis, axillary artery (AA) in short axis, and cephalic vein (CV) in long axis (Fig. 2.8).
5.7 RAPEVA Position 7
Probe position is behind the clavicle (supraclavicular). This position assesses the subclavian vein (SV), external jugular vein (EJ) in long axis, and laterally the subclavian artery in short axis. Probe position moves to the lower neck in transverse plane to assess lower track of the IJV, subclavian artery in long axis as well as visualization of the distal IJV valve (Fig. 2.9).
6 RACEVA Rapid Central Vein Assessment
The RACEVA protocol uses ultrasound for thorough assessment of vascular structures in terms of size, patency and overal vialbility for catheterization. It also provides information on overall anatomy of the area do reduce inadvertant puncture of surrounding anatomic structures (Spencer and Pittiruti 2018). More information on ultrasound scanning and the RACEVA and RAPEVA methods are found in the references (Pittiruti 2012).
6.1 RACEVA Position 1
Position 1—Starting at the mid neck examining the internal jugular vessels and the carotid artery using a transverse view of the vessels. This position assesses the internal jugular vein (IJV) and carotid artery. Assess for compressibility, size, and shape (Figs. 2.10 and 2.11). Where possible and safe to do so for your patient, pressure should be applied to the veins to assess for compressibility and to check for patency and the presence of a thrombosis. The development of a thrombosis is a process, and in the early stages, the thrombus is still compressible (though the vessel is not likely to fully compress). At this stage it may also appear black and, like a normal vessel, only at the later stages does it start to take on a more solid form and then becomes more echogenic and the vein non-compressible. Any suspicion of a thrombus should be referred to the radiology and or vascular team for further investigation including Doppler ultrasound assessment.
Position 2—Still in transverse position, transducer slides down to base of the neck, this allows view of the internal jugular and and carotid artery. The probe in transverse position at the base of the neck (supraclavicular) facilitates view of the lower track of the IJV, subclavian artery in long axis as well as the distal IJV valve. (Figs. 2.12 and 2.13).
6.2 RACEVA Position 3
Position 3—Transverse view, having followed the pathway of the internal jugular down to the base of the neck now place the probe level with the sternal notch on the superior edge of the clavicle. Probe position should be above the clavicle to the side of the sternal notch in transverse plane. This position assesses the brachiocephalic vein. In order to view the brachiocephalic, angle the probe inferiorly toward the heart, and slight pressure may help achieve optimal visualization of the vessel. This is an ideal position for an “in-plane” puncture of the brachiocephalic vein (Figs. 2.14 and 2.15).
6.3 RACEVA Position 4
Position 4—Probe position should be behind the clavicle (supraclavicular). This position assesses the subclavian vein (SV), external jugular vein (EJ) in long axis, and laterally the subclavian artery in short axis (Figs. 2.16 and 2.17). Sliding the probe slightly away from the sternal notch along the superior border of the clavicle and holding the probe in a similar angle used when examining the brachiocephalic allows view of the subclavian vein and artery, using both transverse and longitudinal views.
6.4 RACEVA Position 5
Position 5—Probe position should be perpendicular to the pectoral groove in short access, below the clavicle (lateral third of the clavicle). This position assesses the axillary vein (AV) in short axis, axillary artery (AA) in short axis, and cephalic vein (CV) in long axis. Ideal position for “out of plane”: puncture of axillary vein (Figs. 2.18 and 2.19). Moving to the inferior border of the clavicle, visualize the axillary vein and artery in transverse view. A patient with increased respiratory demand may present with a vein that is opening and collapsing with the changes in intrathoracic pressure during the respiratory cycle. A patient with poor hydration status may also present with a collapsing vein as the venous pressure is low.
6.5 RACEVA Position 6
Position 6—Again visualizing the axillary vein and artery in the deltopectoral or a subclavicular fossa area, view the vessels in a longitudinal perspective. Probe position should be perpendicular to the pectoral groove in long access, below the clavicle (lateral third of the clavicle).
This position assesses the AV in long axis, AA in long axis, and CV vein in long axis. Ideal position for “in-plane”: puncture of the axillary vein (Figs. 2.20 and 2.21).
6.6 RACEVA Position 7
Position 7—Using the second intercostal space, the ultrasound is used to view the pleura and the lung tissue. The aim of identifying these structures is to identify the presence of a pneumothorax with the absence of sliding lung sign outlined in the next chapter with insertion. Sliding lung assessment should be performed pre- and post-CICC insertion, and although this will be covered in a separate section, it is important to note that ultrasound can be used as a reliable technique to assess lung movement and the presence or absence of a pneumothorax (Husain et al. 2012). As noted in the publication, there are several classic sonographic signs that include the “sliding lung” and “comet tail artifacts” that assess the normal function and integrity of the visceral and parietal pleura. Identify the horizontal, equally spaced hyperechogenic reflections of the pleura, and the conical-shaped shadows descending down the pleura deeper into the tissues known as comet tails indicate normal lung function for that lobe (Fig. 2.22).
6.7 RACEVA Position 8
More information on ultrasound scanning and the RACEVA and RAPEVA methods are found in the references (Pittiruti 2012; Pittiruti and Scoppettuolo 2017; Spencer and Pittiruti 2018).
Case Study
A 45-year-old male entered the emergency room with elevated temperature, severe fatigue, weakness, shortness of breath, sleeping problems, some nausea, poor appetite, frequent small quantity urination, dry skin and poor turgor, and swelling to the legs and ankles with some muscle cramping. Laboratory blood work confirms a high creatinine level and a glomerular filtration rate (GFR) of 35 indicating moderate renal dysfunction. The issues noted with this patient include the need for dialysis, cautious fluid replacement for dehydration, and further testing to rule out sepsis. The emergency physician is trained in the RACEVA protocol and evaluates vein options, and configuration knowing the best choice for this patient is an internal jugular placement for the dialysis catheter. After numerous failed attempts at peripheral access, consideration was given to placement of a second axillary central catheter for fluids and antibiotic infusions. Prior to catheter placement, it was discovered that a tri-lumen dialysis catheter was available that included an extra lumen for the intravenous medications. The tri-lumen catheter was placed successfully in the right internal jugular vein under ultrasound guidance, the first infusion of antibiotics given, baseline central venous pressure and oxygen levels obtained, and the patient sent to dialysis for first treatment.
Summary of Key Points
-
1.
The Vessel Health and Preservation process is designed to select the vein, location, and device that has lowest risk, preserves veins, and is most reliable for the treatment.
-
2.
CVAD selection is optimal when individualized for the patient based on treatment plan, medication types, duration, and patient-specific factors.
-
3.
Patients are the focus of VHP with individualized assessment, selection, and placement that reduces unnecessary trauma and VAD-related complications.
-
4.
The use of visualization technology aids in assessment, vein selection, and insertion.
References
Anaya-Ayala JE, Younes HK, Kaiser CL, Syed O, Ismail N, Naoum JJ, et al. Prevalence of variant brachial-basilic vein anatomy and implications for vascular access planning. J Vasc Surg. 2011;53(3):720–4.
Alexandrou E, Ray-Barruel G, Carr PJ, Frost S, Inwood S, Higgins N, et al. Use of short peripheral intravenous catheters: characteristics, management, and outcomes worldwide. J Hosp Med. 2018;13 https://doi.org/10.12788/jhm.3039.
Bodenham A, Babu S, Bennett J, Binks R, Fee P, Fox B, Johnston A, Klein A, Langton J, Mclure H. Association of Anaesthetists of Great Britain and Ireland. Safe vascular access 2016. Anaesthesia. 2016;71:573–85.
Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34:908–18.
Chopra V, Flanders SA, Saint S, Woller SC, O’Grady NP, Safdar N, Trerotola SO, Saran R, Moureau N, Wiseman S, Pittiruti M, Akl EA, Lee AY, Courey A, Swaminathan L, Ledonne J, Becker C, Krein SL, Bernstein SJ, Michigan Appropriateness Guide for Intravenous Catheters Panel. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163:S1–40.
De La Torre-Montero J-C, Montealegre-Sanz M, Faraldo-Cabana A, Oliva-Pellicer B, Garcia-Real I, Fenwick M, Marcos CE, Rivas-Eguia B, Vila-Borrajo C, Valles-Andres J. Venous International Assessment, VIA scale, validated classification procedure for the peripheral venous system. J Vasc Access. 2014;15:45–50.
Emoli A, Cappuccio S, Marche B, Musaro A, Scoppettuolo G, Pittiruti M. [The ISP (Safe Insertion of PICCs) protocol: a bundle of 8 recommendations to minimize the complications related to the peripherally inserted central venous catheters (PICC)]. Assist Inferm Ric. 2014;33:82–9.
Flood S, Bodenham A. Central venous cannulation: ultrasound techniques. Anaesth Intensive Care. 2013;14:1–4.
Gorski L, Hadaway L, Hagle M, Mcgoldrick M, Orr M, Doellman D. Infusion therapy: standards of practice. J Infus Nurs. 2016;39(Suppl 1):S1–S159.
Hallam C, Weston V, Denton A, Hill S, Bodenham A, Dunn H, Jackson T. Development of the UK Vessel Health and Preservation (VHP) framework: a multi-organisational collaborative. J Infect Prev. 2016;17:65–72.
Hanchett M, Poole S. Infusion pathways: planning for success. J Vasc Access Devices. 2001;6:29–37.
Husain LF, Hagopian L, Wayman D, Baker WE, Carmody KA. Sonographic diagnosis of pneumothorax. J Emerg Trauma Shock. 2012;5:76.
Jackson T, Hallam C, Corner T, Hill S. Right line, right patient, right time: every choice matters. Br J Nurs. 2013;22(8):S24, S26–8.
Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, Pirotte T, Karakitsos D, Ledonne J, Doniger S. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38:1105–17.
Lonsway RA. Patient assessment as related to fluid and electrolyte balance. In: Alexander M, Corrigan A, Gorski L, Hankins J, Perucca R, editors. Infusion nursing: an evidenced based approach. 3rd ed. St Louis: Saunders Elsevier; 2010.
Loveday H, Wilson J, Pratt R, Golsorkhi M, Tingle A, Bak A, Browne J, Prieto J, Wilcox M. EPIC3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86:S1–70.
Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–71.
Marsh N, Webster J, Larson E, Cooke M, Mihala G, Rickard C. Observational study of peripheral intravenous catheter outcomes in adult hospitalized patients: a multivariable analysis of peripheral intravenous catheter failure. J Hosp Med. 2017;13(2), 83-89.
Mcgee D, Gould M. Preventing complications of central venous catheterization. New Engl J Med. 2003;348:1123–33.
Moureau NL. Ultrasound anatomy of peripheral veins and ultrasound-guided venipuncture. In: Peripherally inserted central venous catheters. Milan: Springer; 2014.
Moureau NL. Vessel health and preservation: vascular access assessment, selection, insertion, management, evaluation and clinical education. Doctor of Philosophy by Publication (PhD) Thesis and Exegesis (PhD Doctorate), Griffith University; 2017.
Moureau N, King K. Advanced ultrasound assessment for venous access clinical education program and DVD. 2007.
Moureau N, Trick N, Nifong T, Perry C, Kelley C, Carrico R, Leavitt M, Gordon S, Wallace J, Harvill M, Biggar C, Doll M, Papke L, Benton L, Phelan D. Vessel health and preservation (Part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13:351–6.
Pirotte T. Ultrasound-guided vascular access in adults and children: beyond the internal jugular vein puncture. Acta Anaesthesiol Belg. 2008;59:157–66.
Pittiruti M. RaCeVA: a guide for a rational choice of the most appropriate vein for central venous catheterization. Association for Vascular Access 26th Annual Scientific Meeting. San Antonio; 2012.
Pittiruti M, Scoppettuolo G. GAVeCeLT manual of PICC and midline—indications, insertion, management. Edra S.P.A.; 2017.
Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J, Snow P, Kugler J. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;CD006632.
Santolucito J. A retrospective evaluation of the timeliness of physician-initiated PICC referrals: a continuous quality assurance/performance improvement study. J Vasc Access Devices. 2001;6:20–6.
Sharp R, Cummings M, Childs J, Fielder A, Mikocka-Walus A, Grech C, Esterman A. Measurement of vein diameter for peripherally inserted central catheter (PICC) insertion: an observational study. J Infus Nurs. 2015a;38:351–7.
Sharp R, Cummings M, Fielder A, Mikocka-Walus A, Grech C, Esterman A. The catheter to vein ratio and rates of symptomatic venous thromboembolism in patients with a peripherally inserted central catheter (PICC): a prospective cohort study. Int J Nurs Stud. 2015b;52:677–85.
Sharp R, Grech C, Fielder A, Mikocka-Walus A, Esterman A. Vein diameter for peripherally inserted catheter insertion: a scoping review. J Assoc Vasc Access. 2016;21:166–75.
Sou V, Mcmanus C, Mifflin N, Frost SA, Ale J, Alexandrou E. A clinical pathway for the management of difficult venous access. BMC Nurs. 2017;16:64.
Spencer TR, Pittiruti M. Rapid Central Vein Assessment (RaCeVA): a systematic, standardized approach for ultrasound assessment before central venous catheterization. J Vasc Access. 2018;1129729818804718.
Witting MD, Schenkel SM, Lawner BJ, Euerle BD. Effects of vein width and depth on ultrasound-guided peripheral intravenous success rates. J Emerg Med. 2010;39:70–5.
Woller SC, Stevens SM, Evans RS. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative: a summary and review of peripherally inserted central catheter and venous catheter appropriate use. J Hosp Med. 2016;11:306–10.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Alexandrou, E. (2019). Right Assessment and Vein Selection. In: Moureau, N. (eds) Vessel Health and Preservation: The Right Approach for Vascular Access. Springer, Cham. https://doi.org/10.1007/978-3-030-03149-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-03149-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03148-0
Online ISBN: 978-3-030-03149-7
eBook Packages: MedicineMedicine (R0)