Abstract
The Gulf of Mexico has a dynamic ichthyofaunal community of more than 1443 finfish species, over 51 shark species, and at least 49 species of rays and skates. The Gulf of Mexico ecosystem has a relatively high biodiversity and a large number of fish and shark species compared to the Pacific and Atlantic coastal areas of the United States. Many fish stocks of great commercial and recreational importance in the Gulf of Mexico have been determined to be overfished (population levels too low) and/or in the status of overfishing. A wide variety of long-term anthropogenic and natural stressors, such as rapid coastal development, overfishing, shrimp fishery bycatch, climate change, hypoxia, and natural disasters, have negatively affected the Gulf of Mexico finfish and sharks. Of the 13 species that were selected as representative for evaluation, five species were being overfished and/or were in the status of overfishing before 2010. These five species include red snapper, red grouper (some local subpopulations), Atlantic bluefin tuna (most likely but the uncertainty is high), Atlantic blue marlin, and greater amberjack. In addition, many shark species were overfished or were in the status of overfishing immediately before or around April 2010. Management regulations adopted for many fisheries in the 2000s to limit fishing efforts, and shrimp fishery bycatch appear to have been successful for some finfish and shark species, which has reduced the number of overfished fish populations and the frequency of overfishing in the Gulf of Mexico.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
The Gulf of Mexico, surrounded on three sides by continental landmass, is the nineth largest waterbody in the world; it is semi-enclosed with its east connecting to the Atlantic Ocean through the Straits of Florida and its south to the Caribbean Sea through the Yucatán Channel. The Gulf of Mexico basin resembles a large crater with a wide shallow rim. Approximately 38 % of Gulf waters are shallow, intertidal areas. The continental shelf (<200 meters (m) or <656 feet [ft]) and continental slope (200–3,000 m or 656–9,843 ft) represent 22 and 20 % of the Gulf of Mexico basin, respectively, and abyssal regions deeper than 3,000 m (9,843 ft) comprise the remaining 58 % (USEPA 1994). The Sigsbee Deep in the southwestern Gulf of Mexico is the deepest region at 4,384 m (14,383 ft). The average water depth of the Gulf is about 1,615 m (5,299 ft). The boundary of the Gulf of Mexico used in this evaluation follows that defined in McEachran and Fechhelm (2005), which does not exclude the Florida Keys and the northeastern coast of Cuba.
The ichthyofaunal community in the Gulf of Mexico is dynamic and varies greatly, both spatially and temporally, because of fish movement/migration, diversified life-history strategies, fishing pressure, and varying hydrographic, oceanographic, and geographic conditions. It consists of a large number of reef-dependent, demersal species (e.g., snappers and groupers); coastal demersal species (e.g., drums and mullets); demersal species (e.g., tilefishes and porgies); coastal pelagic species (e.g., herrings and jacks); highly migratory, pelagic species (e.g., tunas and billfishes); small and large coastal sharks; and pelagic sharks (McEachran and Fechhelm 2005; Parsons 2006). Because of its unique oceanographic and hydrographic conditions, geological location, and availability of a great diversity of habitats, the Gulf of Mexico ecosystem has a relatively high biodiversity, with a large number of fish and shark species compared to other areas in the United States (Chesney et al. 2000).
Finfish and sharks, both as prey and predators, play significant roles in regulating the dynamics of the Gulf of Mexico ecosystem and the energy flows between organisms of different trophic levels (Hoese and Moore 1998; McEachran and Fechhelm 2005; Parsons 2006). Small coastal pelagic forage fishes, such as herrings and anchovies, filter feed on plankton and play a critical role in transferring primary productivity into fish biomass that is useable for other fish species of higher trophic levels or that directly supports commercial and recreational fisheries (McEachran and Fechhelm 2005; Anderson and McDonald 2007). These fish species form the forage base in the Gulf of Mexico ecosystem. Fish species of higher trophic levels usually prey on forage species, juvenile fish, and other organisms, such as squids, crabs, and shrimps. Many finfish and sharks are apex predators in the Gulf of Mexico ecosystem and are important in regulating the dynamics of their prey species (Hoese and Moore 1998; McEachran and Fechhelm 2005; Parsons 2006). Large oceanic pelagic species, such as tunas, billfishes, and sharks, tend to have few predators and prey on many finfish and invertebrate species. Substantial changes in the dynamics of populations and communities of key forage and apex predator species can have significant cascading effects on Gulf of Mexico ecological processes (Anderson and McDonald 2007).
In the Gulf of Mexico, finfish and sharks support important commercial and recreational fisheries, two of the most important industries in the region, as well as one of the most productive fisheries in the world (Chesney et al. 2000). Overall, approximately 25 % of the U.S. commercial fish landings and 40 % of the recreational harvest occur in the Gulf of Mexico. Commercial landings of finfish and shellfish in the Gulf of Mexico totaled over 590 million kilograms (kg) [1.3 billion pounds (lb)], valued at $661 million in 2008, and 8 of the top 20 fishing ports by value and 4 of the top 7 fishing ports by weight in the United States are located in the Gulf of Mexico (NMFS 2009a). More than 24.1 million recreational fishery trips were made in 2008 in the Gulf of Mexico, resulting in a catch of 190 million fish (NMFS 2009a). Therefore, the economic and social values of fisheries in the Gulf of Mexico are huge and should not be underestimated.
The Gulf of Mexico provides a wide range of habitats for its ichthyofaunal community, but long-term anthropogenic and natural stressors and perturbations, such as rapid coastal development, pollution, overfishing, and natural disasters, have altered the Gulf of Mexico ecosystem and the dynamics of its fish community and populations (O’Connell et al. 2004). However, it is difficult to quantitatively assess and separate the impacts of human and natural perturbations on the resilience of the Gulf of Mexico ecosystem because of the limitations of available data.
The Gulf of Mexico receives about 50 % of all watershed discharge in the United States, and more than 3,100 point-source outfalls discharge into the Gulf of Mexico. Pesticides and nutrients used in the watersheds of the U.S. states bordering the Gulf exceed those used in any of the other coastal zones in the United States. The entire U.S. Gulf of Mexico coastline has been under fish consumption advisory for mercury since 1994 (USEPA 1994). Fifty-nine percent of the estuarine areas of the U.S. Gulf of Mexico, which are essential nursery and spawning grounds for many finfish and sharks, assessed from 1997 through 2000, were considered impaired or threatened (USEPA 2004). Coastal wetlands and nearshore seagrass beds are critical nursery and spawning grounds for many finfish and sharks; however, Lewis et al. (2007) estimated that 78 square kilometers (km2) (30 miles2) of wetlands were being lost annually and that 20–100 % of the seagrasses have been destroyed in some areas of the Gulf of Mexico. The deterioration and even total loss of these critical habitats may greatly reduce the carrying capacity of the Gulf of Mexico for many fish and shark species that depend on these areas as their critical habitat. Overfishing and shrimp fishery bycatch have substantially reduced the population abundance of many fish and shark species of commercial and recreational importance, resulting in some important species being classified as in the status of overfishing and/or being overfished (SEDAR 31 2009; NMFS 2012a).
The objective of this chapter is to provide an overview, synthesis, and evaluation of the life histories, population and community structures, and population dynamics, distribution, and abundance of fish representative of the species and habitat diversity in the Gulf prior to the Deepwater Horizon event. The primary focus is on information believed critical to the overall understanding of the spatiotemporal dynamics and habitat needs of key finfish, shark, and ray species and the major anthropogenic and environmental drivers that influence their conditions in the Gulf of Mexico.
Hoese and Moore (1998) and McEachran and Fechhelm (2005) documented 1,443 finfish species in 223 families in the Gulf of Mexico. A representative subset of 100 key families of finfish were evaluated for their distribution and habitat needs in the Gulf of Mexico (Table 9.1). Finfish families with high to medium importance to commercial and recreational fisheries in the Gulf of Mexico were identified (Table 9.2). Ten finfish families were selected for evaluation based on information in Tables 9.1 and 9.2 and the following criteria: (1) relative importance to the ecosystem of the Gulf of Mexico; (2) importance to commercial and/or recreational fisheries; (3) abundance (high and low population sizes) and range of fish distributions (e.g., coastal waters and estuaries versus open ocean) in the Gulf of Mexico; (4) diversity of life histories (e.g., long-lived versus short-lived, slow growing versus fast growing, and early mature versus late mature); (5) movements (e.g., sedentary/inactive versus highly migratory); and (6) habitat needs (e.g., low salinity versus high salinity, low temperature versus high temperature, habitat generalist versus habitat specialist). The ten finfish families selected included Lutjanidae (snappers), Clupeidae (herrings), Serranidae (seabasses), Scombridae (mackerels and tunas), Xiphiidae (billfishes), Sciaenidae (drums), Malacanthidae (tilefishes), Coryphaenidae (dolphinfishes), Mugilidae (mullets), and Carangidae (jacks) (Table 9.2). Based on their distribution, habitat needs, and commercial and recreational importance, 13 representative species of finfish were selected from the ten families for detailed evaluation in this chapter (Table 9.3). Species selected include red snapper (Lutjanus campechanus); menhaden, including Gulf menhaden (Brevoortia patronus), finescale menhaden (Brevoortia gunteri), and yellowfin menhaden (Brevoortia smithi); red grouper (Epinephelus morio); Atlantic bluefin tuna (Thunnus thynnus); Atlantic blue marlin (Makaira nigricans); Atlantic swordfish (Xiphias gladius); Atlantic sailfish (Istiophorus albicans); red drum (Sciaenops ocellatus); tilefish (Lopholatilus chamaeleonticeps); king mackerel (Scomberomorus cavalla); dolphinfish (Coryphaena hippurus); striped mullet (Mugil cephalus); and greater amberjack (Seriola dumerili). These are representative species that are demersal and reef-dependent (red snapper and red grouper); offshore demersal (tilefish); coastal demersal (red drum and striped mullet); highly migratory and pelagic (Atlantic bluefin tuna, Atlantic blue marlin, Atlantic swordfish, and Atlantic sailfish); offshore pelagic (dolphinfish); and coastal pelagic (menhaden, king mackerel, and greater amberjack). Although many finfish species of great ecological, commercial, and recreational importance, such as many species in the families of snappers, seabasses, tunas, and jacks, were not selected (Table 9.3), they are well represented by the above 13 species with respect to spatiotemporal distributions, life histories, fisheries, and habitat needs.
The status and management of the four groups of shark species in the Gulf of Mexico, Small Coastal Sharks, Large Coastal Sharks, Pelagic Sharks, and Sharks Prohibited from Fisheries, were also evaluated. All of the four species in the Small Coastal Sharks group (Atlantic sharpnose shark, blacknose shark, bonnethead shark, and finetooth shark) were evaluated. Two of the 11 species in the Large Coastal Sharks group (sandbar shark and blacktip shark) were selected for evaluation because they are two of the most abundant and most commercially and recreationally important shark species, and they are widely distributed in the Gulf of Mexico. Rays and skates were also evaluated with three species (giant manta ray, cownose ray, and smalltooth sawfish) being selected because of their abundance and distribution.
Stock assessments to estimate stock abundance and determine stock status are only conducted for a very small number of marine organisms in the Gulf of Mexico (e.g., overfished and/or overfishing). A recent study indicates that of about 60 fish stocks managed in the Gulf of Mexico, information to determine their status is only available for fewer than half (Karnauskas et al. 2013). No formal stock assessments had been done for the vast majority of fish species in the Gulf of Mexico prior to the Deepwater Horizon event, and currently there is limited knowledge about the status of most fish species that live in and/or use the Gulf in part of their lifecycle.
9.2 Overview of the Gulf of Mexico Ecosystem for Finfish
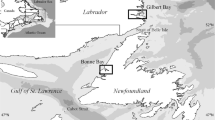
The Gulf of Mexico provides a wide variety of habitats for finfish and sharks (McEachran and Fechhelm 2005), ranging from coastal marsh, seagrasses, mangroves, river mouths, and reefs to man-made structures such as oil and gas platforms, continental shelf, slope, and deepwaters (Figure 9.1). There is large spatiotemporal variability in oceanographic conditions, with the Gulf of Mexico influenced greatly by inflows and discharges from rivers and other land-based sources, including the Mississippi River, and by large-scale oceanographic features, such as the Loop Current and associated core eddies of different thermal conditions (Govoni and Grimes 1992; Sturges and Leben 2000). Combined, these factors result in large spatiotemporal variability in physicochemical conditions, causing primary production to vary markedly within and across areas of different oceanographic conditions in the Gulf of Mexico (Grimes and Finucane 1991; Biggs 1992). Physical–chemical variability affects the distribution, growth, and mortality of pelagic larvae of many fish species in the Gulf of Mexico (Govoni et al. 1989; DeVries et al. 1990; Lang et al. 1994). Higher abundance, increased growth, and reduced mortality have been observed for larvae within frontal features created by riverine discharge and hydrodynamic convergence (Lang et al. 1994; Hoffmayer et al. 2007). The great spatiotemporal variability in oceanographic and physicochemical conditions provides a large diversity of habitat for fish species that often require different habitats in their different stages of life history.
Coral reefs, such as this one on the Flower Garden Banks, are one of a wide variety of habitats available to finfish and sharks in the Gulf of Mexico (photograph by Emma Hickerson, Flower Garden Banks National Marine Sanctuary) (from NMS 2013)
The general movement patterns and key habitat requirements for fish of different life-history stages are responsible for the formulation of fish community structure (O’Connell et al. 2004). Environmental variables, such as temperature, primary production, current, salinity, depth, dissolved oxygen, water clarity, substrate, and geographic area, have been found important in regulating the spatiotemporal dynamics of fish communities (McEachran and Fechhelm 2005). Because of the large spatiotemporal variability in these environmental variables, fish community structure varies temporally among seasons and years and spatially over estuarine categories and geographic areas, such as in areas east and west of the Mississippi River (Hoese and Moore 1998; McEachran and Fechhelm 2005).
For a given fish population, the dynamics of distribution, abundance, and life-history processes are greatly influenced by abiotic factors, such as water temperature, salinity, dissolved oxygen, and substrate, as well as a variety of biotic factors, such as food availability, intra- and interspecific competition, and predator abundance (Briggs 1974; Richards et al. 1989; Ahrenholz 1991). The most profound impacts of these factors on the dynamics of a fish population usually occur during their early life-history stages, when their survival rates are most sensitive to the change in biotic and abiotic environments (Gallaway et al. 2009). For marine fish species that tend to have planktonic early life-history stages (eggs and larvae), their survival rates during the planktonic life stage are usually a function of parental abundance and fecundity and their complex interactions with predation, oceanographic processes, and prey abundance (Richards and Lindeman 1987). Parental abundance can be greatly affected by the level of fishing mortality. The process of fish growing from early life-history stages to catchable sizes, or becoming catchable in commercial and recreational fisheries, is often referred to as recruitment, which consists of largely distinct ecological processes including survival of a cohort of planktonic eggs and larvae, spatiotemporal patterns of demersal settlement of free-swimming juveniles, and natural and fishing mortality of adults and juveniles before they reach the catchable sizes defined by fishing gear selectivity or minimum legal size requirements (Gallaway et al. 2009). Spatiotemporal variations in recruitment, which can be affected by environmental variables and commercial and recreational fisheries, contribute to variability in fish populations and community structure in the Gulf of Mexico ecosystem (O’Connell et al. 2004; McEachran and Fechhelm 2005).
9.2.1 Key Environmental Variables Influencing Spatiotemporal Dynamics of Fish Populations
Several natural environmental gradients result in the diversity of habitats, which contributes to the relatively high species richness in the Gulf of Mexico and spatiotemporal distribution of finfish species (McEachran and Fechhelm 2005). The first gradient is salinity, which tends to increase from west to east along the coastline as a result of spatial variability in rainfall, river output, and temperature. Bottom composition is the second gradient (McEachran and Fechhelm 2005). Large amounts of fine-grained sediments exist in the Gulf of Mexico along the East Texas and Louisiana coasts as a result of large riverine inputs. Bottom sediments become coarse-grained and sandy off the arid South Texas coast and less sandy and muddier away from the barrier islands. Rocky reefs appear on the 40-fathom contour off Texas and on the continental shelf off Louisiana, providing a hard bottom substrate habitat suitable for species of tropical reef fish not typically found in the inshore shallow waters. From east of the Mississippi Delta, the shelf tends to have coarse-grained sandy sediment with large areas of hard bottom and accumulations of shells, which differs greatly from that of most of the western Gulf of Mexico (McEachran and Fechhelm 2005). The Florida West coast mainly has limestone and detrital-derived sediments, which provide suitable habitat for the spread of many coral reef fishes northward. The spatial variation in sediments contributes greatly to the diversity of habitat for fish requiring specific bottom substrates in different stages of their life history in the Gulf of Mexico (McEachran and Fechhelm 2005).
A third gradient is the spatial variability in depth from the shore to the edge of the continental shelf, resulting in large spatiotemporal variability in the temperature regime, which provides habitat diversity for different fishes (McEachran and Fechhelm 2005). The Gulf of Mexico has greater seasonal changes in thermal habitat than regions to the south or east (Backus et al. 1997); these changes provide a diversity of habitat niches for fish species with differing thermal habitat requirements.
Currents play a central role in regulating the sources of fish recruitment, as well as the transportation and distribution of fish larvae, which can have great impacts on the dynamics of fish populations (Richards et al. 1989; DeVries et al. 2006). Many fish species spawning in the Gulf of Mexico depend on seasonal and often wind-driven currents to transport their larvae into estuarine nursery areas. The Loop Current, which enters the Gulf of Mexico from the Yucatán Channel and begins the Gulf Stream, dominates the Gulf of Mexico oceanographic features. It contains a rich variety of larval tropical fishes that grow and settle on the reefs of the eastern Gulf of Mexico, while eddies transport additional species into the western Gulf of Mexico (McEachran and Fechhelm 2005). The Loop Current may further act as an important geographic isolating mechanism that separates inshore fish populations of the eastern and western Gulf of Mexico (Govoni and Grimes 1992; Sturges and Leben 2000). This may result in the degree of endemism found in the western Gulf of Mexico (Shipp 1992). Thus, the spatial structure of Gulf of Mexico fishes is influenced greatly by the Loop Current and its associated anticyclonic rings (Kleisner et al. 2010).
Approximately 4,000 oil and gas platforms exist in the northern Gulf of Mexico, acting as one of the most extensive man-made reef structures in the world. Many of these petroleum platforms have existed for more than 40 years and have greatly affected spatiotemporal distributions of pelagic fish species (Franks 2000). These platforms vary greatly in size and structural complexity from small, single-well platforms to large, multi-well platforms with complex structures that are installed in both inshore shallow waters and in waters more than 250 km (155 miles) offshore and deeper than 2,000 m (6,562 ft) (Cranswick and Regg 1997; Franks 2000). These platforms form additional new habitat in the northern Gulf of Mexico that attracts pelagic and mid-water fish species to form a distinctive ichthyofaunal community different from the faunal assemblage in the surrounding natural habitat (Gallaway and Lewbel 1982; Franks 2000).
Extreme conditions of environmental variables, such as temperature, salinity, and dissolved oxygen, as well as the existence of natural and human-induced toxic substances, can result in significant temporary or even permanent loss of habitats that can lead to die-offs of fishes in the affected areas (McEachran and Fechhelm 2005). Drastic events, such as red tides (Riley et al. 1989), brown tides (Buskey and Hyatt 1995), and extreme freezes (McEachron et al. 1994), in the Gulf of Mexico can significantly increase fish mortality and cause large-scale die-offs. Subtle and long-term changes can cause a gradual shift of the fish community from more temperate species to more tropical species or vice versa for a given region in the Gulf of Mexico (O’Connell et al. 2004). For example, tropical fish tend to be rare inshore, but are commonly found along the South Texas coast (Hoese and Moore 1998). Climatic events, such as hurricanes and floods, can also affect fish community and population dynamics in the Gulf of Mexico; for example, storms are believed to enhance red drum recruitment (Matlock 1987).
9.2.2 The Fish Community in the Gulf of Mexico
The ichthyofaunal community of the Gulf of Mexico has features similar to those of both warm temperate and tropical waters. The Gulf of Mexico has a relatively rich fish fauna for its size and has nearly 10 % of the world’s known marine fish species (Nelson 2006). McEachran and Fechhelm (2005) suggest that the species richness and composition in the Gulf of Mexico is largely similar to that in the West-Central Atlantic region (Cape Hatteras, North Carolina to the equator). Previous studies have documented 1,443 finfish species in 700 genera, 223 families, and 45 orders in the Gulf (Hoese and Moore 1998; McEachran and Fechhelm 2005), which is 200 more species and 54 more genera than what occurs in the eastern Atlantic Ocean between the Arctic and the southern coast of Morocco, including the Mediterranean. This is equal to 64.2 % of the species, 81.6 % of the genera, 92 % of the families, and all of the orders of fish in the West-Central Atlantic Ocean (McEachran and Fechhelm 2005). Species of fish in the West-Central Atlantic Ocean that are not found in the Gulf of Mexico are mostly deep-sea and oceanic fish; temperate fishes rarely occur to the south of Cape Hatteras. Tropical fishes are rare north of Central America or west of the Bahamas or Great Antilles. Relatively large seasonal temperature changes and the lack of extensive reef habitat may exclude species that are not adapted to seasonal changes in thermal habitat and are reef-dependent (McEachran and Fechhelm 2005).
According to McEachran and Fechhelm (2005), only 4.6 % of the 1,443 species (66 species) can be defined as endemic to the Gulf of Mexico. Of these, only nine species are omnipresent and distributed throughout the Gulf of Mexico. The majority of the endemic species are distributed in one or two of the three subregions in the Gulf of Mexico (eastern, northwestern, and southern). Five species of fish that are widely distributed along the U.S. east coast, including Atlantic sturgeon (Acipenser oxyrhynchus), striped bass (Morone saxatilis), black sea bass (Centropristis striata), banded drum (Larimus fasciatus), and shelf flounder (Etropus cyclosquamus), have or have had isolated populations in the northern Gulf of Mexico, suggesting that the Gulf populations are or were remnants of western extremes of once continuous populations (Smith et al. 2002; McEachran and Fechhelm 2005). However, species that limit their distribution in the southern subregion of the Gulf of Mexico tend not to be endemic to the Gulf of Mexico. This indicates that the Yucatán Peninsula, unlike the Florida Peninsula, is not a biogeographic barrier (Smith et al. 2002). The Gulf of Mexico has deep sills in the Straits of Yucatán and in the Straits of Florida, which may allow for easy movement of fishes between the Gulf of Mexico and other areas.
The Gulf of Mexico cannot be defined as a biogeographic province, which requires that more than 10 % of all the species be endemic (Briggs 1974). However, it can be considered a unique biogeographic region because of its high fish species richness and unique community of warm temperate and tropical fish species (McEachran and Fechhelm 2005). This may be due to a combination of diversity of habitats, geological and oceanographic conditions, and geographic location, which makes it accessible to warm temperate and tropical shore fishes and most deep-sea pelagic and benthic fish species (McEachran and Fechhelm 2005).
Almost half of the 1,443 species occurring throughout the Gulf of Mexico can be considered ubiquitous within their respective depth (Table 9.4). These species include wide-ranging epipelagic fishes, e.g., blacktip shark (Carcharhinidae), Gulf menhaden (Clupeidae), Atlantic needlefish (Belonidae), Atlantic flyingfish (Exocoetidae), and common halfbeak (Hemiramphidae); mesopelagic fishes, e.g., Garrick (Gonostomatidae), hatchetfish (Sternoptychidae), lightfish (Phosichthyidae), and smallfin lanternfish (Myctophidae); benthic fishes of the continental shelf, e.g., squirrelfish (Holocentridae), red grouper (Serranidae), red snapper (Lutjanidae), and red drum (Sciaenidae); and benthic fishes of the slope, e.g., blackfin spiderfish (Ipnopidae), Western Atlantic grenadier (Macrouridae), and beardless codling (Moridae) (McEachran and Fechhelm 2005). These families of species also tend to be distributed in other regions of the Atlantic, Pacific, and Indian Oceans. The remaining 51.2 % of the 1,443 fish species mainly limit their spatial distributions within a subregion of the Gulf of Mexico (Table 9.4). For example, a total of 211 species (14.6 %) can be found only in the eastern subregion of the Gulf of Mexico (Table 9.4), and most of these species are mesopelagic fishes that may reflect intrusion of the Loop Current into the eastern Gulf of Mexico. The distribution patterns reflect spatial variability in geological and oceanographic conditions and other habitat variables (Hoese and Moore 1998; McEachran and Fechhelm 2005). For example, benthic species that prefer terrigenous substrates are mainly found in the northern and western Gulf of Mexico, and benthic fishes associated with calcareous substrates tend to be found in the calcareous shelves of Florida and the Yucatán; species preferring warm temperate habitats are usually found in the northern Gulf of Mexico, while those preferring tropical habitats tend to occur in the southern Gulf of Mexico (McEachran and Fechhelm 2005). Although the results may be biased by the difference in sampling efforts, the eastern Gulf of Mexico (Florida Bay to Pensacola, Florida or Mobile Bay, Alabama) appears to have the highest number of species (1,259), followed by the western Gulf of Mexico (Pensacola or Mobile Bays to Cape Rojo, Mexico, 1,056 species), with the southern Gulf of Mexico (Cape Rojo to Cape Catoche, Mexico) having the lowest species diversity (916) (Table 9.4)
More than 1,112 species of finfish, sharks, and rays in the Gulf of Mexico were included in the FishBase database developed by the World Fisheries Center (Froese and Pauly 2009). Although this is not a complete list [the number of finfish species alone is 1,443 as suggested by McEachran and Fechhelm (2005)], the species included in FishBase represent a majority of the finfish, sharks, and rays in the Gulf of Mexico. Based on habitat needs and distribution in the water column, these 1,112 species are divided into seven groups in FishBase: reef-associated, bathydemersal, bathypelagic, benthopelagic, demersal, pelagic-neritic, and pelagic-oceanic. Of the 1,112 fish species in FishBase, more than one-third are reef-associated species, and the benthopelagic and pelagic-oceanic fish species have the lowest species diversity (Table 9.5). The trophic level of fishes associated with each habitat tends to vary greatly (Figure 9.2). The pelagic-oceanic species tend to have the highest average trophic level, while the reef-associated fish species tend to have the widest distribution of trophic levels (Table 9.5; Figure 9.2). The maximum size also varies greatly within each habitat group, with the pelagic-oceanic group having the largest average maximum size and the bathypelagic group having the smallest average size (Table 9.5).
The distribution of trophic levels for fish, shark, and ray species of different habitats in the Gulf of Mexico. Trophic level measures the number of steps the fish, shark, or ray is from the start of the food chain: 1 = primary producers that make their own food, such as plants and algae; 2 = primary consumers, such as herbivores consuming primary producers; 3 = secondary consumers, such as carnivores eating herbivores; 4 = tertiary consumers, such as carnivores eating other carnivores; and 5 = apex predators that are at the top of the food chain with no predators (data from FishBase 2013)
Although various fishery-dependent and fishery-independent monitoring programs have been developed and implemented (McEachran and Fechhelm 2005) and some species, such as red snapper and Gulf menhaden, are well researched, many fish populations in the Gulf of Mexico are still not well understood compared to those of other marine ecosystems in the United States (Rowe and Kennicutt 2009). Therefore, large uncertainty still remains on the dynamics and conditions of many Gulf of Mexico fish populations of commercial and recreational importance (NMFS 2012a).
9.3 Population Dynamics of Key Finfish Species of Ecological, Commercial, and Recreational Importance
Many fish species support highly valued commercial and recreational fisheries. These species range from reef-dependent snappers and groupers, to highly migratory tuna and billfish, to coastal pelagic menhaden and mackerel, and coastal demersal drums and jacks (Hoese and Moore 1998; McEachran and Fechhelm 2005). They differ greatly in their ecological roles, life histories, habitat needs, and contributions to commercial and recreational fisheries. As described earlier, 13 representative species have been selected from ten families for evaluation in this chapter: red snapper; menhaden, including Gulf menhaden, finescale menhaden, and yellowfin menhaden; red grouper; Atlantic bluefin tuna; Atlantic blue marlin; Atlantic swordfish; Atlantic sailfish; red drum; tilefish; king mackerel; dolphinfish; striped mullet, and greater amberjack (Table 9.3). Because of their ecological and fisheries significance in the Gulf of Mexico, snapper and grouper species were also evaluated as families for their distribution, life history, fisheries, and habitat needs.
9.3.1 Snappers (Family Lutjanidae) 
The family Lutjanidae, or snappers, is composed of 17 genera and about 100 species of mostly reef-dwelling marine fishes that are divided into four subfamilies (Allen 1985). Snappers are confined mostly to tropical and subtropical regions of all oceans, while three species occur in freshwater; juveniles of many snapper species inhabit brackish mangrove estuaries and the lower reaches of freshwater streams. Snappers occur in four discrete geographic faunas, and snappers that occur in the western Atlantic Ocean are not found in any other region (Allen 1985). Snappers that occur in the Gulf of Mexico region include 16–17 species in six genera from the family Lutjaninae (Table 9.1) (McEachran and Fechhelm 2005).
Snappers have separate sexes, sexual differentiation remains constant throughout their life span, and sexual dimorphism is rare (Martinez-Andrade 2003). A key reproductive strategy utilized by many species of inshore-dwelling snappers is an extensive migration to selective offshore areas along outer reefs to form seasonal spawning aggregations in the week or so prior to the full moon (Martinez-Andrade 2003). Snapper larvae are most common relatively close to shore, in waters over the continental shelf, or in large coral reef lagoons; they are relatively rare in the more offshore areas at the edge of the shelf and in oceanic waters (Allen 1985). Snappers can grow to about 1 m (3.3 ft) in length, and the typical maximum life span of snappers has been estimated between 4 and 21 years. Most snappers occur in shallow to intermediate depths to 100 m (328 ft), although some are largely confined to deepwater (100–500 m or 328–1,640 ft) (Allen 1985).
Snappers are active predators feeding mostly at night on a variety of prey (Allen 1985). Fishes dominate the diet of most species, and other common prey include crabs, shrimps, other crustaceans, gastropods, cephalopods, and planktons. Generally, the larger, deep-bodied snappers feed on other fishes and large invertebrates on or near the surface of the reef and are usually equipped with large canine teeth adapted for seizing and holding their prey.
Landings of snappers are of significant volume and economic value because of the excellent quality of their meat and high demand, making them some of the most desirable species in the market (Martinez-Andrade 2003). The Gulf of Mexico Fishery Management Council (GMFMC) manages snappers under the Reef Fish Fishery (GMFMC 2004a). The Reef Fish Management Plan (FMP) currently includes 42 species, and snapper species managed under this FMP include red snapper, queen snapper (Etelis oculatus), mutton snapper (Lutjanus analis), schoolmaster (Lutjanus apodus), blackfin snapper (Lutjanus buccanella), cubera snapper (Lutjanus cyanopterus), gray or mangrove snapper (Lutjanus griseus), dog snapper (Lutjanus jocu), mahogany snapper (Lutjanus mahogoni), lane snapper (Lutjanus synagris), silk snapper (Lutjanus vivanus), yellowtail snapper (Ocyurus chrysurus), wenchman (Pristipomoides aquilonaris), and vermilion snapper (Rhomboplites aurorubens).
Because of its recreational and commercial importance as a prized food fish in the Gulf of Mexico, red snapper was selected as the representative snapper species for evaluation (Figure 9.3). Key life-history parameters for red snapper are summarized in Table 9.6 and discussed in the sections below. A summary of red snapper habitat information is presented in Table 9.7, while Table 9.8 includes stock and fisheries information for the red snapper; this information is also discussed in more detail in the following sections.
Red snapper (Lutjanus campechanus) on a coral reef in the Gulf of Mexico (from von Brandis 2013)
9.3.1.1 Key Life-History Processes and Ecology
Red snapper are distributed throughout the Gulf of Mexico and the U.S. Atlantic coast (Figure 9.4). Genetic studies support the hypothesis of a single red snapper stock in the northern Gulf of Mexico (Gold et al. 1997; Heist and Gold 2000).
Range of the red snapper (Lutjanus campechanus) in the Gulf of Mexico and Florida east coast (from USGS 2010a)
Larval abundance is directly related to adult abundance (Lyczkowski-Shultz et al. 2005). During the peak spawning months, the highest larval density is found off the Louisiana coast at depths of 50–100 m (164–328 ft) (Table 9.6), and abundance tends to be lower east of the Mississippi River compared to west of the Mississippi River. According to fall plankton surveys, red snapper larvae can be found less frequently and in lower abundance in the eastern Gulf of Mexico than in the western Gulf. Larvae were also found between the 100 and 200 m (328 and 656 ft) depth contours throughout the Gulf of Mexico, indicating that red snapper spawn from the mid-shelf to the continental slope.
After reaching 50 mm (1.9 in.) total length [TL refers to the length from the tip of the snout to the tip of the longer lobe of the caudal fin or tail, usually measured with the lobes compressed along the midline (FishBase 2013)], these age-0 red snapper are taken as bycatch in the Gulf of Mexico penaeid shrimp fishery and continue to be taken as bycatch as age-1 red snapper. The highest density of age-0 to -1 red snapper is found in the northern Gulf of Mexico at depths between 18 and 55 m (59 and 180 ft) from the Alabama–Florida border to the Texas–Mexico border (Gallaway et al. 1999). They tend to prefer shell and sand substrates (Szedlmayer and Howe 1997). Studies suggest an ontogenetic shift from low-relief to higher-relief habitat with size and age (Szedlmayer and Lee 2004; Wells 2007). The newly settled fish smaller than 40 mm (1.6 in.) TL mostly occur in open habitat, but begin moving onto the reefs as their sizes approach 100 mm (3.9 in.) TL (Table 9.7). They tend to have a high degree of fidelity to these habitats (Workman et al. 2002).
Red snapper enter the targeted commercial and recreational fisheries at age 2 for the rest of their life span (Wilson and Nieland 2001). They can be found across the shelf to the shelf edge and show an affinity for vertical structure (Table 9.7) (Patterson et al. 2001), especially from 2 to 10 years of age. Red snapper older than 8–10 years are no longer totally dependent on structured habitats and are capable of foraging over open habitat (Table 9.7). A National Marine Fisheries Service (NMFS) bottom-longline survey suggests that red snapper tend to be most abundant at depths from 55 to 92 m (180–302 ft) and that older and larger red snapper are found more frequently in the western Gulf of Mexico, while younger and smaller fish are found in the eastern Gulf (Mitchell et al. 2004). Adult red snapper tend to experience a seasonal depth-related movement toward shallower water (inner-mid shelf) in the spring/summer months and offshore (mid-outer shelf) in the winter months (Bradley and Bryan 1975). This movement may be related to spawning-related activity (SEDAR 7 2005).
Red snapper have some rather unique life-history traits (Table 9.6). In the Gulf of Mexico, they can reach maturity at young ages but have a long life span of more than 50 years (Szedlmayer and Shipp 1994; Wilson and Nieland 2001). Red snapper are batch spawners, with an estimated spawning duration of 180 days and a mean spawning frequency of 3.0 in the eastern Gulf of Mexico and 2.9 in the western Gulf of Mexico (SEDAR 7 2005). Lyczkowski-Shultz and Hanisko (2007) suggest that red snapper tend to spawn over a wide depth range from the mid-shelf to the continental slope. The eggs are pelagic and hatch in about 20–27 h after fertilization (Minton et al. 1983). The larvae remain pelagic for about 26–30 days until metamorphosis and settlement (Rooker et al. 2004). After the completion of the pelagic larval stage, red snapper settle and move to structured habitat, such as low-relief, relic-shell habitat (Workman and Foster 1994; Piko and Szedlmayer 2007), and become post-settlement juveniles, ranging from 19 to 50 mm (0.75–1.9 in.) TL in size and 29–66 days in age (Szedlmayer and Conti 1999).
Red snapper experience high rates of growth when they are young but begin to slow down when they reach the age of 8–10 years. There is little evidence for strong sexual dimorphism in growth (Goodyear 1995). The average maximum attainable size in the von Bertalanffy growth equation is less than 900 mm (35.4 in.) TL (Table 9.6).
Females tend to mature at relatively smaller sizes and earlier ages in the eastern Gulf of Mexico compared to those in the western Gulf of Mexico (SEDAR 7 2005). For example, in an analysis done for Southeast Data, Assessment and Review (SEDAR) 7 (2005), over 75 % of females were mature by 300 mm (11.8 in.) FL for samples taken from the eastern Gulf of Mexico, but the proportion in the west was still below 75 % even at 350 mm (13.8 in.) FL. For both regions, all females were mature after reaching 650 mm (25.6 in.) FL. The red snapper is highly fecund and, on average, a female of age 10 can produce over 60 million eggs per year. Fecundity-at-length data can be best quantified with power or exponential functions, but an asymptotic function provides a better fit for fecundity-at-age data, suggesting that fecundity is more dependent upon length, rather than age (SEDAR 7 2005).
Natural mortalityFootnote 1 (M) during the egg stage of the red snapper is estimated at 0.50 per day (Gallaway et al. 2007). The mortality of red snapper larvae is high, and the accumulative M during the larval stage is estimated at 6.76 per year (Gallaway et al. 2007). The estimates of red snapper M for ages 0 and 1 varied greatly among studies (Gallaway et al. 2009), ranging from 0.98 to 3.7 and 0.6 to 1.4 for age-0 and age-1 fish, respectively. An M value of 0.6 per year was used for age-1 fish in recent stock assessments (SEDAR 7 2005). The adult red snapper M was assumed to be 0.1 per year in the assessment.
The newly hatched larval density in the water column is positively related to adult fish abundance, suggesting that spawning stock biomass is positively related to larval abundance. The abundances of age-0 and age-1 red snapper are poorly correlated, indicating the existence of density-dependent mortality in early life history. The availability of low relief, natural habitat for the post-settlement of red snapper (ages 0 and 1) is suggested as a major limiting factor in the observed level of recruitment (Gallaway et al. 2009). However, Cowan et al. (2011) suggest that age-1 red snapper are more vulnerable to shrimp trawl bycatch as compared to age-0 fish, weakening the above argument about the role of low-relief habitats in the shallow Gulf of Mexico. They further state that habitat limitation is not a strong factor in regulating recruitment dynamics of red snapper (Cowan et al. 2011).
The number of recruits, measured as the number of red snapper at age 1, estimated for the eastern U.S. Gulf of Mexico in the stock assessment (SEDAR 7 2009) is much higher than that for the western U.S. Gulf of Mexico (Figure 9.5). The recruitment of red snapper in both the western and eastern Gulf has fluctuated over time. In the eastern Gulf of Mexico, recruitment reached one of the highest values in 2003, but continued to decline from 2003 through 2008, with the recruits in 2008 being less than half of the recruits in 2003.
Abundance of red snapper (Lutjanus campechanus) recruits measured as age-1 fish for the eastern and western U.S. Gulf of Mexico from 1981 to 2008 (data from SEDAR 7 Update 2009)
Red snapper from Alabama tend to mature at smaller sizes and younger ages than those from Louisiana (Woods et al. 2007). Differences in maturation are also found between the eastern Gulf of Mexico (Mississippi, Alabama, and Florida west coast) and western Gulf of Mexico (Louisiana and Texas) (SEDAR 7 2005). Young (to age 8) red snapper in the eastern Gulf of Mexico tend to have a higher reproductive output at age compared to those in the western Gulf. A single stock of red snapper in the Gulf of Mexico has been suggested by genetic studies (Camper et al. 1993; Gold et al. 1997; Heist and Gold 2000), which may result from the lack of sufficient time since the Pleistocene epoch for spatially separated substocks of red snapper in the Gulf of Mexico to have become genetically distinct, or from enough mixing to maintain homogeneity in the population. However, phenotypic differences have been identified in growth, maturation, abundance, age/size compositions, prey compositions, and fishery dynamics between the eastern and western Gulf of Mexico. To account for such differences between the two areas in the stock assessment, the Gulf of Mexico red snapper stock is considered to consist of the two substocks. Although there is evidence of large differences in life history and population dynamics at fine spatial scales, such as among different reefs (Gallaway et al. 2009), more studies are needed to evaluate the potential existence of metapopulation structure.
9.3.1.2 Predators and Prey
Juvenile red snapper prey mainly on fishes and invertebrates from reefs and soft bottom habitats (Table 9.6). A diet shift from open-water prey to reef prey was observed by Szedlmayer and Lee (2004) as fish moved from open to reef habitat, suggesting that reef habitat provides not only protection from predation but also additional food sources. The diet of adult red snapper also includes many species of fishes and invertebrates (Table 9.6).
9.3.1.3 Key Habitat Needs and Distribution
Red snapper eggs are pelagic and float to the surface. Newly hatched larvae are also pelagic and are found to be most abundant from 50 to 100 m (164–328 ft) depths in the Gulf of Mexico west of the Mississippi River. After they reach 16–19 mm (0.6–0.7 in.) TL in about 26–30 days of age, they settle to the bottom. The newly settled fish smaller than 40 mm (1.6 in.) TL mostly occur in open habitat, but begin moving onto the reefs as their sizes approach 100 mm (3.9 in.) TL.
Prior to 8–10 years of age, red snapper tend to prefer shell and sand substrates (Szedlmayer and Howe 1997), are attracted to natural and artificial (e.g., oil and gas platforms) reef habitats, and have a high degree of fidelity to these habitats (Workman et al. 2002). Additional characteristics of juvenile red snapper habitat are described in Table 9.6. After they reach age 8–10, they tend to be less attached to reef habitats and spend most of their time in open waters (Table 9.6).
Essential fish habitat has been designated for Reef Fish, which includes juvenile and adult red snapper. Reef fish essential fish habitat is described in Table 9.7 and shown in Figure 9.6.
The Gulf of Mexico Fishery Management Council’s Gulf of Mexico Reef Fish Essential Fish Habitat for queen snapper (Etelis oculatus), mutton snapper (Lutjanus analis), blackfin snapper (Lutjanus buccanella), red snapper (Lutjanus campechanus), cubera snapper (Lutjanus cyanopterus), gray or mangrove snapper (Lutjanus griseus), lane snapper (Lutjanus synagris), silk snapper (Lutjanus vivanus), yellowtail snapper (Ocyurus chrysurus), wenchman (Pristipomoides aquilonaris), vermilion snapper (Rhomboplites aurorubens), speckled hind (Epinephelus drummondhayi), yellowedge grouper (Epinephelus flavolimbatus), goliath grouper (Epinephelus itajara), red grouper (Epinephelus morio), warsaw grouper (Epinephelus nigritus), snowy grouper (Epinephelus niveatus), black grouper (Mycteroperca bonaci), yellowmouth grouper (Mycteroperca interstitialis), gag (Mycteroperca microlepis), scamp (Mycteroperca phenax), yellowfin grouper (Mycteroperca venenosa), goldface tilefish (Caulolatilus chrysops), blueline tilefish (Caulolatilus microps), tilefish (Lopholatilus chamaeleonticeps), greater amberjack (Seriola dumerili), lesser amberjack (Seriola fasciata), almaco amberjack (Seriola rivoliana), banded rudderfish (Seriola zonata), gray triggerfish (Balistes capriscus), and hogfish (Lachnolaimus maximus) (from GMFMC 2004b)
9.3.1.4 Fisheries
Red snapper support an important commercial fishery in the Gulf of Mexico. The fishery began in Pensacola about 150 years ago (Bortone et al. 1977) and then expanded to the waters off Galveston, Texas, the Campeche Banks, and the Dry Tortugas during the late 1800s (Goodyear 1995).
Commercial landings in the United States are divided into four separate fisheries based on fishing gear (headline and longline) and fishing location (eastern and western Gulf of Mexico): (1) handline east, (2) handline west, (3) longline east, and (4) longline west (Figure 9.7). Most of the catch was landed with handline in the western Gulf of Mexico (Figure 9.7). The total landings tend to have a decreasing trend and reached the lowest value around 1992. The catch doubled for the next 10–12 years, but decreased drastically after 2006 as a result of a large decrease in the western Gulf of Mexico (Figure 9.7).
Commercial landings of red snapper (Lutjanus campechanus) in the U.S. Gulf of Mexico from 1964 through 2009 (data from SEDAR 7 Update 2009)
Red snapper bycatch in the shrimp fishery, mainly consisting of fishes of ages 0 and 1, dominate the catch in numbers of fish (SEDAR 7 2009). The number of red snapper discarded as bycatch has fluctuated between 10 and 60 million fish in most years since the 1970s, and is the lowest in recent years (Figure 9.8). The recreational and commercial fisheries combined take roughly 3–4 million red snapper annually. Targeted commercial and recreational red snapper fisheries dominate removals in weight, accounting for about 4 million kg (9 million lb) in recent years. The annual weight of the shrimp bycatch discarded was estimated to be roughly 1–3 million kg (2–3 million lb) of red snapper (SEDAR 7 2005).
Estimated median number of young red snapper (Lutjanus campechanus) (ages 0–1) discarded in the shrimp fishery in the U.S. Gulf of Mexico using the 2-depth (0–10, 10+ fathoms) and 3-depth (0–10, 10+, 30+ fathoms) zones strata models from 1972 through 2008 (data from SEDAR 7 Update 2009)
Estimates of the recreational catch for red snapper in the Gulf of Mexico since 1981 are obtained from three surveys: (1) the Marine Recreational Fishery Statistics Survey conducted by the NMFS, (2) the Texas Marine Sport-Harvest Monitoring Program by the Texas Parks and Wildlife Department, and (3) the Headboat Survey conducted by the NMFS, Southeast Fisheries Science Center. The estimated recreational landings of red snapper show a decreasing trend over time since 1981 in the U.S. Gulf of Mexico (Figure 9.9). However, it appears to be relatively stable around one half million kg (1.1 million lb) since 2000.
Recreational landings of red snapper (Lutjanus campechanus) in the U.S. Gulf of Mexico from 1981 through 2008 (data from SEDAR 7 Update 2009)
The recreational fishery of Gulf of Mexico red snapper is managed with a size limit, daily bag limit, seasonal length, and allocation quota. For the 2009 recreational fishing season, the size limit was 40.6 cm (16 in.) TL, the daily bag limit was two fish, the fishing season was from June 1 to August 15 (75 days), and the annual quota allocation was 1.11 million kg (2.45 million lb) (SEDAR 7 2009).
The recreational fishery of Gulf of Mexico red snapper is managed with a size limit, daily bag limit, seasonal length, and allocation quota. For the 2009 recreational fishing season, the size limit was 40.6 cm (16 in.) TL, the daily bag limit was two fish, the fishing season was from June 1 to August 15 (75 days), and the annual quota allocation was 1.11 million kg (2.45 million lb) (SEDAR 7 2009).
The red snapper stock is a single management unit in the Gulf of Mexico extending from the U.S.–Mexico border in the west through the northern Gulf waters and west of the Dry Tortugas and the Florida Keys. The assessment assumes there are two sub-units of the red snapper stock within this region, separated roughly by the Mississippi River (SEDAR 7 2009). The GMFMC is responsible for assessing the red snapper stock status in the Gulf of Mexico under Section 303 of the Magnuson-Stevens Act.
In the assessment of the Gulf of Mexico fish stocks, a fishery with fishing mortality higher than the maximum fishing mortality threshold (MFMT) is defined as in the status of “overfishing,” meaning that fish stocks cannot be sustained under such a level of fishing mortality. A fish population with its biomass lower than the minimum spawning stock threshold (MSST) is defined “overfished,” meaning that the stock biomass is too low and reproductive potentials are severely depleted. For the Gulf of Mexico red snapper stock, the MFMT is defined as F SPR26% (SEDAR 7 2009), a fishing mortality (F) at which the reproductive potential is only 26 % of the maximum reproductive potential in the absence of fishing mortality and was estimated at 0.53 per year. The average F from 2006 through 2008 was 1.00, almost two times as high as the MFMT. The fishing mortality of optimal yield (F OY) was determined at 75 % of the MFMT (e.g., 0.39). Thus, recent fishing mortality was much higher than the F OY and the MFMT, suggesting that overfishing occurred in the Gulf of Mexico red snapper fishery prior to 2010 (SEDAR 7 2009). The stock biomass at which maximum sustainable yield (MSY) is achieved (S MSY) was estimated at 4.6 million kg (10.16 million lb), and for the Gulf of Mexico red snapper, the MSST was calculated as (1 − M)S MSY, where M has a value of 0.1 per year, which yields a MSST of 4.1 million kg (9.14 million lb). The biomass as of 2008 was only 1.78, much lower than the MSST (<20 % of the MSST), suggesting that the red snapper stock biomass was severely overfished prior to 2010 (SEDAR 7 2009). Thus, the Gulf of Mexico red snapper stock was overfished, and overfishing occurred in the Gulf of Mexico prior to 2010 (Table 9.8). An early stock assessment (SEDAR 7 2005) also suggests that Gulf of Mexico red snapper were grossly overfished through 2003, and the estimated spawning potential ratio (SPR) was less than 5 %.
|
|
|
9.3.2 Menhaden: Gulf Menhaden (Brevoortia patronus), Finescale Menhaden (Brevoortia gunteri), and Yellowfin Menhaden (Brevoortia smithi)
In the Gulf of Mexico, menhaden play a critical role in linking plankton with upper level predators. Because of their filter feeding abilities, menhaden can consume and redistribute a significant amount of primary production and energy in the Gulf of Mexico. They are small, marine, filter feeding fish belonging to the family Clupeidae (herrings, shads, sardines, hilsa and menhadens). Gulf menhaden are considered the Gulf of Mexico complement to Atlantic menhaden (Brevoortia tyrannus) based on morphological and genetic analyses (Dahlberg 1970; Anderson 2007). Both species support large-scale, commercial reduction fisheries (not directly consumed but used to make fish products), with Gulf menhaden supporting one of the largest fisheries, by weight, in the United States (Pritchard 2005).
Menhaden abundance can greatly influence the population dynamics of many predatory fish species, such as tunas, drums, and sharks; in addition, they are also a very important food source for many birds (Overstreet and Heard 1982). Three species of menhadens, Gulf menhaden, finescale menhaden, and yellowfin menhaden, are distributed in the Gulf of Mexico. Key life-history parameters, habitat preferences and distribution, and general information on the menhaden stock and fishery in the Gulf of Mexico are presented in the tables and paragraphs that follow (Tables 9.9, 9.10, and 9.11).
9.3.2.1 Key Life-History Processes and Ecology
Menhaden are flat and dull silver with a greenish back, have soft flesh, and a deeply forked tail. A prominent black spot is found behind the gill cover, followed by a row of smaller spots. The three species of menhaden, Gulf menhaden, finescale menhaden, and yellowfin menhaden, are distributed in the Gulf of Mexico from estuarine waters outwards to the continental shelf, although they are most likely distributed in less saline waters of estuaries and can be found in bays, lagoons, and river mouths (Table 9.10 and Figure 9.10). Gulf menhaden tend to have larger scales than yellowfin menhaden, and finescale menhaden lack the row of smaller spots that occur on Gulf menhaden. All three species have yellowish fins (McEachran and Fechhelm 2005).
Range of menhaden, including Gulf menhaden (Brevoortia patronus), finescale menhaden (Brevoortia gunteri), and yellowfin menhaden (Brevoortia smithi), in the Gulf of Mexico (USGS 2010b)
The Gulf menhaden occurs throughout the Gulf of Mexico but is mainly distributed in nearshore waters (Table 9.10 and Figure 9.10). Yellowfin menhaden mainly inhabit estuarine or nearshore areas and do not seem to have seasonal migratory behavior. Finescale menhaden also occur in estuarine or nearshore areas. No evidence suggests that finescale menhaden are subject to any systematic seasonal migration, but there appears to be a seasonal shift of larger finescale menhaden between Texas bays (Gunter 1945). In the southern Gulf of Mexico, the range of Gulf menhaden overlaps that of the finescale menhaden (Anderson and McDonald 2007), and it appears that these two species may engage in resource partitioning, a process whereby closely related or trophic-overlapped species occurring in close proximity results in subtle differences in ecological niches (Castillo-Rivera et al. 1996). In the eastern Gulf, the range of Gulf menhaden overlaps that of the yellowfin menhaden, and there is evidence of hybridization between the two species (Anderson and Karel 2007).
All menhaden species are estuarine-dependent and marine migratory species (Anderson and McDonald 2007). In general, spawning usually takes places in the offshore marine environment during winter (Table 9.9) (Gunter 1945; Simmons 1957; Dahlberg 1970; Houde and Swanson 1975). Egg hatch and early growth of larvae usually occur when currents from offshore spawning grounds transport them to low-salinity estuary nursery grounds (Minello and Webb 1997). This process usually takes 1–2 months. The transported larvae enter estuarine bays, sounds, and streams and metamorphose into juveniles. Menhaden juveniles inhabit estuarine areas until the following fall or early winter, when they migrate offshore (Table 9.10). Adults are usually distributed in large schools in nearshore oceanic waters and large estuarine systems. Because the Gulf menhaden has a similar life-history process and is much more abundant and widely distributed than the other two menhaden species, the following discussion is focused on Gulf menhaden.
The spawning season estimated for the Gulf menhaden differs among studies, varying from December through February and October through March (Table 9.9) (Suttkus 1956; Combs 1969; Christmas and Waller 1975; Shaw et al. 1985a, b). This might reflect impacts of environmental conditions, which vary from year to year (SEDAR 27 2011). Gulf menhaden are multiple and intermittent spawners with ova being released in batches over a protracted spawning season (Combs 1969; Lewis and Roithmayer 1981). Spawning can occur from nearshore to 60 miles offshore along the entire U.S. Gulf coast (Table 9.9) (Christmas and Waller 1975). However, Fore (1970) analyzed the distributions of eggs and concluded that spawning of Gulf menhaden occurred mainly over the continental shelf between Sabine Pass, Texas and Alabama, with the greatest concentrations being found in waters between the 8 and 70 m (26.2 and 230 ft) contours off Texas and Louisiana and near the Mississippi Delta.
The eggs of Gulf menhaden are planktonic and drift with prevailing currents for almost 48 h before hatching. Early larvae also drift with the current and feed on phytoplankton. Currents transport Gulf menhaden larvae into low-salinity estuaries for early growth (Minello and Webb 1997). This transportation from spawning grounds to estuarine nursery grounds is critical for the survival of Gulf menhaden larvae. As they grow larger and become able to swim, they shift their diet to zooplankton. After developing gill rakers, they filter-feed on plankton, typically near the surface. In fresh and brackish estuaries and rivers, they grow rapidly in spring and summer, and by fall, they migrate to high-salinity offshore waters no deeper than 100 m (328 ft). No east–west component of annual migration was found for Gulf menhaden in tagging studies (Kroger and Pristas 1975; Pristas et al. 1976); however, Gulf menhaden from the eastern and western extremes of their ranges tend to move toward the center of their range with age (Ahrenholz 1991).
Few Gulf menhaden spawn in their first winter, but almost all fish are mature by their second winter when they reach age 1+. Female Gulf menhaden are generally mature after they reach about 150 mm (5.9 in.) FL and larger (Table 9.9) (Lewis and Roithmayer 1981). The life span of Gulf menhaden is about 5–6 years. The maximum size observed for Gulf menhaden is 223 mm (8.8 in.) FL (Ahrenholz 1991).
Limited information on age and size at maturity is available for finescale and yellowfin menhaden. Female finescale menhaden were found to be mature at the size of 150 mm (5.9 in.) TL (Gunter 1945), and female yellowfin menhaden were found to be mature at 186 mm (7.3 in.) FL (Hellier 1968). The maximum size reported is 281 mm (11 in.) FL for yellowfin menhaden and 289 mm (11.4 in.) FL for finescale menhaden (Ahrenholz 1991).
Younger fish are thought to be more vulnerable to predation, and thus M may decline with size or age (SEDAR 27 2011). In addition to varying with size or age, M also tends to vary from year to year, reflecting annual variability of habitat variables (Figure 9.11).
Annual natural mortality (M) for different age groups of Gulf menhaden (Brevoortia patronus) from 1964 through 2009 (data from SEDAR 27 2011)
Recruitment tends to fluctuate over time without a clear temporal trend. However, large uncertainty appears to be associated with the Gulf menhaden recruitment estimates (Figure 9.12).
Estimates of annual recruitment of Gulf menhaden (Brevoortia patronus), measured as the number of age-0 fish, from 1948 through 2009 (data from SEDAR 27 2011)
Populations of Gulf menhaden throughout the Gulf of Mexico are generally thought to comprise a single genetic stock (SEDAR 27 2011). No evidence supports the existence of multiple stocks for finescale menhaden and yellowfin menhaden within the Gulf of Mexico. There is no strong evidence supporting the existence of metapopulations (groups of spatially separated populations of the same species that interact at some level). However, there appears to be large spatial variability in key life-history parameters, such as M, growth, and maturation. Stock structure also varies over time and space (SEDAR 27 2011).
9.3.2.2 Predators and Prey
Menhaden are omnivorous filter feeders that remove food resources from the water column via their gill rakers while swimming (Table 9.9). Their filtration efficiency is largely a function of branchio-spicule spacing of the gill rakers changing allometrically as menhaden grow (Friedland et al. 2006). Small Gulf menhaden larvae primarily feed on large phytoplankton (e.g., dinoflagellates) and some zooplankton (Govoni et al. 1983). As the larvae grow, phytoplankton become less important in the diet, and large zooplankton, especially copepods, become more important. After metamorphosis into juveniles, Gulf menhaden become filter-feeding omnivores (Table 9.10). However, some of the phytoplankton that the juvenile Gulf menhaden consume is an order of magnitude smaller than the smallest phytoplankton consumed at larval stages (Chipman 1959; June and Carlson 1971). Menhaden may also feed on their own eggs (Nelson et al. 1977), as well as eggs and larvae of other fishes and invertebrates (Peck 1893; McHugh 1967).
Because of their high abundance and schooling behavior, menhaden of all life-history stages from eggs through adults are potential prey for a large number of piscivorous fish and birds (Table 9.9). Many invertebrate predators, especially in oceanic waters, prey upon menhaden larvae, including chaetognaths (arrow worms), squids (mollusks), ctenophores (comb jellies), and jellyfishes (coelenterates).
9.3.2.3 Key Habitat Needs and Distribution
Larvae and early juveniles are often found associated with estuarine marsh edges for forage and protection from predators, and juveniles and adults are typically in open water with non-vegetated bottoms (Table 9.10). Offshore spawning ensures that Gulf menhaden eggs and larvae are euryhaline. Most Gulf menhaden eggs occur in waters with salinities over 25 parts per thousand (ppt) (Fore 1970; Christmas and Waller 1973). Eggs and larvae are found throughout the Gulf of Mexico waters with salinity ranging from 20.7 to 36.6 ppt (Christmas et al. 1982). As the larvae move inshore, they require low salinity waters to complete metamorphosis. The entrance of larvae into estuaries with abundant food and lower salinities may be essential to their survival and to their metamorphosis into juveniles (June and Chamberlin 1959). Temperature may be more critical to egg development than to juveniles and to adults that are distributed widely in the Gulf of Mexico with large spatial variability in temperature. Eggs and larvae have been observed in waters with temperatures ranging from 11 °C (February) to 18 °C (March) in northern Florida, from 16 °C (January) to 23 °C (March) in southern Florida, and from 10 °C (January) to 15 °C (December) in the Mississippi Sound. Menhaden may be subject to cold mortality under freezing winter conditions, especially in narrow or shallow tidal areas. Large fish kills may also occur during the summer, as a result of plankton blooms and low dissolved oxygen or hypoxic conditions (Christmas and Waller 1973; Etzold and Christmas 1979).
Menhaden tend to have high habitat elasticity to adapt to changes in their habitats. In a study examining fish assemblages in an estuary from 1950 to 2000 (O’Connell et al. 2004), Gulf menhaden were found to change little in their frequency or position within the estuarine ecosystem even though the estuary had deteriorated substantially in environmental quality and the fish assemblage shifted from a croaker-dominated complex to an anchovy-dominated complex. This indicates that Gulf menhaden are elastic in their ability to adapt to short- or long-term changes in environmental conditions (O’Connell et al. 2004). Because menhaden are not federally managed, no essential fish habitat has been designated (Table 9.10).
9.3.2.4 Fisheries
The Gulf menhaden fishery has great ecological, economic, and social importance. Although menhaden are bony, oily, and usually not directly consumed by humans, they are an important source of fishmeal and fish oil. Both of these reduction products are used as feed for livestock and aquaculture, such as for salmon, shrimp, tilapia, and catfish. Fish oil made from menhaden is also used as a dietary supplement and as a raw material for products, such as lipstick. Menhaden is one of the best baitfish available. Fresh or frozen menhaden are commonly used as whole or cut bait for snapper and king mackerel fishing (SEDAR 27 2011).
Gulf menhaden supports one of the largest fisheries in the United States (Table 9.11), which dates back to the 1800s. On average, 400–600 kilotons of Gulf menhaden are extracted and used for reduction annually (Figure 9.13), with a much smaller amount being captured for use as bait. Landings have had a decreasing trend since the 1980s, when they were the highest (Figure 9.13). Most of the Gulf menhaden landed in the reduction fishery was ages 1 and 2, representing 57 and 38 % of the annual catch on average, respectively. Commercial reduction fishery catches are landed from areas ranging from Florida to Texas, with the majority of recent catches coming from Louisiana waters (SEDAR 27 2011).
Landings of Gulf menhaden (Brevoortia patronus) in the reduction fishery from 1964 through 2009 (data from SEDAR 27 2011)
The Gulf menhaden reduction fishery has been managed under a regional FMP since 1978. Management of the Gulf menhaden fishery is through partnerships among the NMFS Beaufort Laboratory, the state marine agencies, the menhaden industry, and the Gulf States Marine Fisheries Commission (GSMFC) (Table 9.11). It is one of the most detailed and data-rich fisheries currently operated in the Gulf of Mexico. A statistical catch-at-age model, the Beaufort Assessment Model (BAM), was used as the base model for the most recent stock assessment (SEDAR 27 2011). The BAM model assumes one coast-wide population of Gulf menhaden in the Gulf of Mexico. The BAM model for Gulf menhaden uses annual time steps, including landings data from 1948 to 2010. The 1948 data are from close to the beginning of the fishery and, thus, tend to represent unfished conditions for Gulf menhaden. The BAM model incorporates various fishery-dependent and fishery-independent data, including abundance indices and age compositions derived from various survey programs, commercial catch-at-age data, and biological information on growth, maturation, and M.
Total egg production, a more accurate quantification of population reproductive potential than spawning stock biomass, was estimated in the most recent stock assessment (Figure 9.14) (SEDAR 27 2011). It appears to have a decreasing trend prior to the mid-1980s, but shows an increasing trend since the late 1980s. The total egg production estimates for recent years tend to be higher than those for most years since the mid-1970s, suggesting that the stock is in good condition (Figure 9.14). Based on the most recent stock assessment (SEDAR 27 2011), the Gulf of Mexico Gulf menhaden population was not overfished and overfishing did not occur in 2010 (Table 9.11).
Estimates of Gulf menhaden (Brevoortia patronus) total egg production from 1948 through 2009 (data from SEDAR 27 2011)
9.3.3 Groupers (Family Serranidae, Subfamily Epinephelinae) 
The subfamily Epinephelinae of the family Serranidae consists of about 160 species of marine fishes in 15 genera that are commonly known as the groupers, rockcods, hinds, and seabasses (Heemstra and Randall 1993). Groupers are bottom-associated fishes found in the tropical and subtropical waters of oceans and are of considerable ecological and economic value. Groupers are generally associated with hard or rocky bottoms, and most species occur on coral reefs, occupying caves, ledges, and crevices (Figure 9.15) (Jory and Iverson 1989; Heemstra and Randall 1993). Some species occur in depths of 100–200 m (328–656 ft), with the majority inhabiting depths less than 100 m (328 ft). Most grouper species apparently migrate vertically as they grow, with larger fish living at progressively deeper depths (Jory and Iverson 1989).
Two yellowmouth groupers (Mycteroperca interstitialis) eye one another near a large brain coral in Flower Garden Banks National Marine Sanctuary (from NMS 2013)
As the major predators of coral reef ecosystems, most groupers feed on a variety of fishes, large crustaceans, and cephalopods (Heemstra and Randall 1993). Most groupers are ambush predators, hiding among the coral and rocks until a fish or crustacean goes by, and catch their prey with a quick rush and snap of their powerful jaws. The large head and mouth of the typical grouper enables it to suck in a large volume of water and its prey in less than one second (Heemstra and Randall 1993).
Groupers are typically solitary fishes except for occasional spawning aggregations and are generally resident on a particular reef for many years; this site specificity and their relatively slow growth rate makes them vulnerable to overfishing (Heemstra and Randall 1993). Most groupers are protogynous hermaphrodites, meaning that all fish are first females and then change into males at a certain age/size (Jory and Iverson 1989).
Fifteen species of groupers are managed under the Reef Fish Fishery by the GMFMC (GMFMC 2004b). The fishery is divided into shallow-water and deep-water grouper complexes (SEDAR 12 Update 2009). Species in the shallow-water complex include red grouper, gag grouper (Mycteroperca microlepis), black grouper (Mycteroperca bonaci), scamp (Mycteroperca phenax), yellowfin grouper (Mycteroperca venenosa), yellowmouth grouper (Mycteroperca interstitialis) (Figure 9.15), rock hind (Epinephelus adsensionis), and red hind (Epinephelus guttatus). The deep-water grouper complex includes snowy grouper (Epinephelus niveatus), yellowedge grouper (Epinephelus flavolimbatus), speckled hind (Epinephelus drummondhayi), warsaw grouper (Epinephelus nigritus), and misty grouper (Hyporthodus mystacinus). Nassau grouper (Epinephelus striatus) and goliath grouper (Epinephelus itajara) (Figure 9.16) are managed as individual species and are prohibited from being harvested.
Goliath grouper (Epinephelus itajara) is one of the species of grouper prohibited from being harvested in the Gulf of Mexico (from Puntel 2016)
Red grouper are among the most abundant, popular, and important commercial fish in the Gulf of Mexico; therefore, this species was selected as the representative species of grouper for evaluation (Figure 9.17). Key life-history parameters for red grouper are summarized in Table 9.12 and discussed in detail in the following paragraphs. In addition, information on habitat preferences and distribution of the red grouper stock and fishery is presented in Tables 9.13 and 9.14 and discussed below.
Red grouper (Epinephelus morio) on a coral reef in the Gulf of Mexico (from Dombrowski 2012)
9.3.3.1 Key Life-History Processes and Ecology
In the Gulf of Mexico, red grouper are distributed along the continental shelf, and the center of distribution along the U.S. coast is in the eastern Gulf of Mexico (Table 9.13; Figure 9.18). Genetic differences within the Gulf of Mexico tend to be small, suggesting a single population within the Gulf. This may have resulted from historic bottlenecks in population abundance that helped maintain the most common genotypes (Richardson and Gold 1997).
Range of the red grouper (Epinephelus morio) in the Gulf of Mexico (from NOAA 2013a)
Red grouper spend their larval phase in the plankton. Juveniles occupy nearshore reefs and seagrass beds; adult red grouper leave nearshore reefs and move offshore to rocky bottom habitat (Tables 9.13 and 9.14). Red grouper are usually solitary until spawning time.
Most red grouper exhibit limited movement throughout their life span and can exhibit high site fidelity at older ages upon reaching mid- to outer-shelf depths, which may result from the species habitat-structuring and haremic (territorial) mating behavior (Coleman and Koenig 2010). Limited movement shown by most red grouper throughout their lives could give rise to complex sub-stock structure. However, tagging studies have shown that some red grouper taken inshore during summer feeding and cohort migrations moved in large numbers in response to unusual events, such as hurricanes. For example, following Hurricane Lily in 2002, both juvenile and adult red grouper were abundant on artificial reefs and petroleum platforms off Mississippi where they previously had been absent (Franks 2003). However, since 2002, red grouper off Mississippi have become scarce.
The red grouper is a protogynous hermaphrodite, with all fish beginning life as females. Most of the females transform to males between 7 and 14 years of age after reaching at least 58.4 cm (23 in.) in length (Table 9.12) (Moe 1969; Brule et al. 1999). Females become sexually mature at 4–6 years of age and at a size of 39.9 cm (15.7 in.) standard length [SL, the length of a fish measured from the tip of the snout to the posterior end of the last vertebra, which excludes the tail] (Table 9.12) (FishBase 2013; Bullock and Smith 1991), while males become reproductively significant at age 10 and older. However, in a recent stock assessment (SEDAR 12 Update 2009), 50 % of females were found to be mature at 3 years of age and becoming males, and 50 % of males became mature at 11 years old. Red grouper have a life span of approximately 25–30 years (SEDAR 12 2006) and can grow up to 125 cm (49.2 in.) in length (McGovern et al. 2002). Although abundance of red grouper has changed substantially over time, the sex ratio of the population has not changed greatly since 1975 (Coleman et al. 1996). Peak spawning occurs in late spring, during March and May in the eastern Gulf of Mexico, but spawning may occur from January through June in the Gulf of Mexico and Caribbean Sea (Tables 9.12 and 9.13) (Johnson et al. 1998). Red grouper are indeterminate batch spawners (Johnson et al. 1998; Collins et al. 2002). Fecundity is related to size and ranges from 312,000 to 5,735,700 eggs.
Spawning red grouper release their sperm and eggs in offshore waters (Table 9.13). The fertilized eggs require high salinity (32 ppt) to maintain their buoyancy. The eggs hatch into larvae approximately 30 h after spawning and live as part of the zooplankton that drifts with the ocean currents. The larvae settle to the bottom substrate at about 35–50 days after hatching and reach a size of 20–25 mm (0.8–0.9 in.) SL. The duration of the red grouper larval stage is within the range of 31–66 days for other grouper species (Lindeman et al. 2000).
A constant M rate of 0.2 per year was used in early stock assessments (Schirripa et al. 1999). Using different models (e.g., Jensen 1996; Quinn and Deriso 1999), M was estimated to range from 0.14 to 0.24. Based on a recent estimate of maximum age (29 years) for the Gulf of Mexico red grouper (SEDAR 12 2006), M was estimated to be 0.14 for all age classes using the regression model developed by Hoenig (1983). However, the assumption of having the same M across all the age groups may not be realistic. An age-varying M approach was, thus, developed (Lorenzen 1996), which inversely relates the M-at-age to the mean weight-at-age by a power function incorporating a scaling parameter. Lorenzen (1996) provided point estimates and 90 % confidence intervals of the power and scaling parameters for oceanic fishes, which are used for initial parameterization. The estimated M using the Lorenzen method varies with age and is considered more biologically plausible than a fixed M for all ages (SEDAR 12 2006). The estimate was then re-scaled to the oldest observed age (29 years) so that the cumulative M through this age was equivalent to that of a constant M (M = 0.14) for all ages.
The distribution of major red grouper fishing grounds and the limited movement shown in tagging studies indicate that the spatial distribution of recruitment varies greatly. The Big Bend region of Florida (DeVries et al. 2006; SEDAR 12 2006) and the shallow (<20 m or <65.6 ft) areas off Southwest Florida (Pinellas and Charlotte Counties) were hypothesized to be two primary sources of recruitment.
Significant differences in size and age structure and in growth rates of red grouper were found north and south of 28°N latitude (Lombardi-Carlson et al. 2006). A tagging study conducted by Mote Marine Laboratory strongly suggested that red grouper (age 2–4 years) had limited range. This tendency could contribute to future stock separation given enough time. The large spatial variability in growth and age structure of red grouper also supports the existence of a more complex subpopulation structure that is not genetically distinctive but functionally independent (Fischer et al. 2004).
9.3.3.2 Predators and Prey
In their early juvenile stages, red grouper feed primarily on demersal crustaceans in seagrass beds. As the juveniles become sexually mature, they move out to deeper rocky bottoms and feed on small fishes, such as snappers and porgies, and invertebrates, such as shrimps and crabs (Table 9.12). The proportion of the diet consisting of fish increases with red grouper size. Top predators, such as sharks, prey on juvenile and adult red grouper (Table 9.12). Red grouper are known to be susceptible to red tide poisoning (SEDAR 12 Update 2009).
9.3.3.3 Key Habitat Needs and Distribution
The fertilized eggs of red grouper require high salinity (32 ppt) to maintain their buoyancy. Red grouper larvae are pelagic and are transported by ocean currents from spawning grounds to settlement grounds. Juveniles occupy nearshore reefs and move offshore when they become adults (Table 9.13). Adult red grouper are non-migratory and are often seen resting on the bottom substrate. The designated essential fish habitat for juvenile and adult red grouper, as well as many other managed grouper species, is included in the Reef Fish FMP (Table 9.13 and Figure 9.6).
9.3.3.4 Fisheries
Red grouper is the most abundant grouper species in the Gulf of Mexico, which helps explain its status as the primary commercial grouper species by weight and second most recreationally caught grouper species (GMFMC 2011). Red grouper are managed as a single management unit in the Gulf of Mexico extending from the U.S.–Mexico border in the west through the northern Gulf waters and west of the Dry Tortugas and the Florida Keys (Table 9.14) (SEDAR 12 Update 2009). Landings are regulated through the implementation of allowable biological catch (ABC), size limits, trip limits, quotas, seasonal closures, area closures, and gear restrictions. These regulations have been constantly adjusted over time based on improved understanding of the population dynamics of red grouper and stock status.
Red grouper total landings in the United States are taken from four fleets: longline, commercial handline, commercial trap, and recreational. These combined fleets fluctuated with an overall declining trend, falling from almost 3.9 million gutted kg (8.7 million gutted lb) in 1986 to about 2.1 million gutted kg (4.6 million gutted lb) in 1998 (SEDAR 12 Update 2009). Total landings then increased sharply, reaching almost 3.2 million gutted kg (7.1 million gutted lb) in 1999, while stabilizing at an average of 3.4 million gutted kg (7.5 million gutted lb) until 2005 and nearing the estimated optimal yield (OY) of 3.4 million gutted kg (7.6 million gutted lb) (SEDAR 12 2006; SEDAR 12 Update 2009). Total landings began a decreasing trend in 2006, and reached 2.5 million gutted kg (5.6 million gutted lb) in 2008 (SEDAR 12 Update 2009).
Commercial longline landings in the United States from 1986 to 2005 showed a gradual increase with a range of 0.9–2.0 million gutted kg (2.0–4.3 million gutted lb), while commercial handline landings declined considerably from 1.7 to 0.5 million gutted kg (3.7–0.9 million gutted lb) before stabilizing in 2000 at 0.8 million gutted kg (1.8 million gutted lb) (SEDAR 12 2006). The commercial trap fishery contributed less than either the commercial handline or longline, while only landing about 0.5 million gutted kg (1.1 million gutted lb) annually in 1995 and 2000. Recreational landings, including all components, were equal to a third of all commercial landings from 1986 to 2008.
The annual estimated rate of total fishing mortality (landings and discards combined) for directed fleets increased steadily from 0.25 in 1986 to a peak of 0.29 in 1993, before falling steadily to 0.16 in 1998. The rate of fishing mortality increased slightly in 1999 to around 0.2, followed by a decreasing trend to 0.18 for 2005 (SEDAR 12 Update 2009). Discard mortality is typically 10 % of the landings attributed to directed fleets (SEDAR 12 2006).
The recreational fishery of the Gulf of Mexico red grouper is managed with size limits, daily bag limits, seasonal length, and allocation quotas. For the 2009 recreational fishing season, the size limit was 40.6 cm (16 in.) TL, the daily bag limit was two fish, seasonal length was from June 1 to August 15 (75 days), and the annual quota allocation was 1.1 million gutted kg (2.43 million lb) (SEDAR 12 2006). Some of the regulations implemented have been questioned for their unintended biological implications. For example, Goodyear (1995) raised concerns about the use of a high minimum size limit (50.8 cm or 20 in TL) on red grouper that show great variation in growth, suggesting that the disproportionally high harvest rate of faster growing red grouper may select for the heritable trait for slow growth.
Total stock abundance averaged 27.4 million fish and varied with little trend between 1986 and 1999. However, abundance jumped sharply in 2000 to 39.5 million fish as the strong 1999-year class entered the estimated population at age 1 (SEDAR 12 Update 2009). Total abundance tapered off gradually thereafter to the terminal estimate of 31.2 million fish in 2008 (SEDAR 12 Update 2009). An analysis of stock recruitment and abundance-at-age data from 1986 to 2005 indicated a maturing stock primarily consisting of individuals approximately 10 years old, while older individuals declined in abundance from 1986 to the mid-1990s (SEDAR 12 Addendum 1 2007). Spawning stock is measured as total female gonad weight. Estimated spawning stock gradually improved over the assessment period, from an average of 460 metric tons (1 metric ton = 1.102 U.S. short ton) of eggs in the late 1980s to an average of almost 680 metric tons in the last few years, which included the observed high of 713 metric tons of eggs in 2008 (SEDAR 12 Update 2009). Estimated recruitment at age 1 indicated two notably strong year classes (1996 and 1999), while exhibiting a slightly increasing trend from 1986 to 2005. Recruitment over those years averaged 9.7 million fish, with peak values of 13.2 million in 1997 and 21.1 million in 2000 (SEDAR 12 Update 2009).
Both the 2006 and 2009 updated stock assessments concluded that Gulf of Mexico red grouper stocks were neither overfished nor experiencing overfishing and almost approached OY based on data through 2005 and 2008 (Table 9.14) (SEDAR 12 2006; SEDAR 12 Update 2009). However, the 2009 red grouper stock assessment did indicate a stock decline since 2005, but an episodic 20 % stock mortality event was attributed as the primary source for the decline in concurrence with typical fishing and natural mortality (GMFMC 2011). Successful management and the 50 % U.S. harvest reduction in the last 55 years have encouraged rebounding stocks and allowed the GMFMC to set the 2011 Total Allowable Catch (TAC) at 5.68 million lb gutted weight based on March 2010 projections (GMFMC 2011).
The large variability in the spatial distribution of the red grouper stock within the Gulf of Mexico due to the distribution of suitable habitats, larval transportation patterns, and lack of movement must be considered for these results. Furthermore, both fishery-dependent and fishery-independent monitoring programs clearly have shown that red grouper in the Gulf are characterized by periodic strong year classes, the latest being 1996, 1999, and possibly 2002 (DeVries et al. 2006; SEDAR 12 Update 2009). Understanding the red grouper’s unique life history and continued landings monitoring are critical to management towards OY of this ecologically, socially, and economically important Gulf of Mexico stock.
9.3.4 Atlantic Bluefin Tuna (Thunnus thynnus) 
The Atlantic bluefin tuna is the largest member of the family Scombridae (mackerels and tunas); fishes in this family are generally predators in pelagic ecosystems, are fast swimming, and are some of the most important and familiar food and sport fish species (Figure 9.19). Atlantic bluefin tuna are highly migratory and experience large-scale, transoceanic movements between foraging and spawning grounds over a wide range of pelagic environments from warm tropical to subpolar waters of the North Atlantic Ocean (Figure 9.20) (Mather et al. 1995; Collette et al. 2001; Fromentin and Powers 2005), and the northern Gulf of Mexico is one of the spawning locations of Atlantic bluefin tuna (Table 9.15). Based on genetic and tagging studies, two separate stocks are defined with their separate spawning grounds in the Gulf of Mexico (western stock) and Mediterranean Sea (eastern stock), respectively (Block et al. 2005; Boustany et al. 2007; Carlsson et al. 2007). Information for the western stock or western Atlantic population of bluefin tuna is summarized in the tables and text that follow (Tables 9.15, 9.16, and 9.17).
Atlantic bluefin tuna (Thunnus thynnus) in a net (from DeepAqua 2010)
Range of the Atlantic bluefin tuna (Thunnus thynnus) (modified from Maguire et al. 2006)
9.3.4.1 Key Life-History Processes and Ecology
The western Atlantic bluefin tuna stock, with the Gulf of Mexico as its main spawning grounds, is much smaller than the eastern Atlantic bluefin tuna stock; in addition, its spawning stock biomass has declined by over 90 % in the last 30 years (ICCAT 2012a). The International Commission for the Conservation of Atlantic Tunas (ICCAT) is responsible for the assessment and management of the two Atlantic bluefin tuna stocks. Because the focus of this chapter is the Gulf of Mexico, the western Atlantic bluefin tuna stock has been selected for evaluation (Figure 9.20 and Table 9.17).
The timing and distance traveled to spawning grounds varies among spawning adults of different origins in the eastern and western Atlantic. Individuals of western stock origin move directly from foraging grounds in the western and central North Atlantic to the Gulf of Mexico in the late winter and early spring. Ovaries of western Atlantic bluefin tuna are well developed in April and May (Baglin 1982), and spawning occurs from mid- to late May (Brothers et al. 1983). Most individuals are present on the spawning grounds from March to early July, but the spawning period varies (Table 9.15). A fraction of the stock moves into the highly productive waters of the Gulf of Maine, Scotian Shelf, central North Atlantic, and east of the Flemish Cap before returning to the Gulf of Mexico to spawn. Evidence supports site fidelity to natal areas for fish after reaching reproductive maturity.
Spawning in the Gulf of Mexico occurs in the northern areas, primarily in waters west of the Loop Current in the northern slope waters from 85 and 95°W (Table 9.15) (Block et al. 2005; Teo et al. 2007b). The location and intensity of spawning is influenced by the spatial and temporal variability in the location of major oceanographic features (fronts) and environmental conditions (e.g., waters with sea-surface temperatures above the 24 °C threshold). Thus, changes in the location of the Loop Current from year to year lead to changes in the distribution of bluefin tuna eggs and larvae in the Gulf of Mexico.
Western Atlantic bluefin tuna exhibit distinct behaviors during the three phases (entry, breeding, and exit phases) on their spawning grounds with changes in diving time, depths, and thermal biology (Block et al. 2001a; Teo et al. 2007b). As the bluefin tuna enter and exit the Gulf of Mexico, they tend to dive to significantly deeper daily maximum depths (>500 m or >1,640 ft) and exhibit directed movement paths going to and leaving spawning areas. In the breeding phase, which lasts for about 20 days (Block et al. 2001a; Teo et al. 2007b), the fish exhibit significantly shallower daily maximum depths, perform shallow oscillatory dives, and have movement paths that are significantly more residential and sinuous (Teo et al. 2007b).
High concentrations of Atlantic bluefin tuna larvae have been found in a broad region of the northern Gulf of Mexico, with peaks near the continental shelf break (e.g., 26–28°N latitude, 85–94°W longitude) (Richards 1976, 1997; Turner et al. 1996; Nishida et al. 1998). Atlantic bluefin tuna larvae also occur from the southern Gulf of Mexico to the Yucatán Channel (Richards and Potthoff 1980; McGowan and Richards 1986) and from the Straits of Florida to the Bahamas (Brothers et al. 1983; McGowan and Richards 1989).
Juveniles leave spawning grounds in the Gulf of Mexico in June to begin migration to nursery areas located off the North Carolina and Massachusetts coasts from Cape Hatteras to Cape Cod in waters over the continental shelf (Table 9.16). From June to March, adults inhabit feeding grounds in the central and northern Atlantic (Table 9.16).
The vertical distribution of western Atlantic bluefin tuna is often influenced by their feeding behavior and thermal biology. Atlantic bluefin tuna spend a considerable amount of time in the upper mixed layer, particularly on the inner continental shelf, where diving depths are limited by the bathymetry (Block et al. 2001b). Feeding in the mixed layer above the thermocline is common for both tropical and temperate tunas, and vertical use patterns may vary temporally as a function of shifts in prey distribution (Musyl et al. 2003; Kitagawa et al. 2006). Although Atlantic bluefin tuna spend most of their time in waters shallower than 200 m (656 ft), they are capable of diving to 1,000 m (3,281 ft) when in offshore waters (Block et al. 2001b; Stokesbury et al. 2004; De Metrio et al. 2005). The frequency of deep dives tends to be greatest for Atlantic bluefin tuna when they occur in the warm Gulf of Mexico waters (Block et al. 2001b; Teo et al. 2007b). Because Atlantic bluefin tuna are endothermic and can be thermally stressed in the warm Gulf of Mexico waters, the frequency of deep dives beneath the thermocline in the Gulf of Mexico may result from their efforts to avoid overheating (Block et al. 2005).
Bluefin tuna are oviparous (producing eggs that develop and hatch outside the maternal body), iteroparous (producing offspring several times over many seasons), and are multiple batch spawners (Schaefer 2001). The number of eggs produced is dependent on the size of the fish. Fertilization takes place directly in the water column (Fromentin 2009), and hatching occurs after 2 days. Atlantic bluefin tuna larvae are pelagic and reabsorb the yolk sac within a few days (Fromentin and Powers 2005).
Juvenile bluefin tuna grow rapidly. Growth tends to be linear during the larval phase (2–10 days) at a rate of 0.3–0.4 mm (0.012–0.016 in.)/day (Scott et al. 1993), similar to those reported for other tuna species from temperate and tropical regions, e.g., Pacific bluefin tuna (Thunnus orientalis), 0.33 mm (0.013 in.)/day (Miyashita et al. 2001); yellowfin tuna (Thunnus albacores), 0.47 mm (0.018 in.)/day (Lang et al. 1994); and southern bluefin tuna (Thunnus maccoyii), 0.28–0.36 mm (0.11–0.14 in.)/day (Jenkins and Davis 1990; Jenkins et al. 1991). A growth rate of 1.4 mm (0.06 in.)/day was reported for juveniles in the western Atlantic (267–413 mm or 10.5–16.3 in FL; from 70 to 200 days) (Brothers et al. 1983).
The mean observed length of Atlantic bluefin tuna at ages 1 and 2 in the western Atlantic was 53 and 75 cm (20.9 and 29.5 in.) FL, respectively (Atlantic Bluefin Tuna Status Review 2011). Estimated lengths of Atlantic bluefin tuna at ages 4 and 5 were 118 cm (46.5 in.) and 139 cm (54.7 in.) FL, respectively. Growth trajectories of Atlantic bluefin tuna are similar for young fish (ages 1–5) between eastern and western Atlantic stocks but diverge for older individuals, with size at age being greater for the western Atlantic stock. After age 5, growth trajectories of Atlantic bluefin tuna show marked differences between the eastern and western Atlantic, with the length at age being greater in the western Atlantic than in the eastern Atlantic (Atlantic Bluefin Tuna Status Review 2011). For example, at age 10, mean size in the western Atlantic was 212 cm (83.5 in.) FL, compared to 200 cm (78.7 in.) FL for the eastern Atlantic bluefin tuna. The general trend of greater length at age in the western Atlantic is exhibited in the growth models used for ICCAT assessments in the east (Cort 1991) and west (Turner and Restrepo 1994).
The western spawning stock in the Gulf of Mexico is comprised of large, late-maturing individuals. The estimated age at maturity ranges from 7 to 12 years, with the most commonly used age and size at maturity for the Gulf of Mexico Atlantic bluefin tuna being age 10 and 200 cm (78.7 in.) curved fork length (CFL), the measurement of the length of a tuna taken in a line tracing the contour of the body from the tip of the upper jaw to the fork of the tail, which abuts the upper side of the pectoral fin and the upper side of the caudal keel (FishBase 2013) (Table 9.15). However, Atlantic bluefin tuna reach sexual maturity as early as age 3 or 4 in the eastern Atlantic. Sex-specific differences in growth occur, with males growing slightly faster than females and reaching slightly larger sizes by age 10. Bluefin tuna are a long-lived species, with a life span of about 40 years.
The M of Atlantic bluefin tuna during early life-history stages mainly results from starvation and predation. Daily mortality during the larval stage has been estimated at 0.20 per day for the western stock. This estimate is lower than values reported for more tropical tunas during comparable periods: yellowfin tuna (M = 0.33 per day; Lang et al. 1994) and southern bluefin tuna (M = 0.66 per day; Davis 1991). The mortality of tunas during the juvenile phase is largely a function of size or age rather than species or habitat (Hampton 2000). In the most recent stock assessment, the M rate has been set at 0.14 per year and assumed to be age-independent (NMFS 2012b).
Large uncertainty is associated with the recruitment dynamics estimated in the most recent stock assessment (NMFS 2012a). Two levels of recruitment dynamics were considered in the stock assessment, low and high productivity. These levels could yield very different conclusions about the status of the Atlantic bluefin tuna stock and fishery.
Seasonal differences in growth occur for Atlantic bluefin tuna. The existence of a slowdown in growth during the winter has been confirmed for both juveniles (Mather and Schuck 1960; Furnestin and Dardignac 1962; Cort 1991) and adults (Tiews 1963; Butler et al. 1977). Large differences in growth, maturation, stock structure, and movement have been identified between the eastern and western Atlantic bluefin tuna. Genetic differentiation and natal homing behavior, observed in genetic and archival tagging studies, provide strong evidence for independence of the Gulf of Mexico and Mediterranean Sea Atlantic bluefin tuna stocks (Block et al. 2005; Carlsson et al. 2007; Boustany et al. 2008).
The stock structure is complicated because some fraction of the stock undertakes trans-Atlantic migration annually and/or ontogenetically, but migrants return to their natal sites to spawn. Although resident subpopulations exist in the eastern Atlantic bluefin tuna stock (De Metrio et al. 2005), there is no strong evidence for subpopulations in the western Atlantic bluefin tuna stock.
9.3.4.2 Predators and Prey
Atlantic bluefin tuna are opportunistic feeders and consume a wide variety of prey. As larvae and small juveniles, their diet is comprised primarily of zooplankton, with copepods as the main stomach item (Table 9.15) (Uotani et al. 1981, 1990). Their larvae are capable of feeding on other fish larvae (Miyashita et al. 2001). The diet of older juveniles and adults consists mainly of fishes, cephalopods (mostly squid), and crustaceans (Table 9.15).
Demersal fishes and invertebrates are also found in the stomachs of Atlantic bluefin tuna, especially for those that inhabit nearshore environments. Although no single taxon dominates, as a group, demersal organisms may comprise as much as 20 % of the stomach contents by number (Chase 2002). Large Atlantic bluefin tuna (e.g., >230 cm or >90.5 in CFL) may consume large individual prey items, such as bluefish (Pomatomus saltatrix) and spiny dogfish (Squalus acanthias) (Table 9.15) (Matthews et al. 1977; Chase 2002). The trophic level of adult Atlantic bluefin tuna is one level higher than those of other congeners. Predators of Atlantic bluefin tuna include swordfish, sharks, and whales (Table 9.15).
9.3.4.3 Key Habitat Needs and Distribution
Oceanographic conditions appear important for bluefin tuna spawning, and the actual location of spawning within each basin likely represents a balance between habitat requirements of larvae and the physiological limitations of adults. Key variables include bathymetry, sea surface temperature, eddy kinetic energy, surface chlorophyll concentration, and surface wind speed; sea surface temperature is the most important parameter. The sea surface temperatures reported for Atlantic bluefin tuna on putative spawning grounds in the Gulf of Mexico ranged from approximately 22.6–27.5 °C (Teo et al. 2007b). Because the northern slope waters of the Gulf of Mexico are above the purported 24 °C spawning threshold in early spring (Block et al. 2001a, b, 2005; Teo et al. 2007b), it is not surprising that Atlantic bluefin tuna begin spawning earlier in the Gulf of Mexico. In a study by Teo et al. (2007b), Atlantic bluefin tuna exhibited significant preference for areas with continental slope waters (2,800–3,400 m or 9,186–11,155 ft), moderate sea surface temperatures (24–25 and 26–27 °C), moderate eddy kinetic energy (251–355 cm2/s2), low surface chlorophyll concentrations (0.10–0.16 mg/m3), and moderate wind speeds (6–7 and 9–9.5 m/s or 19.7–22.9 and 29.5–31.2 ft/s).
Temperature and depth are important factors influencing the distribution of Atlantic bluefin tuna in different life-history stages (Table 9.16). Essential fish habitat has been designated for different life-stages of Atlantic bluefin tuna, including eggs, larvae, juveniles, adults, and spawning adults (Table 9.16; Figures 9.21, 9.22, 9.23, and 9.24). In addition, a Habitat Area of Particular Concern has been designated for bluefin tuna (Figure 9.25).
Essential fish habitat for adult Atlantic bluefin tuna (Thunnus thynnus) (from NOAA Fisheries Office of Sustainable Fisheries 2009a)
Essential fish habitat for juvenile Atlantic bluefin tuna (Thunnus thynnus) (from NOAA Fisheries Office of Sustainable Fisheries 2009a)
Essential fish habitat for spawning, eggs, and larval Atlantic bluefin tuna (Thunnus thynnus) (from NOAA Fisheries Office of Sustainable Fisheries 2009a)
Essential fish habitat for all lifestages of Atlantic bluefin tuna (Thunnus thynnus) (from NOAA Fisheries Office of Sustainable Fisheries 2009a)
Highly migratory species habitat area of particular concern for Atlantic bluefin tuna (Thunnus thynnus) (from NOAA Fisheries Office of Sustainable Fisheries 2009a)
9.3.4.4 Fisheries
Atlantic bluefin tuna are very valuable and highly prized; they support an important commercial and recreational fishery in the United States. The total catch for western Atlantic bluefin tuna peaked at 18,671 metric tons in 1964 as a result of the Japanese longline fishery for large fish off Brazil and the U.S. purse seine fishery for juvenile fish (NMFS 2012b). Landings dropped sharply thereafter with the collapse of these two fisheries, but increased again to average over 5,000 metric tons (11 million lb) in the 1970s due to the expansion of the Japanese longline fleet into the Northwest Atlantic and Gulf of Mexico and increased efforts in the purse seine fishery targeting larger fish for the sashimi market. The total catch for western Atlantic bluefin tuna, including discards, has generally been relatively stable since 1982 due to the imposition of quotas (Figure 9.26) (NMFS 2012b). Recent changes in landings mainly result from annual changes in the catch quota. The decline through 2007 was primarily due to considerable reductions in catch levels for U.S. fisheries. The majority of the western Atlantic bluefin tuna catch in recent years is from the commercial longline and sport fisheries (Figure 9.26).
Landed and discarded catch of the western Atlantic bluefin tuna (Thunnus thynnus) stock for different gears from 1987 through 2009 (data from ICCAT 2012a)
Atlantic bluefin tuna are managed domestically by the NMFS’s Highly Migratory Species Management Division (HMSMD) and internationally by the ICCAT (Table 9.17). The spawning stock biomass of the western Atlantic bluefin tuna has declined substantially over the past few decades and is at a very low level despite more than 20 years of strict regulations on the western Atlantic bluefin tuna fishery (NMFS 2012b).
Large uncertainty is associated with the most recent Atlantic bluefin tuna stock assessment, in particular with the estimated recruitment. The status of the population and fishery are dependent on the assumptions made on recruitment dynamics. For the high productivity scenario, the western Atlantic bluefin tuna stock is considered overfished (e.g., population level is too low) and the fishery is in the status of overfishing (e.g., fishing mortality is too high) (Table 9.17) (NMFS 2012a). However, for the low productivity scenario, the western Atlantic bluefin tuna stock is not overfished and the fishery is not in the status of overfishing (NMFS 2012a). Because of the limited information available, it is not clear which scenario more realistically describes the dynamics of Atlantic bluefin tuna recruitment.
Despite the uncertainty in the stock assessment, the stock biomass of the western Atlantic bluefin tuna has decreased greatly since the 1970s, mainly as a result of overfishing (NMFS 2012a). Overfishing over the last several decades has greatly reduced the spawning stock biomass and stock reproductive potential, likely resulting in poor recruitment and current low stock biomass of the Atlantic bluefin tuna. However, the western Atlantic bluefin tuna stock appears to be stable or even slightly increasing over the last 10 years, perhaps resulting from conservation measures and regulations (NMFS 2012a).
9.3.5 Atlantic Blue Marlin (Makaira nigricans)
The Atlantic blue marlin, a species of marlin endemic to the Atlantic Ocean, is widely distributed throughout the tropical and temperate waters of the Atlantic Ocean and Gulf of Mexico and is considered to be a single stock in the Atlantic Ocean (Figure 9.27). The Atlantic blue marlin is an apex predator and is considered a highly prized species in sport fisheries in the Gulf of Mexico. Recent stock assessments of Atlantic blue marlin by the ICCAT suggest that stocks are well below the level to support the MSY. Because of its economic and ecological importance, the Atlantic blue marlin was selected as a representative species to be evaluated in this chapter.
Range of the Atlantic blue marlin (Makaira nigricans) (modified from Maguire et al. 2006)
9.3.5.1 Key Life-History Processes and Ecology
As an apex predator, the Atlantic blue marlin plays a critical role in the ocean ecosystem (ICCAT 2012b). The Atlantic blue marlin is the most tropical of the billfishes and is a blue water fish that spends most of its life in the open sea (Tables 9.18 and 9.19). They rarely aggregate in schools and are usually found as scattered single individuals. Their distributional areas range from about latitude 45°N to about latitude 35°S (Table 9.20). Blue marlin are less abundant in the eastern Atlantic, where they mostly occur off Africa between the latitudes of 25°N and 25°S (NMFS 2009b).
The distribution and movement patterns of Atlantic blue marlin within the Gulf of Mexico tend to vary among individuals. Some may spend considerable time within the Gulf of Mexico for feeding and spawning, while others move seasonally between the Gulf of Mexico and tropical areas, such as the Bahamas. A tagging study with pop-up archival transmitting tags in the Gulf of Mexico suggested that most tagged fish remained in the Gulf of Mexico, with some fish exhibiting egress into Belize (Caribbean Sea) and the U.S. Virgin Islands (Kraus et al. 2011). However, tagged fish showed highly variable movement patterns, regardless of tagging location, season, or egress status. Seasonal changes in distribution suggested a north–south cyclical movement pattern within the Gulf of Mexico that supported a new perspective on Atlantic blue marlin, in which the Gulf of Mexico provides suitable year-round habitat that is utilized by a subset of the Atlantic population. An analysis of otolith chemistry of Atlantic blue marlin also suggested that movement out of the Gulf of Mexico for Atlantic blue marlin may be more limited, as compared to other regions (Wells et al. 2010).
Atlantic blue marlin in the Gulf of Mexico tend to remain in offshore waters (Table 9.19). However, they may move close to the coast from July to September. They spawn in the Gulf of Mexico as early as May and continue to spawn throughout the summer (Table 9.18). Atlantic blue marlin that spawn on spawning grounds off Texas and Louisiana during the summer remain in the Gulf through the fall and winter. Blue marlin tag/recapture data from the Gulf of Mexico indicate that seasonal movements may occur between the Gulf of Mexico (summer) and the Bahamas (winter). Several data sources indicate that the Gulf of Mexico may serve as important spawning and/or nursery habitat for blue marlin (Brown-Peterson et al. 2008; Rooker et al. 2012). Blue marlin larvae were found in a 2005 fishery-independent survey in the areas from 27 to 28°N to 90 to 94°W in July (Rooker et al. 2012). This seems to suggest that blue marlin can spawn in the northern Gulf of Mexico (Brown-Peterson et al. 2008; Kraus et al. 2011). However, larvae mainly are present near the western margin of Loop Current on the continental shelf in relatively warm waters (>27 °C). The presence of young blue marlin larvae along the boundary of the Loop Current may be a result of transport from Caribbean/Straits of Florida spawning events (Kraus et al. 2011; Rooker et al. 2012). Because no spawning-capable adults have been captured in this region, it is unlikely that blue marlin spawn in the Loop Current in the northeastern Gulf of Mexico (Brown-Peterson et al. 2008). Strong histological evidence supports the lack of spawning in the northern Gulf of Mexico east of the Mississippi River, which is augmented by the failure to capture blue marlin larvae in areas not associated with the Loop Current (Kraus and Rooker 2007; Kraus et al. 2011; Rooker et al. 2012). Thus, the likelihood of blue marlin spawning in the northern Gulf of Mexico is slim, although the northern Gulf of Mexico supports an active recreational fishery for blue marlin from May through September (Brown-Peterson et al. 2008).
Limited published information is available on blue marlin biology and life history from the Gulf of Mexico (Table 9.18) (De Sylva et al. 2000). Females are batch spawners and can spawn as many as four times in a spawning season (Brown-Peterson et al. 2004). They often release more than seven million eggs at once, each approximately 1 mm (0.04 in.) in diameter (Brown-Peterson et al. 2008). The larvae may grow as much as 16 mm (0.63 in.) in a day (Brown-Peterson et al. 2008). Males may live for 18 years and females up to 27 years. Females can grow up to four times the weight of males (Wilson et al. 1991; ICCAT 1997).
The M estimated using the Hoenig method (Hoenig 1983) at a maximum age of 30 years is 0.14 (Hill et al. 1989), which was used in the most recent stock assessment. The estimated blue marlin recruitment fluctuates over time and has been low in recent years (Figure 9.28). However, there is great uncertainty associated with the estimated recruitment, which results mainly from uncertainty in the quality of fishery and biological data, as well as the assumed stock structure and population dynamics.
Temporal variability in Atlantic blue marlin (Makaira nigricans) recruitment estimated with the fully integrated stock assessment model (redrawn from Figure 30, ICCAT 2012b)
Given the large distributional area that the Atlantic blue marlin occupies and the existence of multiple spawning grounds, blue marlin in different areas may be subject to different environmental stressors and prey availability. This may result in large spatial variability in key life-history parameters, such as growth and maturation.
Although there is evidence indicating that some Atlantic blue marlin may be able to complete most of their life cycle from spawning to feeding within the Gulf of Mexico, many studies suggest that Atlantic blue marlin larvae are not produced within the Gulf of Mexico; rather, they are transported via the Loop Current from tropical areas. The evidence for the existence of multiple spawning grounds suggests that the stock structure may be more complicated than a one-unit stock assumed in the stock assessment. More evidence is needed to test the hypothesis that the Gulf of Mexico provides suitable year-round habitat that is utilized by a subset of the Atlantic blue marlin population (e.g., existence of a substock of Atlantic blue marlin in the Gulf of Mexico), given the uncertainty regarding whether the Atlantic blue marlin larvae come from within the Gulf of Mexico or originate in Caribbean waters.
9.3.5.2 Predators and Prey
Atlantic blue marlin larvae feed on a variety of zooplankton, along with drifting fish eggs and other larvae. Juvenile and adult Atlantic blue marlin typically feed near the surface but sometimes travel to great depths in search of prey, and feed opportunistically on a wide variety of fish and invertebrates (Table 9.18). Blue marlin have been documented to take prey as large as white marlin, yellowfin, and bigeye tuna in the 45 kg (100 lb) range and are also capable of feeding on small but numerous prey, such as filefish and snipefish. The Atlantic blue marlin has few predators apart from humans (McEachran and Fechhelm 2005).
9.3.5.3 Key Habitat Needs and Distribution
Atlantic blue marlin usually inhabit waters warmer than 24 °C, but have been found at surface water temperatures as high as 30.5 °C and as low as 21.7 °C (Table 9.19). Because Atlantic blue marlin prefers blue water, the clarity of water is also an important factor influencing its distribution (NMFS 2009b). Essential fish habitat has been designated for eggs, larvae, juveniles, adults, and spawning adults of Atlantic blue marlin (Table 9.19; Figures 9.29, 9.30, 9.31, 9.32).
Essential fish habitat for adult Atlantic blue marlin (Makaira nigricans) (from NOAA Fisheries Office of Sustainable Fisheries 2009b)
Essential fish habitat for juvenile Atlantic blue marlin (Makaira nigricans) (from NOAA Fisheries Office of Sustainable Fisheries 2009b)
Essential fish habitat for spawning, eggs, and larval Atlantic blue marlin (Makaira nigricans) (from NOAA Fisheries Office of Sustainable Fisheries 2009b)
Essential fish habitat for all lifestages of Atlantic blue marlin (Makaira nigricans) (from NOAA Fisheries Office of Sustainable Fisheries 2009b)
9.3.5.4 Fisheries
Because of their relative rarity, beauty, and sporting qualities, Atlantic blue marlin are considered one of the most prestigious catches in recreational fisheries, and they support a multi-million dollar industry that includes hundreds of companies and thousands of jobs for boat operators, boat builders, marinas, dealerships, and fishing tackle manufacturers and dealers in the Gulf of Mexico region. The Atlantic blue marlin catch increased abruptly in the early 1960s nearing 9,000 metric tons, but dropped quickly. The catch has been quite stable since the late 1960s, varying between 2,000 and 5,000 metric tons for most years during this time period (Figure 9.33).
Landed and discarded catch of the Atlantic blue marlin (Makaira nigricans) from 1956 through 2009 (data from ICCAT 2012b)
The management of Atlantic blue marlin is subject to domestic and international regulations (Table 9.20). The current Atlantic blue marlin stock assessment indicates that the stock level was low in 2009, fishing mortality was high, and the catch level of 3,431 metric tons in 2010 would likely result in continuing stock decline. A rebuilding plan needs to be developed for the stock of Atlantic blue marlin to reduce the annual total catch below 2,000 metric tons to allow the stock to increase. The Atlantic blue marlin population declined greatly during the last century (Figure 9.34); overfishing is currently occurring, and the stock is overfished (Table 9.20). The International Union for the Conservation of Nature currently considers it a threatened species due to overfishing and the substantially reduced stock abundance.
Temporal variability in Atlantic blue marlin (Makaira nigricans) spawning stock biomass estimated with the fully integrated stock assessment model (redrawn from Figure 29, ICCAT 2012b)
The spawning stock biomass of Atlantic blue marlin has decreased greatly since the 1960s (Figure 9.34). The recent stock biomass is approximately 25 % of the biomass that existed in the 1950s. However, there is large uncertainty associated with the estimates. This uncertainty mainly results from uncertainty in the quality of fishery and biological data, as well as the assumed stock structure and population dynamics.
9.3.6 Atlantic Swordfish (Xiphias gladius) 
The Atlantic swordfish is a highly migratory and circumglobal species; it is widely distributed in the Atlantic Ocean, including tropical, temperate, and some cold water regions from 50°N to 45°S in the western Atlantic and 60°N to 50°S in the eastern Atlantic (Figure 9.35 and Table 9.21) (Palko et al. 1981; Nakamura 1985; NMFS 2009b). Currently, the ICCAT considers the existence of three distinct management units: North Atlantic, South Atlantic, and Mediterranean Sea. The North Atlantic stock is separated from the South Atlantic stock at 5°N. The results of biological (Tserpes and Tsimenides 1995), genetic (Chow and Takeyama 2000; Kasapidis et al. 2007), and tagging (García-Cortés et al. 2003; Neilson et al. 2007) studies clearly supported this delineation of population structure, although intermixing among the three stocks was found in some studies (Alvarado-Bremer et al. 2007). The North Atlantic stock of the Atlantic swordfish was evaluated because it is an apex predator that plays an important role in its marine ecosystems, it supports an important fishery in the United States, and the Gulf of Mexico is an important Atlantic swordfish nursery, feeding, and spawning ground.
Range of the Atlantic swordfish (Xiphias gladius) (modified from Maguire et al. 2006)
9.3.6.1 Key Life-History Processes and Ecology
Because swordfish are difficult to age, there is a lot of uncertainty about some of their basic life-history processes, such as growth and maturation (Table 9.22). In general, juvenile swordfish grow rapidly, reaching about 140 cm (55.1 in.) lower-jaw fork length [LJFL, which is from the tip of the lower jaw to the fork in the tail (FishBase 2013)] by age 3. The growth rate decreases after age 3, perhaps as a result of maturation. There is sexual dimorphism, with females growing faster and reaching larger maximum sizes than males (Table 9.22). Tagging studies have shown that some swordfish can live up to 15 years.
Juvenile Atlantic swordfish of the North Atlantic stock occur year-round in the Gulf of Mexico, the Florida Atlantic coast, and waters near the Charleston Bump (Table 9.23) (Palko et al. 1981; Cramer and Scott 1998). Adult Atlantic swordfish tend to concentrate along boundary currents of the Gulf Stream and the Gulf of Mexico Loop Current (Table 9.23). They are subject to seasonal movement: one group moves northeastward along the U.S. continental shelf in summer and returns southwestward in autumn, and another group moves from deepwater westward toward the continental shelf in summer and back into deepwater in autumn (Palko et al. 1981; Arocha 1997; NMFS 2009b).
Atlantic swordfish tend to move to warmer waters for spawning and cooler waters for feeding. They tend to migrate to the preferred temperatures or areas for spawning during the peak of a spawning season (Palko et al. 1981; Tserpes et al. 2008). Atlantic swordfish appear to spawn throughout the year, and spawning timing tends to vary among different spawning areas (Tables 9.21 and 9.22). Seasonal latitudinal migrations of swordfish, which may result from seasonal changes in sea surface temperature, are well documented (Nakamura 1985; Seki et al. 2002; Takahashi et al. 2003; Neilson et al. 2009).
Although Atlantic swordfish have evolved a specialized muscle that functions like a brain heater and enables them to tolerate a wide range of temperatures and move rapidly between warm surface waters and cold waters at great depths (Carey 1990), their vertical distribution is generally limited by the depth of the thermocline (Block et al. 1992). Takahashi et al. (2003) also indicated that the vertical swimming behavior of swordfish changes in response to near-surface water temperatures.
Limited information is available on the M of the Atlantic swordfish. In the assessment based on the results of the virtual population analysis (VPA) model, M was assumed to be 0.2 per year (Scott and Porch 2007). However, no information or evidence is presented to justify the choice of this value.
9.3.6.2 Predators and Prey
Atlantic swordfish are diurnal feeders rising close to the mixed surface layer at night and descending to deeper waters during the day to feed on pelagic fishes and squids (Carey 1990). Swordfish mainly feed on prey concentrations associated with vertical density discontinuities (Carey and Robison 1981), such as the thermocline (Draganik and Cholyst 1988). Juvenile and adult Atlantic swordfish predate on squids, tunas, dolphinfishes, mackerels, and pelagic crustaceans (Table 9.21). Sperm whales, killer whales, and large sharks prey on swordfish (Table 9.21).
9.3.6.3 Key Habitat Needs and Distribution
Oceanographic variables that may influence the distribution and abundance of Atlantic swordfish include sea surface temperature; depth of the thermocline (Carey 1990); sea surface height anomaly, which is a good indicator of possible oceanographic activities, such as gyres and eddies (Seki et al. 2002; Tserpes et al. 2008); existence of thermal fronts, frontal zones, and eddy fields that can produce locally elevated chlorophyll concentrations and zooplankton abundance that stimulate feeding conditions (Podestá et al. 1993; Logerwell and Smith 2001); and chlorophyll concentrations, which regulate the distribution and abundance of the prey of swordfish (Tserpes et al. 2008; Yáñez et al. 2009). The spatial distribution and abundance of swordfish also may be determined by other factors, such as distinct bathymetric features. Many studies have indicated that the distribution of swordfish is also associated with bottom topographic structures and thermal fronts, such as submarine canyons or hummocky bumps (Carey and Robison 1981; Carey 1990; Podestá et al. 1993; Sedberry and Loefer 2001; Damalas et al. 2007). The average temperature preferred by swordfish during the day can be as low as 10 °C, while it is 28 °C at night when they move up to the near-surface waters (Sedberry and Loefer 2001).
The spatial distributions of essential fish habitat that have been designated for various lifestages of Atlantic swordfish in the Gulf of Mexico, along the U.S. east coast, and around Puerto Rico are shown in Figures 9.36, 9.37, 9.38, and 9.39. Table 9.22 includes the definitions of essential fish habitat that have been established for juvenile, adult, and spawning adult Atlantic swordfish.
Essential fish habitat for adult Atlantic swordfish (Xiphias gladius) (from NOAA Fisheries Office of Sustainable Fisheries 2009c)
Essential fish habitat for juvenile Atlantic swordfish (Xiphias gladius) (from NOAA Fisheries Office of Sustainable Fisheries 2009c)
Essential fish habitat for spawning, eggs, and larval Atlantic swordfish (Xiphias gladius) (from NOAA Fisheries Office of Sustainable Fisheries 2009c)
Essential fish habitat for all lifestages of Atlantic swordfish (Xiphias gladius) (from NOAA Fisheries Office of Sustainable Fisheries 2009c)
9.3.6.4 Fisheries
Atlantic swordfish support an important commercial and recreational fishery in the United States, including the Gulf of Mexico (NMFS 2012a). Canada, Spain, and the United States have operated a targeted pelagic longline Atlantic swordfish fishery since the late 1950s or early 1960s in the North Atlantic (NMFS 2009a, 2012a). The harpoon fisheries have existed at least since the late 1800s in the Northwest Atlantic Ocean. In addition, some driftnet activities for swordfish occur around the Straits of Gibraltar area and in other Atlantic areas (e.g., off the coast of West Africa). The primary fisheries that take swordfish as bycatch are tuna fishing fleets from Taiwan, Japan, Korea, and France (Collette et al. 2012). The tuna longline fishery has operated throughout the Atlantic since 1956, with substantial catches of swordfish as bycatch in some years.
U.S. catches (landings plus dead discards) of swordfish peaked in 1990, with a total of 5,519 metric tons. Since then, U.S. catches have declined, with the lowest catches reported in 2006 (2,057 metric tons). Most (93 % in 2008) of the U.S. swordfish catches have been landed in the pelagic longline fishery operated throughout the western Atlantic, including the Gulf of Mexico and Caribbean Sea, that targeted both yellowfin tuna and swordfish (NMFS 2009a, 2012a).
The U.S. pelagic longline fleet has decreased substantially in size from about 400 active vessels in the 1990s to about 120 vessels in 2008 as a result of regulations, market conditions, and fuel prices. Atlantic swordfish also support a small recreational fishery, which currently lands only a small proportion of total U.S. landings (75 metric tons in 2008). This fishery has, however, expanded in the last few years and is projected to continue to grow (NMFS 2009a, 2012a).
The Atlantic swordfish fishery is managed both domestically and internationally (Table 9.23), and catch limits are one of the regulations used in managing the North Atlantic swordfish stock (NMFS 2010, 2011, 2012a). The total allowable catch (TAC) of 14,000 metric tons per year during the 2007–2009 period was reduced to 13,700 metric tons in 2010 and 12,836 metric tons in 2011. Minimum size limits are also used to manage the fishery. There are two minimum size options that are applied to the entire Atlantic: 125 cm (49.2 in.) LJFL with a 15 % tolerance or 119 cm (46.8 in.) LJFL with zero tolerance and evaluation of the discards (NMFS 2012a). A number of time/area closures went into effect in 2001 for the pelagic longline vessels operating within the U.S. Exclusive Economic Zone (EEZ). Two permanent closures, one in the Gulf of Mexico and the other along the Florida east coast, were established to reduce the bycatch of undersized swordfish. Circle hooks became mandatory for the U.S. pelagic longline fleet in 2004 to reduce mortality of discarded bycatch species, including swordfish (NMFS 2009a, 2012a).
The North Atlantic swordfish fishery is considered to be fully rebuilt, and overfishing is not occurring (Table 9.21) (NMFS 2012a). The latest Standing Committee on Research and Statistics stock assessment indicates that the North Atlantic swordfish stock has greater than 50 % probability to be at or above biomass at MSY (NMFS 2009a). The estimated relative biomass trend shows a consistent increase since 2000. Fishing mortality has been below fishing mortality at MSY since 2005; therefore, the rebuilding objective has been achieved. However, great uncertainty is associated with the stock assessment resulting from the quality of fisheries data and biological parameters (e.g., M and growth), suitability of stock assessment models, lack of understanding of some key life-history process, and assumed stock structure. More data are needed for improved understanding of key biotic and abiotic factors influencing the recruitment dynamics of the North Atlantic swordfish.
9.3.7 Atlantic Sailfish (Istiophorus albicans) 
The Atlantic sailfish is a pelagic-oceanic and highly migratory species (Figure 9.40). It is distributed in tropical and temperate waters about 40°N in the Northwest Atlantic, 50°N in the Northeast Atlantic, 40°S in the Southwest Atlantic, and 32°S in the Southeast Atlantic (Figure 9.41 and Table 9.24). Although the importance of sailfish in commercial fisheries is limited, this species plays an important role in recreational fisheries (Table 9.24). In addition, because it is so important in recreational fisheries, the Atlantic sailfish is the official saltwater fish of Florida. As one of the top predator species that are highly migratory and distributed widely, Atlantic sailfish play an important ecological role in Gulf of Mexico ecosystems. Key information for the Atlantic sailfish is summarized in Tables 9.24, 9.25, and 9.26 and discussed in detail in the following paragraphs.
Atlantic sailfish (Istiophorus albicans) feeding (from NaluPhoto 2012)
Range of the Atlantic sailfish (Istiophorus albicans) (modified from Maguire et al. 2006)
9.3.7.1 Key Life-History Processes and Ecology
Atlantic sailfish usually occur in the upper layers of warm water above the thermocline offshore (NMFS 2009b), but can also descend to deepwater and often migrate into nearshore shallow waters. They occasionally form schools or smaller groups of 3–30 individuals but more frequently appear in loose aggregations over a wide area. Atlantic sailfish are distributed throughout the Gulf of Mexico (Figure 9.41); they can be found year-round in the southern Gulf of Mexico but move into the northern Gulf only during the summer season (Table 9.26). No transatlantic or transequatorial movements have been documented using tag-recapture methods (Orbesen et al. 2010).
Juvenile and adult Atlantic sailfish spend winters in warm waters, often occurring in small schools, and spread out during the summer (Table 9.26). However, there appears to be a year-round Florida east coast population (Beardsley et al. 1975; Nakamura 1985; Bayley and Prince 1993; NMFS 2009b; Orbesen et al. 2010). Atlantic sailfish often move northward in early summer in the western Atlantic to engage in spawning activity (NMFS 2009b). Spawning can begin as early as April, but occurs mainly in summer (Table 9.25). Atlantic sailfish can spawn in various oceanographic conditions from offshore in deepwater to inshore shallow waters near the surface in the warm season (Tables 9.25 and 9.26). The Gulf of Mexico has been identified as an important and critical spawning ground for this species, and large concentrations of sailfish larvae have been found in the Gulf of Mexico during the summer, which suggests that July is the peak of the spawning season for Atlantic sailfish in the Gulf of Mexico (Simms 2009).
Atlantic sailfish grow fast, reaching 137 cm (53.9 in.) in length and 3 kg (6.6 lb) in weight in 6 months and 183 cm (72 in.) and 9 kg (19.8 lb), respectively, in just 1 year. Growth then slows down, and like other billfish, female sailfish grow to be larger than males (Table 9.25). A large female sailfish may release as many as 4.5 million eggs. The M tends to be high during early life-history stages but becomes relatively stable for juvenile sailfish (Luthy et al. 2005; Richardson et al. 2009).
Large variability exists in life-history parameters over the distributional areas of Atlantic sailfish (e.g., East Atlantic versus West Atlantic). However, the growth of juveniles was found to be almost uniform within the Gulf of Mexico (Simms 2009). More studies are needed to evaluate the possible spatial and temporal variability in key life-history parameters. The Atlantic sailfish within the Gulf of Mexico is considered part of the Western Atlantic stock (ICCAT 2010). While studies have indicated the presence of a year-round Florida east coast stock, it is not clear if the Gulf of Mexico has a year-round population.
9.3.7.2 Predators and Prey
Juvenile and adult Atlantic sailfish feed primarily on small pelagic fishes, such as tunas and jacks; they also feed on shrimps, cephalopods, and gastropods (Table 9.25). Feeding can occur at the surface and in mid-water, along reef edges, or along the sea floor. Atlantic sailfish predators include killer whales, bottlenose dolphin, and sharks (Table 9.25).
9.3.7.3 Key Habitat Needs and Distribution
Water temperature and, in some cases, wind conditions are important habitat variables influencing the distribution of Atlantic sailfish (Table 9.26). At the northern and southern extremes of their distribution, Atlantic sailfish occur only in the warmer months. The seasonal changes in distribution may be linked to prey migrations.
The rates of Atlantic sailfish bycatch in the pelagic longline fisheries are two times higher in the Gulf of Mexico than in other areas of the North Atlantic during the spawning season, from May through September (De Sylva and Breder 1997), suggesting that spawning biomass in the Gulf of Mexico tends to be higher than spawning biomass in other areas of the Atlantic. This suggests that the Gulf of Mexico provides an important spawning and larval habitat for Atlantic sailfish.
Essential fish habitat has been designated for juvenile, adult, and spawning Atlantic sailfish. These habitats are described in Table 9.26 and shown in Figures 9.42, 9.43, 9.44, and 9.45.
Essential fish habitat for adult Atlantic sailfish (Istiophorus albicans) (from NOAA Fisheries Office of Sustainable Fisheries 2009d)
Essential fish habitat for juvenile Atlantic sailfish (Istiophorus albicans) (from NOAA Fisheries Office of Sustainable Fisheries 2009d)
Essential fish habitat for spawning, eggs, and larval Atlantic sailfish (Istiophorus albicans) (from NOAA Fisheries Office of Sustainable Fisheries 2009d)
Essential fish habitat for all lifestages of Atlantic sailfish (Istiophorus albicans) (from NOAA Fisheries Office of Sustainable Fisheries 2009d)
9.3.7.4 Fisheries
The United States has historically landed a large quantity of Atlantic sailfish (Figure 9.46). Prior to the mid-1990s, the U.S. share of landings from the West Atlantic sailfish stock varied between 20 and 60 %, with an average of approximately 40 % (Figure 9.47). Beginning around 2000, landings in the United States and the U.S. share of landings dropped dramatically (Figures 9.46 and 9.47). This may reflect the fact that a targeted commercial fishery for Atlantic sailfish is prohibited in the Gulf of Mexico and other U.S. waters. The current Atlantic sailfish commercial catch is bycatch in pelagic longlines, which are commonly used in the Gulf of Mexico to target swordfish and yellowfin tuna.
Landings of the Atlantic sailfish (Istiophorus albicans) stock, West Atlantic sailfish (Istiophorus albicans) stock (WAS), and U.S. landings of the WAS from 1960 through 2007 (data from NMFS 2012b)
Proportion of the U.S. share of the total landings of the West Atlantic sailfish (Istiophorus albicans) stock from 1960 through 2007 (data from ICCAT 2011b)
Atlantic sailfish are subject to domestic and international management regulations (Table 9.24). For the West Atlantic sailfish stock, the most recent stock assessment suggests that overfishing is probably occurring, and the stock may be overfished (Table 9.24) (ICCAT 2011b). However, because of the large uncertainty associated with the data and stock structure, the results are not conclusive. The recent stock assessment (ICCAT 2011b) suggests that the West Atlantic stock suffered great declines in abundance prior to 1990. However, since 1990, different abundance indices tend to suggest conflicting trends, with some indicating declines and others indicating increases or no trends (ICCAT 2011b).
9.3.8 Red Drum (Sciaenops ocellatus) 
Red drum, an estuarine-dependent species, is widely distributed in various habitats throughout the Gulf of Mexico and plays an important ecological role in Gulf of Mexico coastal ecosystems (Figure 9.48) (Powers et al. 2012). In the Gulf of Mexico, red drum occur from northern Mexico into extreme Southwest Florida (Figure 9.49). The overall Gulf of Mexico stock was considered overfished, and overfishing was occurring in the early 2000s (Table 9.29). A harvest moratorium has been implemented on red drum in federal waters since 2007. Thus, there is currently no U.S. commercial fishery targeting this species. This fishing moratorium in federal waters is considered to be one of the main reasons for the recent recovery of red drum abundance. Because the red drum is a highly prized sportfish and supports an important recreational fishery in the Gulf of Mexico and because the fishery is a good example to demonstrate potential impacts of management regulations in federal waters on fish population dynamics, it was selected for evaluation in this chapter. Information on red drum, such as life-history parameters, habitat information, and stock and fisheries information, is summarized in Tables 9.27, 9.28, and 9.29 and discussed in detail in the following paragraphs.
Red drum (Sciaenops ocellatus) in grass flats (from Ftlaudgirl 2016a)
Range of the red drum (Sciaenops ocellatus) in the Gulf of Mexico and along the Florida east coast (from USGS 2010c)
9.3.8.1 Key Life-History Processes and Ecology
Life-history parameters of red drum, such as growth and maturation, vary greatly with location and time; growth rates are likely higher in more southerly estuaries (Table 9.27) (Powers et al. 2012). Depending on their habitat, red drum can be from 271 to 383 mm (10.7–15.1 in.) in size at the end of the first year, and red drum growth is rapid through the ages of 4–5 years. Males tend to mature at younger ages than females (Table 9.27). The maximum age of red drum is around 40 years in Florida (Murphy and Taylor 1990); however, red drum as old as 60 years have been reported in North Carolina waters (Ross et al. 1995).
The fecundity of red drum depends on fish size, and a female red drum can lay eggs ranging from 200,000 to more than three million per batch. Red drum eggs tend to be subject to high mortality (Peters and McMichael 1987; Goodyear 1989). Larval red drum use vertical migrations to ride high salinity tidal currents into tidal creeks and shallow salt marsh nursery habitats (Wenner 1999). They are transported or move to quiet, shallow water with grassy or muddy bottoms to feed on detritus or dead and decomposing organisms (Buckley 1984; Pattillo et al. 1997). A rapid decline in water temperature can cause large mortalities of early juvenile red drum. Tagging studies suggest that they remain in the same area and generally move less than three miles from where they were tagged.
There is large uncertainty associated with the estimation of M of red drum. The M estimated using the observed maximum age ranges from 0.10 to 0.33 per year, and the estimated M based on growth parameters tends to be higher, from 0.42 to 0.92 per year. The estimated M based on age composition data was 0.20 per year, which supports the M estimated from the observed maximum ages (Goodyear 1989).
The distribution of juvenile red drum is typically limited to inshore waters in the Gulf of Mexico, except during fall and winter (Table 9.28). Adult red drum spend less time in bays and estuaries and more time in open Gulf of Mexico waters (Table 9.28). Spawning red drum can be found in both open and nearshore waters in the Gulf of Mexico and tend to spawn near shorelines during late summer and fall (Tables 9.27 and 9.28). There is little evidence of seasonal migration of red drum, and they have been found in rivers and tidal creeks during the winter. Tides and water temperatures influence daily movement from shallow to deepwaters. During the fall, especially during stormy weather, adult red drum can move to the beaches in the Gulf of Mexico.
Genetic studies have concluded that Atlantic and Gulf of Mexico red drum are two distinct subpopulations, likely resulting from oceanographic and geographic conditions in South Florida, which limits genetic exchange between the two coastal groups (Gold and Richardson 1991; Gold et al. 1993; Seyoum et al. 2000). Population structure within the Gulf of Mexico is complicated because red drum have limited coastal movement and migrate back to a natal estuary (Gold et al. 1999; Gold and Turner 2002). Genetic studies indicate significant patterns of heterogeneity in Gulf of Mexico red drum, suggesting that the genetic difference increases with the distances between the estuaries (Gold et al. 1993, 1999). Tagging studies suggest that juvenile red drum have limited dispersal but that adults can travel considerable distances in the Gulf of Mexico (Osburn et al. 1982; Overstreet 1983). Metapopulation structure may exist for the red drum in the Gulf of Mexico, and despite the likely complex spatial structure of the stock, red drum in the Gulf of Mexico is considered as a single stock, which implicitly assumes no spatial heterogeneity in the Gulf of Mexico red drum population. The impacts of this unrealistic assumption on the stock assessment and management of red drum are unknown.
9.3.8.2 Predators and Prey
Red drum generally are bottom feeders, but can feed in the water column when the opportunity arises. Juveniles feed on invertebrates, while adults feed on many species of fish, including menhadens and mullets, as well as invertebrates, including crabs and shrimps (Table 9.27). Red drum predators include piscivorous fishes, sharks, and birds (Table 9.27).
9.3.8.3 Key Habitat Needs and Distribution
The larvae of red drum prefer vegetated muddy bottom. Juvenile red drum prefer rivers, bays, canals, tidal creeks, passes in estuaries, seagrass beds, oyster bars, mud flats, and sand bottom (Table 9.28). As they mature, red drum move farther into the open Gulf of Mexico, and adults can be found along coastal beaches and nearshore shelf waters (Table 9.28). Essential fish habitat, which is shown in Figure 9.50 and described in Table 9.28, has been designated for the red drum.
The Gulf of Mexico Fishery Management Council’s essential fish habitat for red drum (Sciaenops ocellatus) (from GMFMC 2004c)
9.3.8.4 Fisheries
The red drum was designated as a protected game fish in 2007 under Executive Order 13449. The order prohibits the sale of red drum caught in U.S. waters, resulting in the elimination of the commercial fishery targeting red drum in federal waters and in most state waters. In Florida, the recreational hook-and-line fishery has been the sole source of red drum landings since 1988. The Florida landings were about 230,000 kg (0.5 million lb) in 1988, but quickly increased to an average of about 771,000 kg (1.7 million lb) during the 1990s and stabilized in the 2000s at close to 900,000 kg (2 million lb) on average (Figure 9.51).
Total red drum (Sciaenops ocellatus) landings along the Florida Gulf coast from 1982 through 2007. There were no commercial landings after 1988 (data from Murphy and Munyandorero 2009)
Red drum in the Gulf of Mexico are managed by the GMFMC and relevant Gulf state marine resource management agencies (Table 9.29). No Gulf-wide formal stock assessment is available for red drum. However, a stock assessment was conducted in 2009 for red drum in Florida waters. The assessment was done separately for the Florida Gulf and Atlantic coasts. For red drum along the Florida Gulf coast, stock abundance increased substantially over the time after elimination of the commercial fishery. However, recruitment is relatively stable (Figure 9.52).
Estimated red drum (Sciaenops ocellatus) recruitment abundance (number of fish at age 0) and stock abundance (total number of fish age 1 or older) along the Gulf coast of Florida from 1982 through 2007 (data from Murphy and Munyandorero 2009)
The overall Gulf of Mexico red drum stock was considered overfished, and overfishing was occurring in the early 2000s (Table 9.29). However, because of the harvest moratorium on red drum in federal waters, fishery-dependent data are not available in federal waters and fishery-independent data are limited, which makes it difficult to conduct a comprehensive stock assessment. Clearly, large uncertainties are associated with the current assessment of the red drum stock status described above. A bottom longline survey program has been developed to collect data for monitoring the red drum stock dynamics in the Gulf of Mexico (Powers et al. 2012). The condition of red drum within the Gulf of Mexico may also vary greatly from location to location. For example, the red drum population along the Florida Gulf coast appears to be recovered (Figure 9.52). Regardless of the uncertainties, the evolution of this fishery clearly demonstrates the necessity and importance of appropriate management regulations in improving the status of fish populations.
9.3.9 Tilefish (Lopholatilus chamaeleonticeps) 
The tilefish, often referred to as golden tilefish, is a deepwater fish ranging from Nova Scotia to the Gulf of Mexico (Table 9.30) (Dooley 1978); the range of the tilefish in the Gulf of Mexico is shown in Figure 9.53. The tilefish has a unique burrowing behavior and strong habitat preferences (Table 9.31). Tilefish support an important commercial fishery in the Gulf of Mexico and are mainly caught by handline and longline (Table 9.30). Because of their specific habitat requirements and lack of movement, tilefish tend to be sensitive to changes in their local environment. Tilefish was selected as one of the species to be evaluated in this chapter because they represent those benthic demersal species (Table 9.1) that have wide geographic separation and limited movements, require distinct habitats, are sensitive to changes in environment, and support an important commercial fishery.
Range of the tilefish (Lopholatilus chamaeleonticeps) in the Gulf of Mexico (from NOAA 2003)
9.3.9.1 Key Life-History Processes and Ecology
Tilefish are the largest and longest lived of the tilefish species in the family Malacanthidae. They grow slowly and exhibit sexually dimorphic growth, with males having larger sizes (Table 9.32) (Turner et al. 1983; Grimes and Turner 1999; Lombardi et al. 2010). Tilefish can live for more than 40 years, and maximum sizes range from 96.5 to 111.9 cm (37.9–44 in.). Their age at maturity varies greatly over their distributional areas, with tilefish in the northern waters maturing late and at a large size compared to tilefish in the South Atlantic and Gulf of Mexico. Tilefish mature at age 5 in the North-Mid-Atlantic region (Grimes et al. 1988), age 3 in the South Atlantic, and age 2 in the Gulf of Mexico (Table 9.32). It appears that the age/size at maturity for tilefish has declined over time (Palmer et al. 1998). Compared to other species of similar life history and life span, tilefish tend to mature at younger ages and smaller sizes.
The spawning season for tilefish varies greatly among regions and is typically from January to June (Table 9.32). Tilefish are batch spawners and spawn multiple times throughout a spawning season (Palmer et al. 1998). Annual fecundity increases with size from 195,000 to 8 million eggs per female (Grimes et al. 1988; Palmer et al. 1998).
Tilefish in the Gulf of Mexico have demonstrated some evidence of sequential hermaphrodism, suggesting that tilefish tend to be protogynous (Lombardi et al. 2010; SEDAR 22 2011), but the results are not conclusive. Males may be more vulnerable to fishing pressure as they tend to be larger than females, which may result in disrupting spawning behavior of tilefish (Grimes and Turner 1999).
Because of the long life span, slow growth, a complex breeding process, and habitat specificity and limitations, tilefish are susceptible to mass mortality events as a result of sudden changes in their local environment, such as the intrusion of cold water (Harris and Grossman 1985; Barans and Stender 1993). Many methods have been used to estimate M in the stock assessment (SEDAR 22 2011), and the mean M was estimated to be 0.11 per year, which is comparable to other fish species of similar life history.
The number of recruits estimated for tilefish tended to increase gradually over time prior to the mid-1990s for both the East and West U.S. Gulf of Mexico (Figure 9.54). A more than threefold increase in recruitment was believed to occur in 1997, followed by a large decline back to the levels of the 1980s and 1990s. Recruitment continued to decline after 2000, but has recovered slightly since 2005; this temporal pattern is the same for both the East and West Gulf of Mexico (Figure 9.54).
Estimated recruitment abundance, measured as age-0 fish, for tilefish (Lopholatilus chamaeleonticeps) in the East and West U.S. Gulf of Mexico from 1964 through 2009 (data from SEDAR 22 2011)
Wide geographic separation and restricted movements limit possible adult exchanges between the Gulf of Mexico and other regions, which may require the Gulf of Mexico tilefish to be a separate stock in assessment and management. Even within the Gulf of Mexico, because of patchy distribution and the likely lack of movement, tilefish may have much more complex spatial structure, which has not been considered in the current stock assessment and management.
9.3.9.2 Predators and Prey
Tilefish prey on a wide variety of invertebrates and fish, including decapod crustaceans, squids, bivalve mollusks, sea cucumbers, spiny dogfish, and eels (Table 9.32). Sharks, large tilefish, and other predatory fish are the main predators of tilefish.
9.3.9.3 Key Habitat Needs and Distribution
The restrictions of habitat in sediment type, depth, and temperature by adult tilefish may prevent them from moving long distances (Table 9.31). This was shown in tagging studies, which suggested that the movement of tilefish was minimal (Grimes et al. 1983; Katz et al. 1983). This implies that the suitability of local habitat is critical for tilefish. Tilefish are included as one of the Reef Fish species for which essential fish habitat has been designated; this designated habitat is shown in Figure 9.6 and described in Table 9.31.
Tilefish eggs often occur in late spring and summer in the upper water column near the edge of the continental shelf in the Gulf of Mexico (Fahay and Berrien 1981; Fahay 1983; Erickson et al. 1985; Grimes et al. 1988). They hatch in about 40 h at temperatures from 22 to 24.6 °C.
Larval tilefish are pelagic and can be found during the summer in Gulf of Mexico offshore waters (Fahay and Berrien 1981; Fahay 1983; Turner et al. 1983). Early juveniles are still pelagic but start to settle to the bottom at a size of 9–15.5 mm (0.35–0.61 in.) SL (Fahay 1983). The benthic juveniles burrow and occupy simple vertical shafts in the substrate (Able et al. 1982). In the Gulf of Mexico, adults inhabit burrows excavated from firm mud, silt, sand, and clay along the continental slope and shelf, with distinct depth and temperature preferences (Table 9.31).
9.3.9.4 Fisheries
Prior to 1980, tilefish landings were low, but the commercial fishery took off in 1980, reaching the highest level at around 430 metric tons in 1988, which was immediately followed by a large decline (Figure 9.55). Since 1990, tilefish landings have fluctuated between 100 and 250 metric tons.
Vertical and longline commercial landings of tilefish (Lopholatilus chamaeleonticeps) in the U.S. Gulf of Mexico from 1965 through 2009. No data are available for 1970 (data from SEDAR 22 2011)
Tilefish in the Gulf of Mexico are managed under the FMP for the Reef Fish Fishery, which was implemented in 1984. The FMP was developed to: (1) rebuild declining reef fish stocks wherever they occur within the fishery; (2) establish a fishery reporting system for monitoring the Reef Fish Fishery; (3) conserve reef fish habitats, increase reef fish habitats in appropriate areas, and provide protection for juveniles while protecting existing new habitats; and (4) minimize conflicts between user groups of the resource and conflicts for space (SEDAR 22 2011).
Tilefish fishing mortality rates were low prior to 1980, increased quickly after that and reached the highest level in 1988 (Figure 9.56); 1988 was also the year of the highest tilefish landings to date (Figure 9.55). Fishing mortality has decreased since 1988 to around 0.10 during the 1990s and 0.15 during the 2000s (Figure 9.56).
Estimated fishing mortality for Gulf of Mexico tilefish (Lopholatilus chamaeleonticeps) from 1964 through 2009 (data from SEDAR 22 2011)
The stock biomass of tilefish in both the eastern and western U.S. Gulf of Mexico has declined substantially since the 1960s (Figure 9.57). The rate of decline in stock biomass for the western Gulf of Mexico is higher than that for the eastern Gulf. The tilefish stock biomass for the eastern Gulf of Mexico has been higher than that for the western Gulf over most of the years included in the stock assessment. However, in the last 2 years in the assessment, the western Gulf of Mexico tilefish stock biomass was higher than that in the East, which might have resulted from higher landings in the eastern Gulf of Mexico (SEDAR 22 2011). The stock assessment results, however, need to be interpreted cautiously.
Estimated stock biomass for tilefish (Lopholatilus chamaeleonticeps) in East and West U.S. Gulf of Mexico from 1964 through 2009 (data from SEDAR 22 2011)
Most of the tilefish samples were taken from relatively shallow waters, while the stock assessment also covered deep offshore waters from which few samples were taken (SEDAR 22 2011). This inconsistency may result in large uncertainty in the estimation of key life-history parameters, including growth and M, and subsequently the stock dynamics.
Most of the tilefish samples were taken from relatively shallow waters, while the stock assessment also covered deep offshore waters from which few samples were taken (SEDAR 22 2011). This inconsistency may result in large uncertainty in the estimation of key life-history parameters, including growth and M, and subsequently the stock dynamics.
The recent stock assessment suggests that tilefish in the Gulf of Mexico are not overfished (Table 9.30). Most scenarios evaluated in the assessment also suggest that overfishing is not occurring for the Gulf of Mexico tilefish stock. However, at least one scenario considered in the assessment suggests that the Gulf of Mexico tilefish stock is subject to overfishing.
9.3.10 King Mackerel (Scomberomorus cavalla) 
The king mackerel, a subtropical species of mackerel in the family Scombridae, is mainly distributed in tropical and subtropical waters (Table 9.33) (Beaumariage 1973). It is a migratory species of mackerel that occurs in the open waters of the western Atlantic Ocean and Gulf of Mexico (Figure 9.58). Because king mackerel are opportunistic and voracious carnivores and are among the most sought-after gamefish throughout their distributional range (Table 9.33) (Beaumariage 1973), they are the representative mackerel species selected for evaluation. In addition, king mackerel support both commercial and recreational fishing industries in the Gulf of Mexico (SEDAR 16 2009). General stock and fishery information, habitat preferences and life-history parameters for king mackerel are summarized in Tables 9.33, 9.34, and 9.35 and discussed in detail in the paragraphs below.
Range of the king mackerel (Scomberomorus cavalla) in the Gulf of Mexico and along the Florida east coast (from USGS 2010d)
9.3.10.1 Key Life-History Processes and Ecology
The king mackerel inhabits coastal areas, usually in waters less than 73 m (239 ft) deep, and coral reefs, offshore currents, tide rips, and large bays. Two migratory groups of king mackerel have been identified to exist in U.S. waters: the Gulf of Mexico group, ranging from the Texas coast in summer to the middle-east coast of Florida from November through March; and the Atlantic group off North Carolina to southeast Florida that migrates in spring and fall (Figure 9.59). King mackerel spawn from May through October in the coastal waters of the northern and southern Gulf of Mexico in depths ranging from 35 to 183 m (115–600 ft) (Tables 9.34 and 9.35).
Seasonal migratory pattern of king mackerel (Scomberomorus cavalla) in the Gulf of Mexico hypothesized based on tagging data (Figure 4.2 redrawn from SEDAR 16 2009)
Depending on its size, a female may lay from 50,000 to several million eggs over a spawning season (Addis et al. 2011). Eggs of king mackerel are fertilized in the water column and hatch in about 24 h. Little is known about young-of-the-year (YOY) king mackerel (SEDAR 16 2009).
A typical age-1 fish can reach an average weight of 1.4–1.8 kg (3.1–3.9 lb) and a FL of 60 cm (23.6 in.) (SEDAR 16 2009). Female king mackerel can grow much larger than males, and few male king mackerel weigh more than 7 kg (15.4 lb) (Johnson et al. 1983; Finucane et al. 1986). For example, at age 7, females reach an average weight of 9.5 kg (20.9 lb), while males typically weigh 5 kg (11 lb). There is temporal and spatial variation, as well as differences between males and females, in the growth and maturation of king mackerel (Table 9.35).
In the recent stock assessment (SEDAR 16 2009), the M was set at 0.16 and 0.17 for South Atlantic and Gulf of Mexico king mackerel, respectively. Age-specific M was based on a scaled Lorenzen curve. Two migratory units, Gulf of Mexico and South Atlantic, are currently managed (Table 9.33).
9.3.10.2 Predators and Prey
King mackerel are opportunistic carnivores (Table 9.35). They eat a wide variety of schooling pelagic fishes, including sardines, herrings, and anchovies, as well and shrimp and squid (Table 9.35) (Beaumariage 1973; Saloman and Naughton 1983). Predators include sharks and dolphins (Table 9.35).
9.3.10.3 Key Habitat Needs and Distribution
King mackerel commonly occur in depths of 12–45 m (39.4–147.6 ft) (Table 9.34), where the principal fisheries occur. Both juvenile and adult king mackerel prefer epipelagic, neritic tropical, subtropical, and temperate waters (Table 9.34). Larger fish (heavier than 9 kg or 19.8 lb) often occur inshore in the mouths of inlets and harbors; occasionally, they are found at depths of 180 m (590 ft) at the edge of the Gulf Stream. King mackerel prefer water temperatures in the range of 20–29 °C; their distribution may be limited by a minimum water temperature tolerance of 20 °C (Table 9.34). The king mackerel is included as one of the Coastal Migratory Pelagics for which essential fish habitat has been designated (Figure 9.60 and Table 9.34).
Essential fish habitat for king mackerel (Scomberomorus cavalla), Spanish mackerel (Scomberomorus maculatus), cobia (Rachycentron canadum), cero (Scomberomorus regalis), little tunny (Euthynnus alletteratus), dolphinfish (Coryphaena hippurus), and bluefish (Pomatomus saltatrix) (from GMFMC 2004d)
9.3.10.4 Fisheries
In the king mackerel recreational fishery, gear used includes trolling with various live and dead baitfish, spoons, jigs, and other artificial lures (SEDAR 16 2009). Gear used in the king mackerel commercial fishery includes run-around gill nets, trolling with large planers, and heavy tackle and lures similar to those used by sport fishers. The recreational fishery lands more king mackerel than the commercial fishery (Figure 9.61). Fishing mortality for Gulf of Mexico king mackerel also includes discarded bycatch of king mackerel in the Gulf of Mexico shrimp fishery, with most discards being YOY fish, and discarded dead fish in the recreational fishery (Figure 9.62). The number of dead king mackerel discarded in the recreational fishery is much smaller than the bycatch in the shrimp fishery (Figure 9.62).
Landings of king mackerel (Scomberomorus cavalla) in the commercial and recreational fisheries in the U.S. Gulf of Mexico from 1981 through 2007 (data from SEDAR 16 2009)
King mackerel (Scomberomorus cavalla) bycatch in the shrimp fishery and discarded (dead) in the recreational fishery for the migratory group in the Gulf of Mexico from 1981 through 2007 (data from SEDAR 16 2009)
King mackerel in the Gulf of Mexico are managed by the GMFMC and SAFMC under the FMP for Coastal Migratory Pelagic Resources of the Gulf of Mexico and South Atlantic, which was approved in 1982 and implemented in 1983 (Table 9.33). The limit reference points are 0.80 * B MSY (stock biomass that can produce MSY) for biomass, which is baseline to determine if fish stock is overfished, and F MSY for fishing mortality, which is the baseline to determine if overfishing occurs (SEDAR 16 2009). Overfishing has occurred in the past (Table 9.33), but the Gulf of Mexico migratory group of king mackerel was not overfished and was not experiencing overfishing in fishing year 2006/07 (Figure 9.63).
King mackerel (Scomberomorus cavalla) fishing mortality and the ratio of current fishing mortality (F) versus the maximum fishing mortality threshold (MFMT), which is used to determine if the fishery is subject to overfishing of the migratory group in the Gulf of Mexico, from 1983 through 2007 (data from SEDAR 16 2009)
The recruitment of Gulf of Mexico king mackerel has fluctuated but has had an increasing trend since the early 1980s (Figure 9.64). The stock abundance of all fish age 1 or older in the Gulf of Mexico group also increased over time. A large increase in stock abundance appeared to occur in fishing year 2003/04 (Figure 9.64). However, the estimation of the recent stock abundance and recruitment tends to be subject to large uncertainty and even biases (most often overestimation) because of retrospective errors in stock assessment (Mohn 1999). Therefore, the large increase in stock abundance and recruitment in recent years should be interpreted cautiously.
Recruitment measured as the abundance of king mackerel (Scomberomorus cavalla) at age 0 and stock abundance measured as number of fish age 1 or older for the migratory group in the Gulf of Mexico from 1981 through 2007 (data from SEDAR 16 2009)
The king mackerel spawning stock has also increased over time. The ratio of spawning stock to the minimum spawning stock threshold has become larger than one in recent years (Figure 9.65), suggesting that the Gulf of Mexico king mackerel is not overfished.
King mackerel (Scomberomorus cavalla) spawning stock measured as the abundance of hydrated eggs (SSB) and the ratio of spawning stock versus minimum spawning stock threshold (MSST), which is a pre-set spawning stock biomass used to determine if the stock is overfished for the migratory group in the Gulf of Mexico from 1981 through 2007 (data from SEDAR 16 2009)
9.3.11 Dolphinfish (Coryphaena hippurus)
Dolphinfish, often referred to as mahi-mahi or dorado, are large, fast-swimming, and surface-dwelling fishes found in off-shore temperate, tropical, and ocean waters worldwide (Figure 9.66) (NMFS 2009c). The range of dolphinfish in the Gulf of Mexico is shown in Figure 9.67. As one of the top coastal pelagic predators, dolphinfish play an important role in the Gulf of Mexico ecosystem; in addition, they are a significant species in both commercial and recreational fisheries in the Gulf of Mexico (NMFS 2009c). While details are included in the paragraphs that follow, life history, habitat, and stock and fisheries information for dolphinfish are summarized in Tables 9.36, 9.37, and 9.38.
Dolphinfish (Coryphaena hippurus) underwater off the Florida coast (from Ftlaudgirl 2016b)
Range of the dolphinfish (Coryphaena hippurus) in the Gulf of Mexico (from NOAA 2013b)
9.3.11.1 Key Life-History Processes and Ecology
Females may spawn 2–3 times per year, and produce between 80,000 and 1,000,000 eggs per spawning event, depending on their sizes (Beardsley 1967). Dolphinfish spawn in warm ocean currents (water temperatures greater than 24 °C) throughout much of the year (Tables 9.36, 9.37, and 9.38), and spawning occurs in the open water when the water temperature rises. The spawning season varies greatly with latitude. In the northern Gulf of Mexico, spawning occurs at least from April through December, while spawning takes place throughout the year in the southern Gulf of Mexico, and oceanic waters are preferred (Tables 9.36 and 9.37).
Larval and juvenile dolphinfish are commonly found in floating seaweed (Gibbs and Collette 1959; Beardsley 1967; Rose and Hassler 1974). In waters above 34 °C, dolphinfish larvae are found year-round, with greater numbers detected in spring and fall. In a study conducted in the northern Gulf of Mexico, 70 % of the youngest larvae collected were found at a depth greater than 180 m (590 ft).
Dolphinfish are among the fastest-growing fish, and they become sexually mature within a few months (Table 9.36). The length at maturity varies spatially, ranging from 35 to 53 cm (13.8–20.9 in.) FL (Table 9.36).
The estimated M ranged from 0.68 to 0.80 per year in a previous stock assessment (Prager 2000). This range is consistent with the values used for yellowfin tuna that are also a wide-ranging, fast-growing, and predatory species found in similar warm ocean waters.
Some studies suggest that there might be multiple dolphinfish stocks based on the analysis of biological and morphological variables (Oxenford and Hunt 1986; Duarte-Neto et al. 2008; Lessa et al. 2009). However, genetic connectivity was found between migratory groups in the Atlantic, Caribbean, and Gulf of Mexico, which leads to the definition of a single stock in the Atlantic, U.S. Caribbean, and Gulf of Mexico for dolphinfish (GMFMC and SAFMC 2011). Impacts of uncertainty regarding stock structure were evaluated in an assessment by the Caribbean Regional Fisheries Mechanism (CRFM 2006).
9.3.11.2 Predators and Prey
Dolphinfish are carnivorous and feed on a variety of fish and invertebrates; examples include crabs and shrimps associated with Sargassum and juvenile tunas, billfishes, jacks, and dolphinfish (Table 9.36). Predators of dolphinfish include large tunas, marlins, sailfishes, and swordfishes, as well as sharks (Table 9.36).
9.3.11.3 Key Habitat Needs and Distribution
Both juvenile and adult dolphinfish prefer tropical and subtropical oceanic waters and are closely associated with floating objects and Sargassum (Table 9.37). While juveniles are restricted to waters that are warmer than 20 °C, adults can tolerate temperatures ranging from 15 to 29.3 °C.
Essential fish habitat has been designated for seven species managed as Coastal Migratory Pelagics, and dolphinfish is included as one of the species. Table 9.37 contains a description of the designated habitat for dolphinfish; it is shown in Figure 9.60, with that of several other Gulf of Mexico fish species.
9.3.11.4 Fisheries
The dolphinfish supports an important recreational and commercial fishery in the Gulf of Mexico (Table 9.38). From 1998 to 2006, on average, 6,240 metric tons (13.8 million lb) of dolphinfish were landed in the recreational fishery, which consisted of 94 % of the total dolphinfish landings; commercial fishermen landed 415 metric tons (914,909 lb) (NMFS 2009c). The total landings of dolphinfish increased from 2,100 metric tons (4.6 million lb) in 1981 to a peak of 11,300 metric tons (24.9 million lb) in 1997. Dolphinfish landings decreased to 5,800 metric tons (12.8 million lb) in 2006 (NMFS 2009c). Multiple councils manage dolphinfish (Table 9.38).
A time series of relative abundance index data was developed based on U.S. longline fishery data, which was then used for the assessment of dolphinfish using a surplus production model (Prager 2000). The estimated MSY was about 12,000 metric tons (26.5 million lb)/year, and the estimated fishing mortality that yielded MSY (F MSY) was about 0.5 per year. The estimated stock biomass in 1998 was above B MSY, suggesting that the stock was not overfished in 1998. This assessment suggested some increase in stock size relative to previous estimates and that the fishery was sustainable (Prager 2000). Although a large uncertainty may exist in the assessment as a result of the quality and quantity of data and uncertainty about the stock structure, a recent assessment suggested that there was no decline in catch per unit effort (CPUE) indices and that the current fishing mortality level appears to be sustainable (Collette et al. 2012). The life history of fast growth, early maturation, and high M suggests that dolphinfish may be able to withstand a relatively high exploitation rate. Overfishing appears not to be occurring, and the dolphinfish stock was not overfished from 2000 through 2011 (Table 9.38).
9.3.12 Striped Mullet (Mugil cephalus) 
The striped mullet is a cosmopolitan species distributed worldwide throughout estuarine, coastal tropical, and warm temperate waters (Figure 9.68) (Addis et al. 2011); its distribution throughout the Gulf of Mexico is shown in Figure 9.69. Striped mullet are catadromous, which means they spawn in saltwater, but return to freshwater to feed and grow (De Silva 1980).
A school of striped mullet (Mugil cephalus) swim along the bottom of Fanning Springs, Florida (from Wood 2016)
Range of the striped mullet (Mugil cephalus) in the Gulf of Mexico and along the Florida east coast (from USGS 2010b)
As a widely distributed, abundant, and low trophic level fish, striped mullet play an important role in Gulf of Mexico coastal ecosystems (McEachran and Fechhelm 2005). It captures and transfers food and energy that cannot be utilized by other finfish species of higher trophic levels and is an important prey species for many finfish and sharks. In addition, striped mullet support one of the most important inshore commercial finfish fisheries in Florida (Mahmoudi 2000, 2005, 2008). In the tables (Tables 9.39, 9.40, and 9.41) and text that follow, life history, habitat, and stock and fisheries information for striped mullet are summarized.
9.3.12.1 Key Life-History Processes and Ecology
The movement of spawning adult to offshore spawning areas may be linked to lunar or tidal cycles (Rivas 1980). In the northern Gulf of Mexico, peak spawning occurs in November and December; spawning occurs slightly later in the more southern areas of the eastern and western Gulf of Mexico (Table 9.39).
The fecundity of a female depends on its size and ranges from 250,000 to 2.2 million eggs. Striped mullet appear to spawn only once each year, and eggs are small, non-adhesive, and pelagic (Collins 1985; Greeley et al. 1987). Fertilization is external in the water column (Ross 2001), and fertilized eggs hatch in about 48 h. Nocturnal spawning, followed by the rapid development of fertilized eggs, may reflect possible adaptations minimizing the probability of eggs being exposed to heavy waves (Martin and Drewry 1978).
Juvenile and adult striped mullet can be found in freshwater, as well as shallow marine and estuarine waters (Table 9.40). Both males and females approach sexual maturity in freshwater (Ross 2001), which occurs after 2–3 years of age (Table 9.39). The life span of striped mullet is about 5 or 6 years, but few striped mullet live past 4 years (Rivas 1980).
The M of striped mullet was assumed to be 0.3 per year, constant for all age groups and for all years, in the Florida stock assessment (Mahmoudi 2005). However, the reliability of this assumed M remains unknown.
Striped mullet distributed in the U.S. Gulf of Mexico are considered a unit stock. However, because of limited movements and wide distribution in various habitats of the Gulf of Mexico, their key life-history parameters, such as growth and maturation, may have large spatial variability, and they tend to be assessed and managed under regional or state-specific management programs (Leard et al. 1995).
9.3.12.2 Predators and Prey
The striped mullet is a detritivore/invertivore and a filter feeder (Goldstein and Simon 1999), and common food sources for juveniles and adults include microalgae, diatoms, dinoflagellates, plant detritus, and organic sediments (Table 9.39). They usually feed at surface boundaries by sucking up mud surfaces or grazing on diatoms or algae attached to rock or plant surfaces (Odum 1970; Ross 2001). Larvae tend to feed on microcrustaceans, such as copepods and insect larvae (Etnier and Starnes 1993). When striped mullet reach 40 mm (1.6 in.) SL, their feeding shifts from grazing on surface/subsurface materials to digging into bottom sediments. Fish reaching 110 mm (4.3 in.) SL can dig 5–7 mm (0.2–0.3 in.) into the sediment, and a striped mullet of 200 mm (7.9 in.) SL may filter over 450 kg (992 lb) of bottom sediment in a year. Sand grains can consist of 50–60 % of the diet of fish larger than 40 mm (1.6 in.) SL (Odum 1970; Eggold and Motta 1992). Adult striped mullet may feed opportunistically on animal prey when highly abundant (e.g., spawning aggregations of marine bristleworms) (Bishop and Miglarese 1978). Bacteria may be important in the diet of striped mullet in muddy areas (Moriarity 1976). Feeding becomes active during the daytime, peaking near midday, and starts to decline in the afternoon. Digestion rates were found to be lower for fish inhabiting freshwater, compared to those in saltwater (Perera and De Silva 1978).
Striped mullet have many predators (Table 9.39). They include snooks, seatrouts, drums, catfishes, flounders, sharks, and gars, as well as sea birds and marine mammals.
9.3.12.3 Key Habitat Needs and Distribution
Striped mullet often enter and inhabit estuaries and freshwater environments (McEachran and Fechhelm 2005). Adult mullet can be found in waters ranging from 0 to 75 ppt salinity, but juveniles cannot tolerate such wide salinity ranges. The habitat preferences of juvenile and adult striped mullet include shallow estuarine and marine waters, as well as contiguous freshwaters (Table 9.40). Because it is not federally managed, essential fish habitat has not been designated for striped mullet in the Gulf of Mexico.
9.3.12.4 Fisheries
The commercial fishery for ripe striped mullet increased significantly in the early 1980s as a result of development of the roe export market (Mahmoudi 2000, 2005, 2008). Various regulations have been implemented in the management of the striped mullet fishery since 1989, and commercial fishing has been strictly restricted since 1995, when Florida prohibited the use of gill and other entangling nets in state waters. This caused a rapid decline in landings and fishing effort since 1995, especially on the Florida Gulf coast. Important regulations developed for managing the mullet fishery included seasonal closures in the early 1950s, a minimum size in 1989, gear restrictions and temporal closures in the early 1990s, and the elimination of the use of gill nets in 1995 (Mahmoudi 2000, 2005, 2008).
The striped mullet is a very important species targeted by the recreational fishery for food and bait in the Gulf of Mexico (Mahmoudi 2000, 2005, 2008). Cast nets are used almost exclusively in the striped mullet recreational fishery. In Florida, landings in the recreational fishery were less than 14 % of the total statewide striped mullet landings from 1998 through 2001 and fluctuated widely from year to year. Since 1995, annual recreational harvests have averaged 356,909 fish (169,250 kg or 373,132 lb) and 425,055 fish (352,713 kg or 777,600 lb) on the Northwest and Southwest Florida Gulf coasts, respectively.
The striped mullet fishery is managed by multiple entities, and the Florida striped mullet stocks have not been overfished and were not subject to overfishing in recent years (Table 9.41). No formal stock assessment has been conducted for striped mullet in other parts of the Gulf of Mexico.
The fishing mortality rate of striped mullet in Florida waters has declined significantly since the net ban was implemented on both coasts of Florida in 1995. The recent fishing mortality rates were below the management target levels (Mahmoudi 2008). This has resulted in a gradual increase of the spawning stock biomass especially along the Florida Gulf coast, where over 85 % of striped mullet are landed. The current striped mullet stocks appear to be healthy, and current levels of fishing effort appear to be sustainable (Mahmoudi 2008).
9.3.13 Greater Amberjack (Seriola dumerili) 
The greater amberjack is the largest genus in the family Carangidae, with a maximum length of 200 cm (78.7 in.) (Figure 9.70) (Murie and Parkyn 2010). It is a popular fish targeted in recreational fisheries, as well as in commercial fisheries and was selected as the representative jack species for evaluation in this chapter.
School of greater amberjack (Seriola dumerili) around a shipwreck (from semet 2013)
Greater amberjack are widely distributed in the Gulf of Mexico (Figure 9.71). In the southern Gulf of Mexico, they sometimes move to nearshore waters (Harris et al. 2007). Greater amberjack are often found near reefs, including artificial reefs, floating wrecks, and offshore oil and gas platforms in the northern Gulf of Mexico. Information regarding the life history, habitat preferences, and stock and fisheries for the greater amberjack is summarized in the tables (Tables 9.42, 9.43, and 9.44) and paragraphs below
Range of the greater amberjack (Seriola dumerili) in the Gulf of Mexico (from NOAA 2013c)
9.3.13.1 Key Life-History Processes and Ecology
Greater amberjack spawn from March through June, and little is known regarding the spawning aggregations of the Gulf of Mexico population. The age and length of maturity of female greater amberjack is variable and ranges from 1 to 6 years (Table 9.42).
The daily instantaneous M was estimated at 0.005 for YOY greater amberjack from 40 to 130 days old, resulting in a cumulative M of 36 % for a 100-day period (Wells and Rooker 2004b). Greater amberjack in the Gulf of Mexico tend to have a life span of at least 15 years, based on age samples available (Manooch and Potts 1997b; Thompson et al. 1999). Using the method of Hoenig (1983), this yields a value for M of 0.28. The M used in the stock assessments is 0.25 (Turner et al. 2000; SEDAR 9 2006).
9.3.13.2 Predators and Prey
Juvenile greater amberjack feed mainly on planktonic decapods and other small invertebrates (Andovora and Pipitone 1997). Adult greater amberjack are opportunistic predators, feeding on benthic and pelagic fishes and invertebrates, such as scads, sardines, and squids (Table 9.42). Predators of the greater amberjack in the Gulf of Mexico often include larger fishes, such as tunas, and seabirds, such as brown noddys and sooty terns (Table 9.42).
9.3.13.3 Key Habitat Needs and Distribution
Juvenile greater amberjack are associated with pelagic Sargassum mats until about 6 months of age (Table 9.43). The habitat of adults includes pelagic and epibenthic waters, and greater amberjack congregate around reefs, rock outcrops, and wrecks (Ingram, 2006). Some greater amberjack are full-time residents along the Florida Gulf and Atlantic coasts, while others may migrate from the South Atlantic Bight into inshore waters during certain times of the year. Greater amberjack tend to congregate in schools when they are young; however, the schooling behavior changes as the fish grow older, and old fish are primarily solitary.
The greater amberjack is managed under the Reef Fish FMP by the GMFMC (GMFMC 2004a). Essential fish habitat that has been designated for Reef Fish is shown in Figure 9.6 and described in Table 9.43.
9.3.13.4 Fisheries
Greater amberjack are caught primarily with hydraulic reels, handlines, rods-and-reels, longlines, and traps. For stock assessment and management, greater amberjack are considered as two stocks in the United States (Table 9.44). The Gulf of Mexico stock inhabits the northern Gulf of Mexico and along the Southwest Florida coast, while the South Atlantic stock inhabits South Florida, the Florida Keys, and the U.S. South Atlantic region (Gold and Richardson 1998; SEDAR 9 2006).
Various management regulations have been in place since 1998 within the Gulf of Mexico fishery (SEDAR 9 2006). The bag limit is one fish per day in the recreational fishery, with a 71.1 cm (28 in.) minimum legal length. In the commercial fishery, the minimum legal size is 91.4 cm (36 in.), and there is a seasonal closure from March through May when greater amberjack spawn. The majority of greater amberjack commercial landings are from handline (Figure 9.72). The landings increased dramatically during the 1980s, but have exhibited a decreasing trend since the late 1980s. Since 1998, the landings have fluctuated from year to year, but are relatively stable compared to the variability in landings observed in the 1980s and 1990s (Figure 9.72).
Landings of greater amberjack (Seriola dumerili) by gear type (longline and handline) and area (western Gulf of Mexico and eastern Gulf of Mexico) in the Gulf of Mexico from 1963 through 2004 (data from SEDAR 9 2006)
The abundance of greater amberjack in the Gulf of Mexico decreased in the late 1980s through the mid-1990s (Figure 9.73). However, the stock assessment conducted in 2006 (SEDAR 9 2006) indicated that the stock abundance increased after 1998, reaching a high level in 2000, but then decreased again (Figure 9.73). Spawning stock fecundity had similar temporal trends as recruitment prior to 1998 (Figure 9.74) (SEDAR 9 2006). However, after 1998, the stock fecundity had an opposite temporal trend compared to the recruitment (e.g., an increased stock fecundity versus an increased and then decreased recruitment) (Figure 9.74). The cause for this difference is unclear.
Greater amberjack (Seriola dumerili) abundance for different age groups in the Gulf of Mexico from 1987 through 2005 (data from SEDAR 9 2006)
Greater amberjack (Seriola dumerili) recruitment (age-0 fish) and spawning stock fecundity in the Gulf of Mexico from 1987 through 2004 (data from SEDAR 9 2006a)
9.4 Population Dynamics of Shark and Ray Species
The great diversity of oceanographic and bathymetric conditions, geology, topology, and ecosystems in the Gulf of Mexico provides suitable habitats for many shark, ray, and skate species. More than 51 shark species are distributed throughout the Gulf of Mexico region, with the highest abundances in the central Gulf of Mexico from Louisiana to Alabama (Parsons 2006). There are at least 49 species of rays and skates in the Gulf of Mexico, and six species are endemic (McEachran 2009).
9.4.1 General Introduction
Three groups of sharks are defined based on their habitats for the assessment and management of sharks in the Gulf of Mexico: Large Coastal Sharks, Small Coastal Sharks, and Pelagic Sharks (SEDAR 29 2012). The Large Coastal Sharks group is divided into two subgroups: Ridgeback Species, which include sandbar (Carcharhinus plumbeus), silky (Carcharhinus falciformis), and tiger (Galeocerdo cuvier) sharks; and Non-Ridgeback Species, which include blacktip (Carcharhinus limbatus), spinner (Carcharhinus brevipinna), bull (Carcharhinus leucas), lemon (Negaprion brevirostris), nurse (Ginglymostoma cirratum), scalloped hammerhead (Sphyrna lewini), great hammerhead (Sphyrna mokarran), and smooth hammerhead (Sphyrna zygaena) sharks (SEDAR 29 2012. The Small Coastal Sharks group includes Atlantic sharpnose (Rhizoprionodon terraenovae), blacknose (Carcharhinus acronotus), bonnethead (Sphyrna tiburo), and finetooth (Carcharhinus isodon) sharks (SEDAR 29 2012). The Pelagic Sharks group includes blue (Prionace glauca), oceanic whitetip (Carcharhinus longimanus), porbeagle (Lamna nasus), shortfin mako (Isurus oxyrinchus), and common thresher (Alopias vulpinus) sharks (SEDAR 29 2012).
In addition to these three groups of sharks, because of very low population biomass and poor stock conditions (e.g., overfished), the following 19 shark species are listed as commercially and recreationally prohibited species: sand tiger (Odontaspis taurus), bigeye sand tiger (Odontaspis noronhai), whale (Rhincodon typus), basking (Cetorhinus maximus), great white (Carcharodon carcharias), dusky (Carcharhinus obscurus), bignose (Carcharhinus altimus), Galapagos (Carcharhinus galapagensis), night (Carcharhinus signatus), Caribbean reef (Carcharhinus perezi), narrowtooth (Carcharhinus brachyurus), Atlantic angel (Squatina dumerili), Caribbean sharpnose (Rhizoprionodon porosus), smalltail (Carcharhinus porosus), bigeye sixgill (Hexanchus nakamurai), bigeye thresher (Alopias superciliosus), longfin mako (Isurus paucus), sevengill (Heptranchias perlo), and sixgill (Hexanchus griseus) sharks. The first five species were part of the Large Coastal Sharks group until 1997, the second nine species were part of Large Coastal Sharks group until 1999, and the last five species were part of the Pelagic Sharks group until 1999. All of these species are prohibited in commercial and recreational fisheries (NMFS 2006b, 2009b).
The abundance of a given shark species and species composition tends to vary spatially and temporally, corresponding to spatial and temporal variability in their habitats (Parsons 2006). For example, blacktip shark, spinner shark, Atlantic sharpnose shark, and bull shark are abundant in coastal waters. Adult blacktip shark is more abundant in the central Gulf of Mexico than any other region. Tiger shark is not reported to utilize coastal nursery areas; however, their young are distributed offshore. Whale shark is distributed along much of the Gulf of Mexico, with highest concentrations off the Louisiana Delta; their distribution can be both near coastal areas and well offshore (Parsons 2006). In the southern Gulf of Mexico and along the Florida Gulf coast, the most common coastal shark is the bonnethead shark; blacktip, blacknose, and lemon sharks are also abundant. In the northern Gulf of Mexico, the Atlantic sharpnose is the dominant species, and blacktip and finetooth sharks are also common. The bull shark is most common in and around the marshes of Louisiana (Parsons 2006). Many coastal sharks experience seasonal inshore–offshore movements to avoid unfavorable thermal habitats. For example, in the northern Gulf of Mexico, coastal shark species tend to move offshore in the fall and winter into warmer and deeper offshore waters, and then return to inshore nursery areas for the spring and summer months. Such seasonal inshore–offshore movements are less clear in the southern Gulf of Mexico, where water temperatures are less variable seasonally (Parsons 2006).
In general, sharks tend to have a life history with slow growth, late maturity, and low reproductive rates, making them vulnerable to exploitation. Heavy exploitation can greatly reduce the biomass of shark stocks, resulting in overfished populations. Baum and Myers (2004) suggested that fishing might drive some shark populations in the Gulf of Mexico to extremely low abundances. However, their analyses and conclusions were criticized as flawed. Burgess et al. (2005) suggested that Baum and Myers (2004) overstated the severity of low shark population levels in the Gulf of Mexico; however, even though they questioned the severity of overfishing, they agreed that many shark stocks in the Gulf of Mexico had been overfished. Of 39 species included in the shark FMP in the Gulf of Mexico, 19 species are listed as commercially and recreationally prohibited species. In addition, the Large Coastal Sharks, Small Coastal Sharks, and Pelagic Sharks groups are now subject to strict management regulations for both commercial and recreational fisheries, including limitations on the type of fishing gear that can be used, size limits, temporally and/or spatially allocated catch quota, requirements for landing conditions to prevent shark finning and species identification, requirements for reporting to improve catch data quality, license requirements, and restrictions of catch and fishing times/locations for research.
9.4.2 Stock Assessment and Management History
The FMP was developed in 1993 for sharks of the Atlantic Ocean (NMFS 1993). It includes the following major management measures: (1) establishing a fishery management unit (FMU) consisting of 39 frequently caught species of Atlantic sharks, separated into three groups for assessment and regulatory purposes (Large Coastal Sharks, Small Coastal Sharks, and Pelagic Sharks); (2) developing assessment protocols for determining annual quotas and other management regulations for commercial fisheries for the Large Coastal Sharks and Pelagic Sharks groups; and (3) defining management regulations for the recreational shark fisheries. The 1993 plan also identified 34 additional species of sharks that were not included in the FMU but were included in the fishery for data reporting purposes (NMFS 1993).
The Large Coastal Sharks group was determined to be overfished based on a 1992 stock assessment and a rebuilding plan was developed, which forms the basis for determining subsequent annual catch quotas for the Large Coastal Sharks stocks. The 1996 stock assessment suggested that Large Coastal Sharks stocks were not on the path for rebuilding (SEFSC 1996). In 1996, the NMFS developed a new rebuilding plan for the Large Coastal Sharks and Small Coastal Sharks stocks to be consistent with the revised definition of overfishing and establishment of new provisions for rebuilding overfished stocks, minimizing bycatch mortality, and protecting essential fish habitat in the amendments to the Magnuson-Stevens Act.
In 62 FR 16648, April 7, 1997, the NMFS issued the final rule prohibiting the directed commercial fishing for, landing of, or sale of five species of sharks from the Atlantic Large Coastal Sharks group (NMFS 1999). These five species were placed in a new Prohibited Species group that included sand tiger, bigeye sand tiger, whale, basking, and great white sharks. These shark species were excluded from directed fishing as a precautionary measure to prevent directed fisheries and markets from developing (50 CFR Part 678, proposed rule). Of the five prohibited species, only sand tiger and bigeye sand tiger sharks were exploited commercially, accounting for less than 1 % of the landings in the directed Large Coastal Sharks fishery (50 CFR Part 678, proposed rule). Sand tiger and bigeye sand tiger sharks were determined to be highly vulnerable to overfishing due to a maximum litter size of only two pups (SEFSC 1998; NMFS 1999). Whale and basking sharks were particularly vulnerable to indiscriminate mortality due to their habit of swimming near the surface (50 CFR Part 678, proposed rule). Great white shark was determined to be susceptible to overfishing due to low reproductive potential, although limited information was available at the time. Because a recreational fishery already existed for the great white shark in parts of its range, the fishery was restricted to catch and release only (50 CFR Part 678, final rule) (NMFS 2003).
In April 1999, the NMFS published the Final FMP for Atlantic Tuna, Swordfish, and Sharks (NMFS 1999). A court order prevented implementation of shark specific rules until a settlement agreement was reached resolving several 1997 and 1999 lawsuits in 2000. The settlement agreement did not address any regulations affecting prohibited shark species (NMFS 2006b). Differing from the previous legislation that prohibited the possession of species known to be vulnerable to fishing pressures, this legislation allowed possession of only those species with stock sizes known to be able to withstand fishing mortality (NMFS 1999). This 1999 FMP increased the total number of prohibited shark species to 19, which included whale, basking, sand tiger, bigeye sand tiger, great white, dusky, night, bignose, Galapagos, Caribbean reef, and narrowtooth sharks from the Large Coastal Sharks group; Caribbean sharpnose, smalltail, and Atlantic angel sharks from the Small Coastal Sharks group; and longfin mako, bigeye thresher, sevengill, sixgill, and bigeye sixgill sharks from the Pelagic Sharks group (NMFS 1999). The goal of this action was to prevent the development of directed fisheries or markets for uncommon or seriously depleted species, as well as those thought to be highly susceptible to exploitation (NMFS 2006b). This FMP defined a new Deepwater and Other Sharks management group to extend the protection of the finning prohibition to all species of sharks, including the 34 species previously included in the fishery in 1993 only for data collection purposes. The 1999 FMP also included life-history information and designated essential fish habitat for highly migratory species, including many shark species within the FMU; however, limited life-history information for some shark species prevented the definition of essential fish habitat at that time (NMFS 1999). Based on a stock assessment conducted for the Large Coastal Sharks and Small Coastal Sharks stocks in 2002, Amendment 1 to the 1999 FMP (for tunas, billfish, and sharks) was added in 2003, which included: (1) aggregating the Large Coastal Sharks group; (2) using MSY as a basis for setting commercial quotas; (3) eliminating the commercial minimum size; and (4) developing various management regulations, including area-specific catch quotas and temporal and spatial closures to reduce fishing and bycatch mortality (NMFS 2003).
The 2006 Consolidated FMP required that the owners and operators using pelagic and demersal longline gear take mandatory workshops and certifications to reduce bycatch mortality and that all federally permitted shark dealers be trained in the identification of shark carcasses (NMFS 2006b). This FMP also included a plan for preventing the overfishing of finetooth sharks by expanding observer coverage and collecting more information on finetooth shark catch. A stock assessment was conducted in 2006 on the Large Coastal Sharks group, which included sandbar, blacktip, porbeagle, and dusky sharks (SEDAR 11 2006). The assessment suggested that dusky and sandbar sharks were overfished, with overfishing still occurring, and that porbeagle sharks were overfished. Amendment 1 to the Consolidated FMP of 2006 updated and expanded upon the life-history information and essential fish habitat for sharks within the FMU (NMFS 2009b). Amendment 2 was added to the 2006 Consolidated FMP for developing rebuilding plans for overfished shark species. Amendment 3 was added to the 2006 Consolidated FMP to address issues raised in the Small Coastal Sharks stock assessment in 2007, which assessed finetooth, Atlantic sharpnose, blacknose, and bonnethead sharks separately. Blacknose sharks were considered overfished with overfishing occurring; however, Atlantic sharpnose, bonnethead, and finetooth sharks were not overfished and overfishing was not occurring (NMFS 2009b).
9.4.3 Small Coastal Sharks Group
The Small Coastal Sharks group currently includes four species: Atlantic sharpnose, blacknose, bonnethead, and finetooth sharks (SEDAR 29 2012). They are widely distributed in coastal waters of the Gulf of Mexico and experience seasonal inshore–offshore movements, usually leaving inshore waters in October and November for warm offshore waters and moving back into inshore waters in spring. These species tend to be smaller than 150 cm (59 in.) TL and have a maximum life span of less than 12 years. They become sexually mature at relatively young ages (2–3 years old), with males often maturing sooner than females. The reproductive cycle is usually annual within the Gulf of Mexico, with an average litter size ranging from 3 to 10 pups. One single stock is assumed in the assessment. Tagging studies provide little evidence to support mixing between the sharks of this group in the South Atlantic and Gulf of Mexico, which suggests that most small coastal sharks complete their life cycles within the Gulf of Mexico. Species in the Small Coastal Sharks group support important commercial and recreational fisheries and are often taken as bycatch in finfish fisheries (SEDAR 13 2007). In the most recent stock assessment (SEDAR 13 2007), sharpnose, finetooth, and bonnethead sharks were determined healthy, with no overfishing occurring and stocks were not overfished. However, the blacknose shark was considered to be overfished, with overfishing still occurring. Detailed descriptions of the distributions and life histories of these species are provided below.
9.4.3.1 Atlantic Sharpnose Shark
The Atlantic sharpnose shark is mainly distributed in waters from the Bay of Fundy to the Straits of Florida and the Gulf of Mexico (SEDAR 13 2007). Even though no genetic differences were found (Heist et al. 1996), based on tagging and life-history studies, a two-stock hypothesis has been proposed: an Atlantic stock distributed from North Carolina to the Straits of Florida and a Gulf of Mexico stock from the Florida Keys throughout the Gulf of Mexico. Little mixing was found in tagging studies between these two stocks (SEDAR 13 2007), suggesting that the two stocks are rather independent. However, large differences have been observed in the life histories between samples collected from the two areas, which might have resulted from differences in the sampling times, locations, and habitats. Most catch of this species is from the Florida east coast, and a single working stock was assumed for the assessment (SEDAR 13 2007).
The Atlantic sharpnose shark is the most common shark in the northern Gulf of Mexico (SEDAR 13 2007). They tend to engage in seasonal inshore–offshore movement, leaving the coast in October and November for warmer offshore waters and returning in March and April. Most adult females tend to be found just offshore in deepwaters. It appears that there is sex and size segregation in the distribution of the population in the Gulf of Mexico (Parsons 2006). Young-of-the-year, juvenile, and adult Atlantic sharpnose sharks prefer sandy and seagrass bottoms, but also can be found on muddy grounds (Bethea et al. 2009). The shallow waters in the extensive barrier islands of the northern Gulf of Mexico are important Atlantic sharpnose shark pupping and nursery grounds.
Age and growth of the Atlantic sharpnose shark in the Atlantic Ocean and Gulf of Mexico have been based on vertebral age analysis (Parsons 1983; Branstetter 1987; Carlson and Baremore 2003). The Atlantic sharpnose shark has a maximum length of about 107 cm (42.1 in.) and a maximum age of 11 years (Loefer and Sedberry 2003). Tagging studies, however, suggest that the maximum age should be 12 years. Nearly all females and males become sexually mature at the age of 2.5 years in the Gulf of Mexico and at age 3.5 in the South Atlantic. Peak mating activity occurs in June and July, and the gestation period is 10–12 months. Reproductive periodicity is annual for both the Gulf of Mexico and the Atlantic Ocean. Fecundity is 4.1 pups per year, with pupping occurring in June. The annual survival rate is about 0.7 for age-1 sharks and slightly higher (around 0.75) for adults (SEDAR 13 2007).
9.4.3.2 Blacknose Shark
Blacknose sharks occur from North Carolina to Brazil, including the Gulf of Mexico (SEDAR 13 2007). They can usually be found in inshore shallow waters in the northern Gulf of Mexico, although not very abundant. Genetic studies suggest that the reproductive cycles differ by basin, but tagging data show no mixing, so they are considered as one unit stock in the assessment (SEDAR 13 2007).
The blacknose shark is small, with a maximum size around 150 cm (59 in.) TL (Parsons 2006). Age and growth was studied for the blacknose shark in the Atlantic Ocean and Gulf of Mexico (Carlson et al. 1999; Driggers et al. 2004). Males mature at about 100–110 cm (39.4–43.3 in.) TL and females at 110–115 cm (43.3–45.3 in.) TL. Mating occurs in late summer or early fall. The gestation period is about 9–10 months. The fecundity is about 3–6 pups, with pupping months in May and June. The reproductive periodicity in the Gulf of Mexico is annual, while the periodicity is considered biennial in the South Atlantic. The annual survival rates are 0.72 for age-1 sharks and 0.76–0.83 for adults. Their main prey includes fish, squid, shrimp, and other invertebrates.
9.4.3.3 Bonnethead Shark
The bonnethead shark is distributed from New England to south of Brazil and commonly occurs in the Gulf of Mexico (SEDAR 13 2007). Bonnethead sharks are considered in the most recent stock assessment a single stock from North Carolina through the Straits of Florida and the Gulf of Mexico (SEDAR 13, 2007). However, there are no data supporting this single-stock hypothesis. In the Gulf of Mexico, it is especially abundant east of Mobile Bay and is the dominant shark species in the shallow coastal waters of the Florida Gulf coast. Like most coastal sharks in the Gulf of Mexico, bonnethead sharks are also subject to seasonal inshore–offshore movements, leaving the coastal waters in October and November for warm offshore waters and moving back to inshore areas in the spring.
Bonnethead sharks are small, with a maximum size of about 109 cm (42.9 in.) TL for males and 124 cm (48.8 in.) TL for females. Age and growth have only been studied for bonnethead sharks in the eastern Gulf of Mexico (Parsons 1993; Carlson and Parsons 1997; Lombardi-Carlson et al. 2003). The maximum age is estimated at 7.5 years based on vertebral age analysis. However, tagging studies suggest that a maximum age of 12 years is a more reasonable estimate. Males become sexually mature at around 2 years of age and females at 2.5 years. They may mate in the fall, with the mated females storing sperm until the following spring when their eggs ovulate for fertilization. Gestation is 4–5 months, the shortest of any placental viviparous (give birth to young) shark species. Their reproductive cycle is annual, with pupping time in August (Parsons 1993; Carlson and Parsons 1997; Lombardi-Carlson et al. 2003). The average size of a litter is about 10 pups.
Juvenile bonnethead sharks tend to be associated with sandy and seagrass bottoms, and adults can be found on muddy, sandy, and seagrass bottoms. Although they feed mainly on blue crabs, shrimp, and squid, occasionally, fish can be found in their stomachs. The first-year survival rate is about 0.66 per year, and survival rates of adults range from 0.66 to 0.81 per year.
9.4.3.4 Finetooth Shark
Finetooth sharks are distributed in the western Atlantic from New York to southern Brazil (SEDAR 13 2007). They are abundant in the northern Gulf of Mexico. Finetooth sharks from North Carolina through the Straits of Florida and into the Gulf of Mexico are considered a single stock because of the lack of genetic differences. However, there is a low exchange of individuals between the Gulf of Mexico and South Atlantic (SEDAR 13 2007). They are one of the most abundant species in inshore waters of the northern Gulf of Mexico.
The finetooth shark is a medium-sized shark, reaching a maximum size of 180 cm (70.9 in.) TL and a maximum age of 12 years (Parsons 2006; SEDAR 13 2007). Males and females become sexually mature at 120 and 137 cm (47.2 and 53.9 in.) TL, respectively, and at ages of 6–7 years old (Parsons 2006; SEDAR 13 2007). Mating occurs in late spring and early summer. The reproductive cycle is biennial, with pupping in June and an average litter size of 3–4 pups (Neer and Thompson 2004). The gestation period likely lasts 11–12 months. They mainly feed on finfish, including mackerel, whiting, and sea trout. Young-of-the-year finetooth sharks prefer muddy bottoms; juveniles also mainly exist on the muddy bottom but can also be found on sandy and seagrass bottoms, while adults usually are associated with seagrass and sandy bottoms (Bethea et al. 2009).
9.4.4 Large Coastal Sharks Group
Currently, the Large Coastal Sharks group consists of 11 shark species that are widely distributed throughout the world (SEDAR 29 2012). In the western Atlantic Ocean, they can be found from along the U.S. Atlantic coast all the way to the south of Brazil. All of the Large Coastal Sharks can be found in the Gulf of Mexico (SEDAR 29 2012). They are considered either as part of the South Atlantic stock or as an independent stock in the assessment and management. Most species of Large Coastal Sharks use the inshore shallow waters of the northern Gulf of Mexico as their spawning and nursery grounds. They tend to move into the inshore shallow waters in the Gulf of Mexico during the spring to give birth to their offspring. The inshore shallow waters provide refuges for their newborn offspring from potential predators (usually large sharks). The young sharks spend summers in the inshore waters for feeding. The preferred bottoms range from sand to mud to seagrass. Young-of-the-year sharks tend to occur more frequently in shallower water with higher temperatures, lower salinities, and more turbid conditions compared to the habitats for juveniles and adults. Small and young sharks may select these habitats as a refuge from larger and more active predators (Bethea et al. 2009). Most sharks move into warmer offshore waters in the fall. Compared to species of Small Coastal Sharks, most of the Large Coastal Sharks tend to become sexually mature at a later age.
The Large Coastal Sharks group supports several important commercial and recreational fisheries in the Gulf of Mexico (SEDAR 29 2012). Although most species are not overfished and overfishing is not occurring, most stock abundances have been reduced over time. The catch quota for the Large Coastal Sharks stocks has been reduced continuously over time since the 1990s but has become relatively stable since the mid-2000s (Figure 9.75), perhaps reflecting a stabilized Large Coastal Sharks group.
Annual commercial catch quota for the Large Coastal Sharks group in the Atlantic Ocean and Gulf of Mexico from 1993 through 2009. The catch quota data do not include bycatch and discards (data from Table 8 in SEDAR 29 2012)
Sandbar and blacktip sharks are two of the most abundant and most commercially and recreationally important shark species in the Gulf of Mexico (SEDAR 29 2012). They both belong to the Large Coastal Sharks group and are widely distributed in the Gulf of Mexico. As top predators that are abundant in Gulf of Mexico coastal ecosystems, sandbar and blacktip sharks play an important role in regulating the ecosystem dynamics of the Gulf of Mexico; therefore, they were selected as representative species for evaluation.
9.4.4.1 Sandbar Shark
Sandbar sharks, one of the largest coastal sharks in the world, can be found in the subtropical waters of the western Atlantic from southern Massachusetts in the United States to southern Brazil, including the Gulf of Mexico between 44°N and 36°S (SEDAR 21 2011). They usually prefer waters ranging from 23 to 27 °C in temperature. Sandbar sharks occur over muddy or sandy bottoms in shallow coastal waters, such as estuaries, bays, river mouths, and harbors, and on continental and insular shelves. They spend most of the time in waters 20–65 m (65.6–213 ft) deep; they also occur in deeper waters (200 m or 656 ft or more), as well as intertidal zones. However, they tend to avoid the surf zone and beach areas. Sandbar sharks usually swim alone or aggregate in sex-segregated schools varying in size.
Sandbar sharks are viviparous (SEDAR 21 2011). Males reach maturity between 1.3 and 1.8 m (4.3–5.9 ft) in size, while females mature at 1.45–1.8 m (4.8–5.9 ft). Birth sizes of pups range from 55 to 70 cm (21.6–27.6 in.) long. Mating occurs in the spring or early summer (May through June). Once fertilization occurs, the gestation period is 8–9 months in the western Atlantic population, where pups are born between June and August. The female has a triennial reproductive cycle. The litter size is typically between 6 and 13 pups, depending on the size of the mother. In the northern Gulf of Mexico, an important nursery area exists around Cape San Blas, Florida. Juvenile sandbar sharks are also captured off Mississippi and Alabama, suggesting the existence of other nursery grounds. Females give birth in shallow water nursing grounds so that YOY and juveniles sharks can be protected from predation by larger sharks, such as bull sharks. Juveniles remain in or near the nursery grounds until late fall after which they form schools and migrate to deeper waters. They return to the nursery grounds during warmer months. After reaching the age of 5 years, they begin to follow the wider migrations of adults. Sandbar sharks are opportunistic bottom feeders preying on bony fishes, smaller sharks, rays, cephalopods, gastropods, crabs, and shrimps. Sandbar sharks feed throughout the day but become more active at night. Predators of sandbar sharks include tiger sharks and great white sharks, on occasion. Sandbar shark M was assumed to be 0.14 in the most recent stock assessment (SEDAR 21 2011).
Sandbar sharks in the South Atlantic and Gulf of Mexico are assessed and managed as a single stock (SEDAR 21 2011). Mexican fisheries and U.S. recreational fishing dominated the catch prior to the mid-1980s (Figure 9.76). After 1985, the commercial catch in the Gulf of Mexico increased quickly and comprised almost half of the total catch between 1985 and 1995. After the mid-1990s, catch in the Gulf of Mexico decreased rapidly (Figure 9.76). Sandbar shark stock abundance has decreased substantially since 1960, and stock abundance in 2009, which is the most recent year covered in the most recent stock assessment (SEDAR 21 2011), is only about 25 % of the stock biomass in the 1960s. Spawning stock fecundity (calculated as numbers × proportion mature × fecundity in numbers) describing the stock reproductive potential also has the same trend as stock abundance (Figure 9.77).
Sandbar shark (Carcharhinus plumbeus) catch by recreational and commercial fisheries in the Mid-Atlantic, South Atlantic, and Gulf of Mexico from 1960 through 2009 (data from SEDAR 21 2011b)
Estimated stock abundance and spawning stock fecundity for the sandbar shark (Carcharhinus plumbeus) in the Gulf of Mexico from 1960 through 2009 (data from SEDAR 21 2011b)
The most recent stock assessment suggests that the sandbar shark stock was overfished and, therefore, subject to rebuilding. However, in the base run and in most sensitivity runs, the stock was found not to be currently subject to overfishing (F2009/FMSY ranges from 0.29 to 0.93) (SEDAR 21 2011). Overfishing was found to be occurring (F2009/F MSY of 2.62) only for the low productivity scenario (SEDAR 21 2011).
9.4.4.2 Blacktip Shark
The blacktip shark (Figure 9.78), a fast-swimming and highly active shark species, is widely distributed in coastal tropical and subtropical waters around the world, including brackish habitats (SEDAR 29 2012). In the western Atlantic, their distribution ranges from southern New England to southern Brazil, including the Gulf of Mexico and the Caribbean. The blacktip shark is one of the most abundant shark species in the Gulf of Mexico. Based on tagging and genetic studies, two stocks are defined in the stock assessment: the Atlantic stock distributed from Delaware to the Straits of Florida and the Gulf of Mexico stock. Although adult blacktip sharks are highly mobile and often disperse over long distances, tagging studies provide little evidence to support mixing between the two stocks (Keeney et al. 2005; SEDAR 11 2006). They are philopatric (behavior of remaining in, or returning to, their birthplace) and return to their original nursery areas to give birth, which can result in subgroups of genetically distinct breeding stocks that overlap in geographic distributional ranges (Keeney et al. 2003, 2005).
Blacktip shark (Carcharhinus limbatus) (from Block 2011)
Blacktip sharks are targeted as a prized and high quality food fish, and are captured in targeted commercial and recreational fisheries (SEDAR 29 2012). The majority of landings are from the demersal longline fishery. Another major source of mortality in the Gulf of Mexico is discards in the Gulf of Mexico menhaden fishery. The landings of blacktip shark in the Gulf of Mexico increased rapidly in the late 1980s but have decreased substantially since 1990 (Figure 9.79). The lowest catch level occurred in 2008, and landings have increased slightly since then (Figure 9.79).
Blacktip shark (Carcharhinus limbatus) catch in the Gulf of Mexico in recreational and commercial fisheries from 1981 through 2009 (data from SEDAR 29 2012)
Female blacktip sharks can reach up to 200 cm (78.7 in.) TL, while males can reach up to about 180 cm (70.9 in.) TL (Parsons 2006). Maximum ages found in the most recent stock assessment (SEDAR 29 2012) were 18.5 years for females and 23.5 years for males, a significant increase of 6 years and 12 years for females and males, respectively, compared to ages observed by Carlson et al. (2006). The M is assumed to be between 0.1 and 0.2, decreasing with age (Figure 9.80).
Age-specific natural mortality (M) assumed in the assessment of the Gulf of Mexico blacktip shark (Carcharhinus limbatus) stock (data from SEDAR 29 2012)
The blacktip shark is a synchronous, seasonally reproducing species with reproductive activity (e.g., mating and parturition) mainly occurring in March through May. Length and age at 50 % maturity are 105.8 cm (41.6 in.) FL and 4.8 years for males and 119.2 cm (46.9 in.) FL and 6.3 years for females, respectively. Female blacktip sharks have a biennial ovarian cycle. The gestation period ranges from 10 months (Parsons 2006) to approximately 12 months (SEDAR 29 2012); the average fecundity is 4.5 pups (ranging from 1 to 10 pups), with the average size at birth at 38 cm (14.9 in.) FL (or about 60 cm or 23.6 in TL). Fecundity was found to increase with both maternal size and age. Females are also capable of asexual reproduction in the absence of males.
Blacktip sharks mainly feed on fishes, squids, and sometimes crustaceans. In the Gulf of Mexico, the most important prey of the blacktip shark is the Gulf menhaden, followed by the Atlantic croaker (Micropogonias undulatus) (Barry 2002). Juveniles may be prey of other large sharks, but adults have no known predators.
Blacktip sharks do not inhabit oceanic waters, although some individuals may be found some distance offshore (Compagno 1984). Most blacktip sharks are found in water less than 30 m (98.4 ft) deep over continental and insular shelves; though, they may dive to 64 m (210 ft) (Froese and Pauly 2009). Their favored habitats include muddy bays, island lagoons, and the drop-offs near coral reefs. Juvenile blacktip shark abundance showed significant correlation with turbidity/water clarity (Bethea et al. 2009). They can also be tolerant of low salinity, moving into estuaries and mangrove swamps. Seasonal migration has been documented to avoid unfavorable thermal habitats, usually moving into warm waters during the fall and returning to inshore feeding/nursery grounds in the spring. Newborn and juvenile blacktip sharks can be found on muddy/sandy/seagrass grounds in inshore shallow nurseries in late spring and early summer, and grown females tend to return to the nurseries where they were born to give birth. Young blacktip sharks are most likely to form aggregations in early summer to avoid predators (Heupel and Simpfendorfer 2005). There tends to be segregation by sex and age, with adult males and nonpregnant females being found apart from pregnant females; juveniles are separated from both groups in the winter (Castro 1996).
The abundance of blacktip sharks in the Gulf of Mexico has had a relatively small decrease since the 1980s and seems to have stabilized or slightly increased since 2000 (Figure 9.81). The spawning stock fecundity that describes stock reproductive potential has the same trend as stock abundance. The most recent stock assessment concluded that the Gulf of Mexico blacktip shark stock was not overfished, and overfishing was not occurring (SEDAR 29 2012). This conclusion is robust with respect to all of the uncertainty in data quality and quantity and assumptions considered in the assessment (SEDAR 29 2012).
Estimated blacktip shark (Carcharhinus limbatus) abundance and spawning stock fecundity for the Gulf of Mexico from 1981 through 2009 (data from SEDAR 29 2012)
9.4.5 Pelagic Sharks Group
The Pelagic Sharks group was initially identified in the 1993 FMP and included the following ten species: shortfin mako, longfin mako, porbeagle, common thresher, bigeye thresher, blue, oceanic whitetip, sevengill, sixgill, and bigeye sixgill (NMFS 1993). Since 1993, five species have been moved to the group of sharks that are prohibited from fishing because of low population levels. Therefore, the Pelagic Sharks group currently includes blue, oceanic whitetip, porbeagle, shortfin mako, and common thresher sharks.
Sharks included in the Pelagic Sharks group are transoceanic, cosmopolitan species that, in general, are highly migratory. In the Western Atlantic, most of these species can be found from Maine to Argentina, including the Gulf of Mexico and the Caribbean. They tend to stay in oceanic deepwater areas but sometimes come close to shore. They can be found most frequently from the surface to depths of at least 200 m (656 ft), but also appear at depths over 1,000 m (3,281 ft). This group of sharks tends to have the largest body sizes. For example, the common thresher can be as large as over 700 cm (275.6 in.) TL (FishBase 2013).
Pelagic sharks are the top predators in the marine ecosystem, feeding mostly on oceanic bony fishes, but also on threadfins, stingrays, sea turtles, sea birds, gastropods, squids, crustaceans, mammalian carrion, tunas, and dolphinfish. Like other shark species, they are viviparous and may be subject to partial segregation by size and sex in some areas.
Pelagic sharks are often caught as bycatch in the North Atlantic Ocean by fishing fleets from several nations. In the U.S. Atlantic, Gulf of Mexico, and the Caribbean, the combined commercial and recreational catch and discards tend to fluctuate greatly over time (Figure 9.82). Since the mid-1990s, the catch has been fairly stable, remaining around 20,000 sharks per year. For the most recent year included in the time series (e.g., 2006), the majority of the catch was from the recreational fishery.
Catch of the Pelagic Shark group in the U.S. Atlantic, Gulf of Mexico, and Caribbean from 1981 through 2006 (data from Cortés 2008)
9.4.6 Prohibited Sharks
Many species of sharks are prohibited from being commercially and recreationally fished because of low populations and poor stock conditions. These sharks, which are now in the Prohibited Species group, were formerly in other managed groups and are described below.
9.4.6.1 Prohibited Sharks Formerly in the Small Coastal Sharks Group
The 1993 FMP for Sharks of the Atlantic Ocean listed seven species of sharks in the Small Coastal Sharks group. Of these seven species, three were moved into the newly created Prohibited Species group in the 1999 FMP for Atlantic Tuna, Swordfish, and Sharks. Since 1999, commercial and recreational fishermen have been prohibited from possessing these three species, which include the Atlantic angel, Caribbean sharpnose, and smalltail sharks. Atlantic angel, Caribbean sharpnose, and smalltail sharks occupy shallow coastal waters and estuaries from the Gulf of Mexico south throughout the Caribbean. The Atlantic angel shark can be found in waters as far north as New England in the western North Atlantic, and the Caribbean sharpnose inhabits waters between 24°N and 35°S. The Atlantic angel shark is dorsoventrally flattened, resembling a ray. Angel sharks reproduce biennially, bearing as many as 16 pups per litter (Castro 1983). The Caribbean sharpnose shark is closely related to the Atlantic sharpnose, with similar biology and life history, differing only in the number of precaudal vertebrae and geographic range (Springer 1964). All of these shark species have long gestation periods of about 10 months (Carlson et al. 2004).
9.4.6.2 Prohibited Sharks Formerly in the Large Coastal Sharks Group
The 1993 FMP for Sharks of the Atlantic Ocean listed 22 species of sharks in the Large Coastal Sharks group. Through legislation in 1997 by the NMFS, five species from the Large Coastal Sharks group were moved into the Prohibited Species group. Of the remaining 17 species in the Large Coastal Sharks group, six were added to the Prohibited Species group in the 1999 FMP for Atlantic Tuna, Swordfish, and Sharks. Since 1999, commercial and recreational fishermen have been prohibited from possessing these 11 shark species: basking, bigeye sand tiger, bignose, Caribbean reef, dusky, Galapagos, narrowtooth, night, sand tiger, great white, and whale sharks. These sharks tend to be widely distributed globally and can be found throughout the western North Atlantic, Gulf of Mexico, Caribbean, and south to Brazil. The majority of these 11 species inhabit coastal to pelagic waters, but the group also includes a few deepwater species. These sharks tend to mature late, and many have long gestation periods and biennial reproductive cycles. Feeding strategies in this group range from apex predator, as in the great white shark, to generalist feeders and scavengers, and filter feeders, such as the basking and whale sharks. Great white and whale sharks were selected as representative Large Coastal Sharks that were moved to the Prohibited Sharks group and are briefly discussed in the following paragraphs.
The great white shark occurs sporadically throughout cold and warm temperate seas (Figure 9.83). In the western North Atlantic, the great white shark ranges from Newfoundland to the Gulf of Mexico, with highest abundances in the Mid-Atlantic Bight region (Casey and Pratt 1985). It has been observed in the Gulf of Mexico from January to September. Seasonal movements appear to be related to water temperature changes, preferring water temperatures of 12–25 °C (Miles 1971). Higher proportions of juvenile great white sharks in the Mid-Atlantic Bight region suggest that this area may serve as a nursery area (Casey and Pratt 1985). Great white sharks are an apex predator feeding primarily on fish as juveniles and switching to primarily marine mammals after reaching a length of over 300 cm (118 in.) (Klimley 1985; McCosker 1985). Little is known about great white shark reproduction, as few gravid females have been examined. Great white sharks carry 7–10 embryos and are thought to reach maturity at 9–10 years (Cailliet et al. 1985; Francis 1996; Uchida et al. 1996). Small localized populations, susceptibility to longlines, and limited reproductive potential contribute to making the great white shark vulnerable to overfishing (Strong et al. 1992).
Great white shark (Carcharodon carcharias) (from Dascher 2013)
The whale shark is the largest fish in the ocean, reaching lengths of over 12 m (39.4 ft) (Figure 9.84). It is a slow-moving filter feeder distributed throughout the world in tropical seas (Castro 1983). The range of the whale shark includes the northern Gulf of Mexico, and they appear to be more abundant in the western Gulf of Mexico than the eastern Gulf (Burks et al. 2006). Whale sharks sometimes form large feeding aggregations near the surface, and as many as 100 individuals or more join these aggregations. Very little is known about whale shark reproduction. One gravid female has been described, carrying 300 young in various stages of development. Due to its wide range, whale shark populations may have to be managed as an ocean-wide population. The whale shark has been demonstrated to be susceptible to overfishing based on records of the Taiwanese fishery.
Whale shark (Rhincodon typus) (from crisod 2013)
9.4.6.3 Prohibited Sharks Formerly in the Pelagic Sharks Group
Ten species of sharks were included in the Pelagic Sharks group in the 1993 FMP for Sharks of the Atlantic Ocean (NMFS 1993). Of these 10 species, five were moved into the Prohibited Species group in the 1999 FMP for Atlantic Tuna, Swordfish, and Sharks (NMFS 1999). Since 1999, commercial and recreational fishermen have been prohibited from possessing the following five shark species: longfin mako, bigeye thresher, sevengill, sixgill, and bigeye sixgill sharks. These sharks tend to occur in waters 100s to 1,000s of meters deep and have very wide global distributions, including the Gulf of Mexico, Caribbean, and western North Atlantic. They tend to have a generalist feeding strategy, preying on various bony and cartilaginous fishes, squids, and crustaceans, as well as scavenging carrion. This group tends to have slow growth rates, a late age at maturity, and small litter sizes, with the exception of the sixgill shark, which can have as many as 20–100 pups in a single litter. The longfin mako and bigeye sixgill sharks were not described by science until the 1960s, and very little is known about them. The pelagic sharks of the Prohibited Species group are susceptible to bycatch in fisheries utilizing bottom trawls and longlines, such as those used in the tuna, swordfish, and tilefish fisheries. Due to low fecundity, slow maturation rates, and likelihood of bycatch mortality, these sharks are highly susceptible to overfishing (NMFS 2006b, 2009b).
9.4.7 Rays and Skates
Rays and skates are a diverse group of cartilaginous fishes and inhabit marine ecosystems in the Gulf of Mexico, primarily along the bottom, ranging from shoreline depths to 2000 m (6,562 ft) deep (Figure 9.85) (McEachran 2009). Only three species (Family Mobulidae) inhabit the oceanic surface and epipelagic zone. They are primarily specialized for a bottom-dwelling lifestyle, feeding primarily on benthic invertebrates. This lends to their unique physiology, which includes teeth resembling flat-crowned plates for crushing shells and exoskeletons, a highly protrusible mouth advantageous for the suction of benthic invertebrates from the substrate, and varying degrees of dorsoventral flattening conducive to camouflage and ambushing prey (Pough et al. 2008). Similar to what has been suggested for the skull structure of a hammerhead shark, this flattening increases the distribution of ampullae of Lorenzini across a larger surface area, which may be more conducive to seeking out prey along the bottom (Pough et al. 2008). However, some rays are highly specialized zooplankton strainers; one species even uses an elongated snout with tooth-like structures for slashing at schools of fish (McEachran and de Carvalho 2002). Rays and skates can vary greatly in size from 13 cm (5.1 in.) to 7 m (23 ft), and can weigh from 10 g (35 oz) to more than 2,700 kg (6,000 lb). Rays and skates are separated for both their reproductive strategy and tail differences. Rays are viviparous, while skates are oviparous and lay collagenous egg cases that are commonly called “mermaid’s purses.” A skate tail is typically long, thick, and finned, while a typical ray tail is more whip-like and often contains a specialized venomous or serrated dorsal barb. No direct fishery exists for rays and skates in the Gulf of Mexico; however, intensive bycatch during shrimp trawling and bottom longlining can negatively impact rays and skates (Sheperd and Myers 2005). Three representative species of rays and skates were selected for further discussion in the paragraphs that follow.
Southern sting rays (Dasyatis americana) often rest in the valleys between pinnacles and Stetson Bank, which is located within the Flower Garden Banks National Marine Sanctuary (photograph by Emma Hickerson) (from NMS 2013)
The giant manta ray (Manta birostris) is the world’s largest ray reaching 1,814 kg (4,000 lb), with an average wingspan of 6.7 m (22 ft) (Figure 9.86). It primarily inhabits pelagic waters from 0 to 100 m (328 ft) deep, but can also be found near reefs, over deeper waters, as well as in muddy, intertidal habitats (McEachran 2009; FFWCC 2013a). Giant manta rays are considered highly transient, migratory, and circumglobal; however, some debate exists on whether records from other oceans may be for different species (McEachran and de Carvalho 2002). The giant manta ray belongs to Family Mobulidae, which is unique from other ray families because they feed almost exclusively on zooplankton while slowly swimming in the epipelagic and oceanic surface zones using funneling fins near the mouth called rostra and specialized gill rakers (FFWCC 2013a). This family has a low reproductive potential; giant manta rays can viviparously produce up to two pups per litter, although one is considered more typical, with a gestation period of 10–14 months, and a possible life span of approximately 20 years (Bigelow and Schroeder 1953; Homma et al. 1999; Ebert 2003).
Manta ray (Manta birostris) (from Scubaguys 2016)
The cownose ray (Rhinoptera bonasus) is a semi-pelagic species that can form large schools anywhere from 100s to 1,000s of individuals (Neer 2005; FFWCC 2013b). Cownose rays have an average wingspan of 0.9 m (3 ft) and are often seen actively swimming or leaping out of the water. They primarily inhabit pelagic waters from 0 to 25 m (82 ft) deep in bays, estuaries, river mouths, and even in the open ocean (McEachran 2009; FFWCC 2013b). In the Western Atlantic, cownose rays can be found from southern New England to Argentina (McEachran and de Carvalho 2002; McEachran 2009). Cownose rays are considered migratory but can be found in some estuaries throughout the year (McEachran and de Carvalho 2002). Their diet consists of bivalve mollusks, crustaceans, and polychaetes (FFWCC 2013b). Like other rays and skates, cownose rays have a low reproductive potential and can viviparously produce up to 2–6 pups per litter, although one may be considered more typical (McEachran and de Carvalho 2002; FFWCC 2013b).
The smalltooth sawfish (Pristis pectinata) is a recognizable species given its elongated snout that can measure up to one-quarter of its total length and be lined with 24–28 unpaired teeth (FFWCC 2013c). It can grow up to 5.5 m (18 ft) and primarily inhabits shallow coastal waters near river mouths, estuaries, bays, or depths up to 125 m (410 ft) (Bigelow and Schroeder 1953; Simpfendorfer and Wiley 2005; McEachran 2009). Historically, in the Western Atlantic, smalltooth sawfish ranged from New York to Brazil, but there has been significant population reduction and range contraction. Today, it is found for the most part only between the Caloosahatchee River in Florida and the Florida Keys (NMFS 2009d). The diet of the smalltooth sawfish consists mostly of small schooling fishes, such as anchovies or mullets, which are injured or killed as the snout is slashed through the school (Bigelow and Schroeder 1953). Smalltooth sawfish have a low reproductive potential, but can potentially produce up to 20 pups per litter, with an approximate gestation period of 5 months (Bigelow and Schroeder 1953; NMFS 2009d).
9.5 Summary
The Gulf of Mexico is one of the most productive marine ecosystems in the world, with high fish species richness and high fishery productivity supported by a great diversity of habitat types. Finfish and shark species play critical roles in the Gulf of Mexico ecosystem and in the spatiotemporal dynamics of their populations. They are strongly influenced by habitat quality and biotic and abiotic factors, including hydrographic and geographic conditions, predation, food supply, fishing, natural weather, geochemical cycles, and the impacts of human activity and coastal development. However, heavy fishing over the last several decades and the long-term effects of anthropogenic and natural stressors on the finfish and shark species and their habitats in the Gulf of Mexico have resulted in populations of great commercial and recreational importance being defined as being overfished and/or undergoing overfishing (Table 9.45).
This chapter has evaluated the distribution, life history, habitat needs, fisheries, and population status of some of the most important finfish and shark species in the Gulf of Mexico prior to the Deepwater Horizon event and has attempted to analyze factors that have most influenced their health and productivity. The 13 finfish species selected for detailed evaluation in this chapter are representative of the Gulf of Mexico. They vary greatly in life history and distribution, are important to commercial and recreational fisheries, and consist of fish species of almost all habitat types. A summary of the status of the 13 fish species selected for evaluation is presented in Table 9.45. Some important general conclusions for finfish and shark in the Gulf of Mexico include the following:
-
Inshore estuaries in the Gulf of Mexico are critical habitats because they are ideal nursery and feeding grounds for most finfish and shark species, providing food and refuge from juvenile predation.
-
The Gulf of Mexico provides critical spawning grounds for many highly migratory fish and shark species of great ecological, commercial, and recreational importance.
-
Only a small fraction (4.6 %) of finfish is endemic to the Gulf of Mexico, although some fish species of commercial and recreational importance can complete their entire life cycle within the Gulf of Mexico ecosystem.
-
Almost half of finfish species in the Gulf of Mexico are omnipresent and can be found throughout the Gulf; the other half is limited to parts of the Gulf of Mexico.
-
Many fish species in the Gulf of Mexico experience inshore–offshore movement in response to changes in environmental conditions (e.g., thermal habitat) and/or needs of life-history processes (e.g., spawning).
-
Fish species differ widely in their ability to adapt to changes in the biotic and abiotic environment in the Gulf of Mexico; omnipresent species are the most robust in adapting to changes in habitat.
-
There is large spatiotemporal variability in key life-history parameters (e.g., growth and maturation) for many Gulf of Mexico fish species.
-
Many fish species in the Gulf are often considered a unit fish stock, which implicitly assumes adequate Gulf-wide mixing for fisheries stock assessment and management, even though evidence suggests complex stock structure within the Gulf of Mexico (e.g., existence of meta-population or local populations as a result of spatial isolations).
-
A wide variety of short-term and long-term anthropogenic and natural stressors, such as rapid coastal development, pollution, heavy fishing, climate change, and natural disasters, have reduced the resilience and robustness of the Gulf of Mexico ecosystem with respect to human and natural perturbations.
-
Different fish species tend to respond to fishing and changes in habitat in different ways. Some species are more sensitive, and others are more robust with respect to changes in fishing mortality and habitat.
-
High fishing pressure and degraded environment have changed key life-history parameters of important fish species in the Gulf of Mexico, e.g., reduced size at age and earlier maturation, reduced stock reproductive potential, increased temporal fluctuation of recruitment, and impaired ability of fish stocks to recover from low stock abundance.
-
Many finfish and shark species of great ecological, commercial, and recreational importance have been subject to overfishing and being overfished. Their present population abundances tend to be much lower compared to historic levels, which may be the result of a combination of factors, including high fishing pressure, large bycatch mortality, and recruitment failure likely due to low spawning stock biomass and/or unfavorable environmental conditions.
-
Recent management regulations appear to have been effective in improving the population levels of some important fish species, which have recovered or are moving towards recovery from overfishing and/or overfished status.
-
No formal stock assessment had been done for the vast majority of fish species in the Gulf of Mexico immediately prior to the Deepwater Horizon event, and subsequently, there is limited knowledge about the status of these fish species (e.g., if they are overfished and/or if overfishing is occurring).
As discussed above, numerous long-term anthropogenic stressors, including fishing pressure, as well as a variety of natural stressors, affect finfish in the Gulf of Mexico. Of the 13 finfish species evaluated in this chapter, five species were being overfished and/or were in the status of overfishing immediately prior to the Deepwater Horizon event. The five species included red snapper, red grouper (some local subpopulations), Atlantic bluefin tuna (most likely but the uncertainty is high), Atlantic blue marlin, and greater amberjack. In addition, many shark species were overfished or were in the status of overfishing immediately before or around April 2010. Of 39 shark species included in the shark Fisheries Management Plan in the Gulf of Mexico, 19 species are listed as commercially and recreationally prohibited species because of very low population biomass and poor stock conditions. Finfish species evaluated in this chapter that were determined to have healthy stocks in the Gulf of Mexico immediately before the Deepwater Horizon event included menhaden, Atlantic swordfish, Atlantic sailfish, red drum, striped mullet, tilefish, king mackerel, and dolphinfish.
Notes
- 1.
Mortality is usually measured as an instantaneous rate. Total mortality (Z) is the sum of fishing mortality (F) and natural mortality (M). The proportion of fish dead as a result of Z can be calculated as 1 − exp(−Z). Thus, in the absence of fishing mortality (F = 0), an M of 0.50 per day for red snapper eggs is equivalent to 39.3 % of red snapper eggs dying per day and an accumulative M of 6.76 per year during the larval stage is equivalent to an annual mortality rate of 99.9 %.
References
Able KW, Grimes CB, Cooper RA, Uzmann JR (1982) Burrow construction and behavior of tilefish, Lopholatilus chamaeleonticeps, in the Hudson Submarine Canyon. Environ Biol Fish 7:199–205
Addis D, Chagaris D, Cooper W, Mahmoudi B, Muller RG, Munyandorero J, Murphy MD, O’Hop J (2011) Florida’s inshore and nearshore species: 2010 status and trends report. Florida Fish and Wildlife Conservation Commission, Fish Wildlife Research Institute, St. Petersburg, FL, USA. March. 334 p
Ahrenholz DW (1981) Recruitment and exploitation of Gulf menhaden, Brevoortia patronus. Fish Bull 79:325–335
Ahrenholz DW (1991) Population biology and life history of the North American menhadens, Brevoortia spp. Mar Fish Rev 53:3–19
Allen GR (1985) FAO species catalogue: An annotated and illustrated catalogue of Lutjanid species known to date. FAO fisheries synopsis no. 125, vol 6. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, 208 p
Alvarado-Bremer JR, Mejuto J, Gomez-Marquez J, Pla-Znuy C, Vinas J, Marques C, Grieg TW (2007) Genetic population structure of the Atlantic swordfish: Current status and future directions. Collect Vol Sci Pap ICCAT 6:107–118
Anderson WW (1958) Larval development, growth, and spawning of striped mullet (Mugil cephalus) along the South Atlantic Coast of the United States. Fish Bull 58:501–519
Anderson JD (2007) Systematics of the North American menhadens: Molecular evolutionary reconstructions in the genus Brevoortia (Clupeiformes: Clupeidae). Fish Bull 105:368–378
Anderson JD, Karel WJ (2007) Genetic evidence for asymmetric hybridization between menhadens (Brevoortia spp.) from Peninsular Florida. J Fish Biol 71:235–249
Anderson JD, McDonald DL (2007) Morphological and genetic investigations of two western Gulf of Mexico menhadens (Brevoortia spp.). J Fish Biol 70:139–147
Andovora F, Pipitone C (1997) Food and feeding habits of the amberjack, Seriola dumerili, in the central Mediterranean Sea during the spawning season. Cah Biol Mar 38:9196
Arnold EL Jr, Thompson JR (1958) Offshore spawning of the striped mullet (Mugil cephalus) in the Gulf of Mexico. Copeia 1958:130–132
Arocha F (1997) The reproductive dynamics of swordfish Xiphias gladius L. and management implications in the Northwestern Atlantic. PhD Thesis, University of Miami, Coral Gables, FL, USA, 383 p
Arrenguín-Sánchez F, Cabrera MA, Aguilar FA (1995) Population dynamics of the king mackerel (Scomberomorus cavalla) of the Campeche Bank, Mexico. Sci Mar 59:637–645
Atlantic Bluefin Tuna Status Review Team (2011) Status review report of Atlantic bluefin tuna (Thunnus thynnus). Report to National Marine Fisheries Service, Northeast Regional Office, March 22, 104 p
Backus S, Durfee CG III, Mourou G, Kapteyn HC, Murnane MM (1997) 0.2-TW laser system at 1kHz. Opt Lett 22:1256–1258
Baglin RE Jr (1982) Reproductive biology of western Atlantic bluefin tuna (Thunnus thynnus). Fish Bull 80:121–134
Barans CA, Stender BW (1993) Trends in tilefish distribution and relative abundance off South Carolina and Georgia. Trans Am Fish Soc 122:165–178
Barry KP (2002) Feeding habits of Blacktip Sharks, Brevoortia gunteri, and Atlantic Sharpnose Sharks, Rhizoprionodon terraenovae, in Louisiana Coastal Waters. MS Thesis, Louisiana State University, Baton Rouge, LA, USA, 72 p
Bartlett MR, Haedrich RL (1968) Neuston nets and South Atlantic blue marlin Makaira nigricans. Copeia 1968:469–474
Bass RJ, Avault JW Jr (1975) Food habit, length-weight relationship, condition factor, and growth of juvenile red drum, Sciaenops ocellatus, in Louisiana. Trans Am Fish Soc 104:35–45
Baum JK, Myers RA (2004) Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol Lett 7:135–145
Bayley RE, Prince ED (1993) A review of tag release and recapture files for Istiophoridae from the Southeast Fisheries Center’s cooperatives game fish tagging program, 1954 to present. Collect Vol Sci Pap ICCAT 41:527–548
Beardsley GL Jr (1967) Age, growth, and reproduction of the dolphin, Coryphaena hippurus, in the Straits of Florida. Copeia 1967:441–451
Beardsley GL Jr, Merrett NR, Richards WJ (1975) Synopsis of the biology of the Sailfish Istiophorus platypterus (Shaw and Nodder, 1791). In: Proceedings, International Billfish Symposium, Kailua-Kona, HI, USA, August, Part 3, pp 95–120
Beasley M (1993) Age and growth of greater amberjack, Seriola dumerili, from the northern Gulf of Mexico. MS Thesis, Louisiana State University, Baton Rouge, LA, USA, 85 p
Beaumariage DS (1969a) Returns from the 1965 Schlitz Tagging Program including a cumulative analysis of previous results. Florida Board of Conservation, Marine Research Lab, Technical Series No. 59. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 38 p
Beaumariage DS (1969b) Current status of biological investigations of Florida’s Mackerel Fisheries. In: Proceedings, 22nd Annual Meeting of the Gulf and Caribbean Fisheries Institute, Coral Gables, FL, USA, May, pp 79–86
Beaumariage DS (1973) Age, growth, and reproduction of King Mackerel, Scomberomorus cavallas, in Florida. Florida Marine Research Lab, Publication No. I. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 45 p
Beaumariage DS, Bullock LH (1976) Biological research on snappers and groupers as related to fishery management requirements. In: Bullis HR Jr, Jones AC (eds) Colloquium on snapper-grouper fishery resources of the western Central Atlantic Ocean. Florida Sea Grant College Report No. 17. St. Petersburg, FL, USA, pp 86–94
Beaumariage DS, Wittich AC (1966) Returns from the 1964 Schlitz tagging program. Florida Board of Conservation, Marine Research Lab, Technical series no. 47. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 50 p
Bentivoglio AA (1988) Investigations into the growth, maturity, mortality rates and occurrence of the Dolphin (Coryphaena hippurus) in the Gulf of Mexico. MS Thesis, University College of North Wales, Bangor, Wales, UK, 37 p
Bethea DM, Hollensead L, Carlson JK, Ajemian MJ, Grubbs RD, Hoffmayer ER, Del Rio R, Peterson GW, Baltz DM, Romine J (2009) Shark nursery grounds and essential fish habitat studies: Gulfspan Gulf of Mexico FY’08 Cooperative Gulf of Mexico States shark pupping and nursery survey. Contribution report PCB-08/02 to the National Oceanic and Atmospheric Administration, Highly Migratory Species Division, Narragansett, RI, USA, 64 p
Bigelow HB, Schroeder WC (1953) Fishes of the Gulf of Maine. Fish Bull 54:1–577
Biggs DC (1992) Nutrients, plankton, and productivity in a warm-core ring in the western Gulf of Mexico. J Geophys Res 97:2143–2154
Bishop JM, Miglarese JV (1978) Carnivorous feeding in adult striped mullet. Copeia 1978:705–707
Block B (2011) Blacktip reef shark. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/stock-photo-7471636-blacktip-reef-shark.php?st=808bdc3. Accessed 5 December 2016
Block BA, Booth DT, Carey FG (1992) Depth and temperature of the blue marlin, Makaira nigricans, observed by acoustic telemetry. Mar Biol 114:175–183
Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Boustany A, Farwell CJ, Dau DJ, Seitz A (2001a) Archival and pop-up satellite tagging of Atlantic bluefin tuna. In: Sibert JR, Nielsen JL (eds) Electronic tagging and tracking in marine fisheries. Kluwer Academic, Dordrecht, The Netherlands, pp 65–88
Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Farwell CJ, Boustany A, Teo SLH, Seitz A, Walli A, Fudge D (2001b) Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science 293:1310–1314
Block BA, Teo SLH, Walli A, Boustany A, Stokesbury MJW, Farwell CJ, Weng KC, Dewar H, Williams TD (2005) Electronic tagging and population structure of Atlantic Bluefin Tuna. Nature 34:1121–1127
Boland GS, Gallaway BJ, Baker JS, Lewbel GS (1983) Ecological effects of energy development on Reef Fish of the Flower Garden Banks. Report No. NOAA-83120106. NTIS PB84-128289, Contract No. NA80-GA-C-00057. National Marine Fisheries Service, Galveston, TX, USA, 466 p
Boothby RN, Avault WJ Jr (1971) Food habits, length-weight relationship, and condition factor of the red drum (Sciaenops ocellata) in southeastern Louisiana. Trans Am Fish Soc 100:290–295
Bortone SA, Hastings PA, Collard SB (1977) The pelagic-Sargassum ichthyofauna of the eastern Gulf of Mexico. Northeast Gulf Sci 1:60–67
Boustany AM, Reeb CA, Teo SL, De Metrio G, Block BA (2007) Genetic data and electronic tagging indicate that the Gulf of Mexico and Mediterranean Sea are reproductively isolated stocks of bluefin tuna (Thunnus thynnus). Collect Vol Sci Pap ICCAT 60:1154–1159
Boustany AM, Reeb CA, Block BA (2008) Mitochondrial DNA and electronic tracking reveal population structure of Atlantic bluefin tuna (Thunnus thynnus). Mar Biol 156:13–24
Bradley E, Bryan CE (1975) Life history and fishery of the red snapper (Lutjanus campechanus) in the Northwestern Gulf of Mexico. In: Proceedings, 27th annual Gulf and Caribbean Fisheries Institute and the 17th annual international game fish research conference, Miami, FL, USA, November, pp 77–106
Branstetter S (1987) Age and growth estimates for blacktip, Brevoortia gunteri, and spinner, C. brevipinna, sharks from the northwestern Gulf of Mexico. Copeia 1987:964–974
Breder CM Jr (1940) The spawning of Mugil cephalus on the Florida West Coast. Copeia 1940:138–139
Breuer JP (1957) An ecological survey of Baffin and Alazan Bays, Texas. Publ Inst Mar Sci Univ Texas 4:134–155
Briggs JC (1974) Marine zoogeography. McGraw-Hill Companies, New York, NY, USA, 475 p
Broadhead GC (1953) Investigations of the Black Mullet, Mugil cephalus L., in Northwest Florida. Florida Board of Conservation, Marine Research Lab, Technical Series No. 7. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 33 p
Broadhead GC (1958) Growth of the Black Mullet, (Mugil cephalus L.) in West and Northwest Florida. Florida Board of Conservation, Marine Research Lab, Technical Series No. 25. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 29 p
Broadhead GC, Mefford HP (1956) The migration and exploitation of the Black Mullet, Mugil cephalus, Linnaeus, in Florida as determined from tagging during 1949–1953. Florida Board of Conservation, Marine Research Lab, Technical Series No. 18. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 31 p
Brothers EB, Prince ED, Lee DW (1983) Age and growth of young-of-the-year Bluefin Tuna, Thunnus thynnus, from Otolith Microstructure. In: Proceedings, International Workshop on Age Determination of Oceanic Pelagic Fishes: Tunas, Billfishes and Sharks, Miami, FL, USA, February, pp 49–59
Brown-Peterson NJ, Franks JS, Comyns BH, Hendon LA, Hoffmayer ER, Hendon JR, Waller RS (2004) Aspects of the reproduction of large pelagic fishes in the Northern Gulf of Mexico. In: Proceedings, 55th Annual Meeting of the Gulf and Caribbean Fisheries Institute, Xel Ha, Quintana Roo, Mexico, November, pp 1016–1017
Brown-Peterson NJ, Franks JS, Comyns BH, McDowell JR (2008) Do Blue Marlin spawn in the Northern Gulf of Mexico. In: Proceedings, 60th Annual Meeting of the Gulf and Caribbean Fisheries Institute, Punta Cana, Dominican Republic, November, pp 372–378
Brule T, Deniel C, Colas-Marrufo T, Sanchez-Crespo M (1999) Red grouper reproduction in the southern Gulf of Mexico. Trans Am Fish Soc 128:385–402
Buckley J (1984) Habitat suitability index models: Larval and juvenile red drum. FWS/OBS 82/10.74, U.S. Department of the Interior, Fish and Wildlife Service, Washington, DC, USA, 15 p
Bullock LH, Smith GB (1991) Seabasses (Pisces: Serranidae), Memoirs of the Hourglass Cruises, vol VIII, Part II. Florida Marine Research Institute, Department of Natural Resources, St. Petersburg, FL, USA, 243 p
Bumguardner BW, Anderson JD (2008) Age and growth, reproduction and genetics of Billfish in Gulf of Mexico Waters off Texas. In: Proceedings, Atlantic Billfish Research Program Symposium-Gulf States Marine Fisheries Commission Spring Meeting, Galveston, TX, USA, March, pp 52–66
Burgess GH, Beerkircher LR, Cailliet GM, Carlson JK, Cortés E, Goldman KJ, Simpfendorfer CA (2005) Is the collapse of shark populations in the Northwest Atlantic Ocean and Gulf of Mexico real? Fish 30:19–26
Burgos JM (2001) Life history of the Red Grouper (Epinephelus morio) off the North Carolina and South Carolina Coast. University of Charleston, Charleston, SC, USA, 90 p
Burks CM, Driggers WB III, Mullin KD (2006) Abundance and distribution of whale sharks (Rhincodon typus) in the northern Gulf of Mexico. Fish Bull 104:570–584
Burns KM (1981) Seasonal and areal distribution of scombrid larvae in the vicinity of Palm Beach, Florida. MA Thesis, University of South Florida, Tampa, FL, USA, 66 p
Buskey EJ, Hyatt CJ (1995) Effects of The Texas (USA) “brown tide” alga on planktonic grazers. Mar Ecol Prog Ser 126:285–292
Butler MJA, Caddy JF, Dickson CA, Hunt JJ, Burnett CD (1977) Apparent age and growth, based on otolith analysis of giant bluefin tuna (Thunnus thynnus) in the 1975–1976 Canadian catch. Collect Vol Sci Pap ICCAT 5:318–330
Cailliet GM, Natanson LJ, Welden BA, Ebert DA (1985) Preliminary studies on the age and growth of the white shark, Carcharodon carcharias, using vertebral bands. Mem South Calif Acad Sci 9:49–60
Camper JD, Barber RC, Richardson LR, Gold JR (1993) Mitochondrial DNA variation among red snapper (Lutjanus campechanus) from the Gulf of Mexico. Mol Mar Biol Biotechnol 2:154–161
Carey FG (1990) Further acoustic telemetry observations of swordfish. Mar Recreat Fish 13:103–122
Carey FG, Robison BH (1981) Daily patterns in the activities of swordfish, Xiphias gladius, observed by acoustic telemetry. Fish Bull 79:277–292
Carlson JK, Baremore IE (2003) Changes in biological parameters of Atlantic sharpnose shark Rhizoprionodon terraenovae in the Gulf of Mexico: Evidence for density-dependent growth and maturity? Mar Freshw Res 54:227–234
Carlson JK, Parsons GR (1997) Age and growth of the bonnethead shark, Sphyrna tiburo, from Northwest Florida, with comments on clinal variation. Environ Biol Fish 50:331–341
Carlson JK, Cort E, Johnson AG (1999) Age and growth of the blacknose shark, Carcharhinus acronotus, in the eastern Gulf of Mexico. Copeia 1999:684–691
Carlson JK, Bethea DM, Middlemiss A, Baremore IE (2004) Shark nursery grounds and essential fish habitat studies, Gulfspan Gulf of Mexico-FY04. An internal report to NOAA’s Highly Migratory Species Office, Sustainable Fisheries Division Contribution No. PCB-04/06. Panama City Beach, FL, USA, 21 p
Carlson JK, Sulikowski JR, Baremore IE (2006) Do differences in life history exist for blacktip sharks, Carcharhinus limbatus, from the United States South Atlantic Bight and eastern Gulf of Mexico? Environ Biol Fish 77:279–292
Carlsson J, McDowell JR, Carlsson JE, Graves JE (2007) Genetic identity of YOY bluefin tuna from the eastern and western Atlantic spawning areas. J Hered 98:23–28
Carpenter KE (2002) FAO species identification guide for fishery purposes: The living marine resources of the Western Central Atlantic: Bony fishes, Part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. American Society of Ichthyologists and Herpetologists Special Publication No. 5, vol 3. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, pp 1375–2127
Casey JG, Pratt HL Jr (1985) Distribution of the white shark, Carcharodon carcharias, in the western North Atlantic. Mem South Calif Acad Sci 9:2–14
Castillo-Rivera M, Kobelkowsky A, Zamayoa V (1996) Food resource partitioning and trophic morphology of Brevoortia gunteri and B. patronus. J Fish Biol 49:1102–1111
Castro JI (1983) The sharks of North American Waters. Texas A&M University Press, College Station, TX, USA, 180 p
Castro JI (1996) Biology of the blacktip shark, Brevoortia gunteri, off the southeastern United States. Bull Mar Sci 59:508–522
Castro Aguirre JL (1978) Catalogo Sistematico Peces Marinos Pentran a los Advas Contenentals de Mexico con Aspectos Geograficos Etologicos. Cientifica No. 19, Instituto Nacional de Pesca, Benito Juárez, Mexico, 298 p
Cervigon F (1966) Los Peces Marinos de Venezuela. Fundacion La Salle de Ciencias Naturales. Caracas, Venezuela, 951 p
Chase BC (2002) Differences in the diet of Atlantic bluefin tuna (Thunnus thynnus) at five seasonal feeding grounds on the New England continental shelf. Fish Bull 100:168–180
Chesney EJ, Baltz DM, Thomas RG (2000) Louisiana estuarine and coastal fisheries and habitats: Perspectives from a fish’s eye view. Ecol Appl 10:350–366
Chipman WA (1959) Use of radioisotopes in studies of the foods and feeding activities of marine animals. Pubbl Stn Zool Napoli II 31(Suppl):154–175
Chow S, Takeyama H (2000) Nuclear and mitochondrial DNA analyses reveal four genetically separated breeding units of the swordfish. J Fish Biol 56:1087–1098
Christmas JY Jr, Gunter G (1960) Distribution of menhaden, genus Brevoortia, in the Gulf of Mexico. Trans Am Fish Soc 89:338–343
Christmas JY, Waller RS (1973) Mississippi: Estuarine vertebrates. In: Christmas JY (ed) Cooperative Gulf of Mexico Estuarine Inventory and Study, Mississippi. Gulf Coast Research Laboratory, Ocean Springs, MS, USA, pp 320–434
Christmas JY, Waller RS (1975) Location and time of menhaden spawning in the Gulf of Mexico. Gulf Coast Research Laboratory, Ocean Springs, MS, USA, 20 p
Christmas JY, McBee JT, Waller RS, Sutter FC, III (1982) Habitat suitability index models: Gulf menhaden. FWS/OBS 82/10.23. U.S. Department of Interior, Fish and Wildlife Service, Washington, DC, USA, 23 p
Coleman FC, Koenig CC (2010) The effects of fishing, climate change, and other anthropogenic disturbances on red grouper and other reef fishes in the Gulf of Mexico. Integr Comp Biol 50:201–212
Coleman FC, Koenig CC, Collins LA (1996) Reproductive styles of shallow-water groupers (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environ Biol Fish 47:129–141
Collette BB, Nauen CE (1983) FAO species catalogue: An annotated and illustrated catalogue of tunas, mackerels, bonitos and related species known to date. FAO Fisheries Synopsis No. 125, vol 2. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, 137 p
Collette BB, Russo JL (1984) Morphology, systematics, and biology of the Spanish mackerels (Scomberomorus, Scombridae). Fish Bull 82:545–689
Collette BB, Reeb C, Block B (2001) Systematics of the tuna and mackerels (Scombridae). In: Block BA, Stevens ED (eds) Tuna: Physiology, ecology, and evolution. Academic Press, San Diego, CA, USA, pp 5–35
Collette B, Acero A, Amorim AF, Boustany A, Canales RC, Cardenas G, Carpenter N, Di Natale A, Fox W, Fredou FL, Graves J, Viera Hazin FH, Juan Jorda M, Minte Vera C, Miyabe N, Cruz R, Nelson R, Oxenford H, Schaefer K, Serra R, Sun C, Teixeira RP, Pires F, Travassos PE, Uozumi Y, Yanez E (2012) Coryphaena hippurus. In: IUCN (International Union for the Conservation of Nature). 2012 IUCN red list of threatened species, Version 2012.2. http://www.iucnredlist.org/. Accessed 31 May 2013
Collins MR (1981) The feeding periodicity of striped mullet Mugil cephalus, Linnaeus, in two Florida habitats. J Fish Biol 19:307–315
Collins MR (1985) Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (South Florida), striped mullet. U.S. Fish and Wildlife Service Biology Report 82(11.34), TR EL-82-4. U.S. Army Corps of Engineers, Coastal Ecology Group, Waterways Experiment Station, Vicksburg, MS, USA. 11 p
Collins LA, Johnson AG, Keim CP (1996) Spawning and annual fecundity of the red snapper (Lutjanus campechanus) from the northeastern Gulf of Mexico. In: Arreguín-Sánchez F, Munro JL, Balgos MC, Pauly D (eds) Biology, fisheries and culture of tropical groupers and snappers. ICLARM Conference Proceedings 48, Campeche, Mexico, pp 174–188
Collins LA, Fitzhugh GR, Mourand L, Lombardi LA, Walling WT Jr, Fable WA, Burnett MR, Allman RJ (2001) Preliminary results from a continuing study of spawning and fecundity in the red snapper (Lutjanidae: Lutjanus campechanus) from the Gulf of Mexico, 1998–1999. In Proceedings, 52nd Annual Meeting of the Gulf and Caribbean Fisheries Institute, Key West, FL, USA, November, pp 34–47
Collins LA, Fitzhugh GR, Lombardi-Carlson LA, Lyon HM, Walling WT, Oliver DW (2002) Characterization of Red Grouper (Serranidae: Epinephelus morio) reproduction from the Eastern Gulf of Mexico. Panama City Laboratory Contribution Series 2002-07, National Marine Fisheries Service, Southeastern Fisheries Science Center, Panama City Beach, FL, USA, 20 p
Combs RM (1969) Embryogenesis, histology and organology of the ovary of Brevoortia patronus. Gulf Res Rep 2:333–434
Compagno LJV (1984) FAO species catalogue: An annotated and illustrated catalogue of shark species known to date, Part 2: Carcharhiniformes. FAO Fisheries Synopsis No. 12, vol 4. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, pp 251–655
Cook M, Barnett BK, Duncan MS, Allman RJ, Porch CE, Fitzhugh GR (2009) Characterization of red snapper (Lutjanus campechanus) size and age at sexual maturity for the 2009 Gulf of Mexico SEDAR Update, Draft working document. Panama City Laboratory Contribution Series 2009–16, National Marine Fisheries Service, Southeastern Fisheries Science Center, Panama City Beach, FL, USA, August, 16 p
Copeland BJ, Bechtel TJ (1974) Some environmental limits of six Gulf Coast estuarine organisms. Contrib Mar Sci 18:169–203
Cort JL (1991) Age and growth of the bluefin tuna, Thunnus thynnus (L.) of the Northeast Atlantic. Collect Vol Sci Pap ICCAT 35:213–230
Cortés E (2008) Catches of pelagic sharks from the western North Atlantic Ocean, including the Gulf of Mexico and Caribbean Sea. Collect Vol Sci Pap ICCAT 62:1434–1446
Cowan JH, Grimes CB, Patterson WF, Walters CJ, Jones AC, Lindberg WJ, Rose KA (2011) Red snapper management in the Gulf of Mexico: Science-or faith-based? Rev Fish Biol Fish 21:87–204
Cramer J, Scott G (1998) Summarization of catch and effort in the pelagic longline fishery and analysis of the effect of two degree square closures on swordfish and discards landings. Sustainable Fisheries Division Contribution MIA-97/98-17, Southeast Fisheries Science Center, Miami, FL, USA, 22 p
Crane J (1936) Notes on the biology and ecology of giant tuna Thunnus thynnus, L., observed at Portland, Maine. Zoologica 21:207–212
Cranswick D, Regg J (1997) Deep-water in the Gulf of Mexico: America’s new frontier. OCS Rep. 97-0004. U.S. Department of the Interior, Minerals Management Service, New Orleans, LA, USA, 41 p
CRFM (Caribbean Regional Fisheries Mechanism) (2006) Report of the Second Annual Scientific Meeting—Port of Spain, Trinidad and Tobago, 13–22 March 2006, vol 1, Suppl 1. CRFM Secretariat, Belize and St. Vincent and the Grenadines, 48 p
crisod (2013) White shark. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/stock-photo-17993543-white-shark.php?st=33b18. Accessed 5 December 2016
Dahlberg MD (1970) Atlantic and Gulf of Mexico menhadens, genus Brevoortia (Pisces: Clupeidae). Bull Fla State Mus Biol Sci 15:91–162
Damalas D, Megalofonou P, Apostolopoulou M (2007) Environmental, spatial, temporal and operational effects on swordfish (Xiphias gladius) catch rates of eastern Mediterranean Sea longline fisheries. Fish Res 84:233–246
Darnell RM (1958) Food habits of fishes and larger vertebrates of Lake Pontchartrain, Louisiana, an estuarine community. Publ Inst Mar Sci Univ Texas 5:354–416
Dascher C (2013) Great white shark [Internet]. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/stock-photo-7436967-great-white-shark.php?st=290490c. Accessed 5 December 2016
Davies JH, Bortone SA (1976) Partial food list of 3 species of Istiophoridae (Pisces) from the northeastern Gulf of Mexico. Fla Sci 39:249–253
Davis TL (1991) Advection, dispersion and mortality of a patch of southern bluefin tuna larvae Thunnus maccoyii in the East Indian Ocean. Mar Ecol Prog Ser 73:33–45
De Metrio G, Arnold GP, de la Serna JM, Block BA, Megalofonou P, Lutcavage M, Oray I, Deflorio M (2005) Movements of bluefin tuna (Thunnus thynnus L.) tagged in the Mediterranean Sea with pop-up satellite tags. Collect Vol Sci Pap ICCAT 58:1337–1340
De Silva SS (1980) Biology of juvenile grey mullet: A short review. Aquaculture 19:21–36
De Sylva DP, Breder PR (1997) Reproduction, gonad histology, and spawning cycles of North Atlantic billfishes (Istiophoridae). Bull Mar Sci 60:668–697
De Sylva DP, Richards WJ, Capo TR, Serafy JE (2000) Potential effects of human activities on billfishes (Istiophoridae and Xiphiidae) in the western Atlantic Ocean. Bull Mar Sci 66:187–198
Deegan LA (1985) The population ecology and nutrient transport in Gulf Menhaden in Fourleague Bay, Louisiana. PhD Thesis, Louisiana State University, Baton Rouge, LA, USA, 134 p
DeepAqua (2010) Bluefin tuna in net. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/photo/bluefin-tuna-in-net-gm95662678-11554516?clarity=false. Accessed December 4, 2016
DeVries DA, Grimes CB (1997) Spatial and temporal variation in age and growth of king mackerel, Scomberomorus cavallas, 1977–1992. Fish Bull 95:694–708
DeVries DA, Grimes CB, Lang KL, White BDW (1990) Age and growth of king and Spanish mackerel larvae and juveniles from the Gulf of Mexico and U.S. South Atlantic Bight. Environ Biol Fish 29:135–143
DeVries DA, Brusher JH, Fitzhugh GR (2006) Spatial and temporal patterns in demographics and catch rates of red grouper from a fishery-independent trap survey in the Northeast Gulf of Mexico, 2004–2005. SEDAR 12-DW-08, Panama City Laboratory Contribution Series 2006-13, National Marine Fisheries Service, Southeastern Fisheries Science Center, Panama City Beach, FL, USA, 7 p
Ditty JG, Shaw RF (1996) Spatial and temporal distribution of larval striped mullet (Mugil cephalus) and white mullet (Mugil curema, Family: Mugilidae) in the northern Gulf of Mexico, with notes on mountain mullet, Gonostomus monticola. Bull Mar Sci 59:271–288
Ditty JG, Shaw RF, Grimes CB, Cope JS (1994) Larval development, distribution, and abundance of common dolphin, Coryphaena hippurus, and pompano dolphin, C. equiselis (Family: Coryphaenidae), in the northern Gulf of Mexico. Fish Bull 92:275–291
Dombrowski B (2012) Red grouper swimming in coral reef underwater. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/photo/red-grouper-swimming-in-coral-reef-underwater-gm139547530-377657. Accessed December 4, 2016
Dooley JK (1978) Systematics and biology of the tilefishes (Perciformes: Branchiostegidae and Malacanthidae), with description of two new species. NOAA Technical Report Circular No. 411. National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Washington, DC, USA, 78 p
Draganik B, Cholyst J (1988) Temperature and moonlight as stimulators for feeding activity by swordfish. Collect Vol Sci Pap ICCAT 27:305–314
Dragovich A (1970) The food of bluefin tuna (Thunnus thynnus) in the western North Atlantic Ocean. Trans Am Fish Soc 99:723–731
Driggers W, Carlson J, Cullum B, Dean J, Oakley D (2004) Age and growth of the blacknose shark, Carcharhinus acronotus, in the western North Atlantic Ocean with comments on regional variation in growth rates. Environ Biol Fish 71:171–178
Duarte-Neto P, Lessa R, Stosic B, Morize E (2008) The use of sagittal otoliths in discriminating stocks of common dolphinfish (Coryphaena hippurus) off northeastern Brazil using multishape descriptors. ICES J Mar Sci 65:1144–1152
Ebert DA (2003) Sharks, rays and chimaeras of California. University of California Press, Berkeley, CA, USA, 287 p
Eggleston DB, Bochenek EA (1990) Stomach contents and parasite infestation of school bluefin tuna, Thunnus thynnus, collected from the Middle Atlantic Bight, Virginia. Fish Bull 88:389–395
Eggold BT, Motta PJ (1992) Ontogenetic dietary shifts and morphological correlates in striped mullet, Mugil cephalus. Environ Biol Fish 34:139–158
Erickson DL, Harris MJ, Grossman GD (1985) Ovarian cycling of tilefish, Lopholatilus chamaeleonticeps Goode and Bean, from the South Atlantic Bight, USA. J Fish Biol 27:131–146
Etnier DA, Starnes WC (1993) The fishes of Tennessee. University of Tennessee Press, Knoxville, TN, USA, 681 p
Etzold DJ, Christmas JY (1979) A Mississippi marine finfish management plan. MASGP-78-046, Mississippi-Alabama Sea Grant Consortium, Ocean Springs, MS, USA, 36 p
Fable WA Jr, Trent L, Bane GW, Ellsworth SW (1987) Movements of king mackerel, Scomberomorus cavallas, tagged in Southeast Louisiana, 1983–1985. Mar Fish Rev 49:98–101
Fahay MP (1983) Guide to the early stages of marine fishes occurring in the western North Atlantic Ocean, Cape Hatteras to the southern Scotian Shelf. J Northw Atl Fish Sci 4:1–423
Fahay MP, Berrien P (1981) Preliminary description of larval tilefish (Lopholatilus chamaeleonticeps). Rapp PV Cons Int Explor Mer 178:600–602
FFWCC (Florida Fish and Wildlife Conservation Commission) (2013a) Eagle rays: Cownose rays. http://myfwc.com/research/saltwater/sharks-rays/ray-species/cownose-ray/. Accessed 31 May 2013
FFWCC (2013b) Manta rays: Giant Manta. http://myfwc.com/research/saltwater/sharks-rays/ray-species/giant-manta/. Accessed 31 May 2013
FFWCC (2013c) Sawfishes: Smalltooth Sawfish. http://myfwc.com/research/saltwater/sharks-rays/ray-species/smalltooth-sawfish/. Accessed 31 May 2013
Finucane JH, Collins LA, Barger LE (1978) Spawning of the striped mullet, Mugil cephalus, in the northwestern Gulf of Mexico. Northeast Gulf Sci 2:148–151
Finucane JH, Collins LA, Brusher HA, Saloman CH (1986) Reproductive biology of king mackerel, Scomberomorus cavallas, from the southeastern United States. Fish Bull 84:841–850
Fischer W, Bianchi G, Scott WB (eds) (1978) FAO species identification sheets for fishery purposes: Western Central Atlantic, Fishing Area 31, vol IV. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy
Fischer AJ, Baker MS Jr, Wilson CA (2004) Red snapper (Lutjanus campechanus) demographic structure in the northern Gulf of Mexico based on spatial patterns in growth rates and morphometrics. Fish Bull 102:593–603
FishBase (2013) http://fishbase.org/. Accessed 31 May 2013
Fitzhugh GR, Duncan MS, Collins LA, Walling WT, Oliver DW (2004) Characterization of red snapper (Lutjanus campechanus) reproduction for the 2004 Gulf of Mexico SEDAR. SEDAR 7-DW-35, Panama City Laboratory Contribution Series 2004-01. National Marine Fisheries Service, Southeastern Fisheries Science Center, Panama City Beach, FL, USA, 29 p
Fitzhugh GR, Lyon HM, Walling WT, Levins CF, Lombardi-Carlson LA (2006) An update of Gulf of Mexico red grouper reproductive data and parameters for SEDAR 12. SEDAR 12-DW-04, Panama City Laboratory Contribution Series 2006-14, National Marine Fisheries Service, Southeastern Fisheries Science Center, Panama City Beach, FL, USA, 18 p
Fitzhugh GR, Levins CF, Walling WT, Gamby M, Lyon H, DeVries DA (2008) Batch fecundity and an attempt to estimate spawning frequency of king mackerel (Scomberomorus cavallas) in U.S. Waters. SEDAR 16-DW-06, Southeast Data, Assessment and Review, North Charleston, SC, USA, 17 p
Fore PL (1970) Oceanic distribution of eggs and larvae of the Gulf menhaden. Report of the Bureau of Commercial Fisheries Biology Laboratory, Beaufort, North Carolina, for the Fiscal Year Ending June 30, 1968, U.S. Fish and Wildlife Service Circular 341, pp 11–13
Fore PL, Baxter KN (1972) Diel fluctuations in the catch of larval Gulf menhaden, Brevoortia patronus, at Galveston Entrance, Texas. Trans Am Fish Soc 101:729–732
Francis M (1996) Observations of a pregnant white shark with a review of reproductive biology. In: Klimley AP, Ainley DG (eds) Great White Sharks: The biology of Carcharodon carcharias. Academic Press, San Diego, CA, USA, pp 157–172
Franks JS (2000) A review: Pelagic fishes at petroleum platforms in the Northern Gulf of Mexico; diversity, interrelationships, and perspective. Pêche thonière et dispositifs de concentration de poissons, Caribbean-Martinique, 15–19 Oct 1999
Franks JS (2003) First record of goliath grouper, Epinephelus itajara, in Mississippi coastal waters with comments on the first documented occurrence of red grouper, Epinephelus morio, off Mississippi. In: Proceedings, 56th Annual Meeting of the Gulf Caribbean Fisheries Institute, Tortola, British Virgin Islands, November, pp 295–306
Freeman BL, Turner SC (1977) Biological and fisheries data on tilefish, Lopholatilus chamaeleonticeps Goode and Bean. Sandy Hook Laboratory Technical Series Report No. 5. Northeast Fisheries Science Center, National Marine Fisheries Service, Sandy Hook, NJ, USA, 41 p
Friedland KD, Ahrenholz DW, Smith JW, Manning M, Ryan J (2006) Sieving functional morphology of the gill raker feeding apparatus of Atlantic menhaden. J Exp Zool A Comp Exp Biol 305:974–985
Froese R, Pauly D (eds) (2009) FishBase 2009: Concepts, design and data sources. www.fishbase.org. Accessed 31 May 2013
Fromentin JM (2009) Lessons from the past: Investigating historical data from bluefin tuna fisheries. Fish 10:197–216
Fromentin JM, Powers JE (2005) Atlantic bluefin tuna: Population dynamics, ecology, fisheries and management. Fish 6:281–306
Ftlaudgirl (2016a) Redfish is swimming in the grass flats ocean. Bigstockphoto, New York, NY, USA. http://www.bigstockphoto.com/image-41736652/stock-photo-redfish-is-swimming-in-the-grass-flats-ocean. Accessed 5 December 2016
Ftlaudgirl (2016b) Mahi mahi swimming underwater in blue ocean. Bigstockphoto, New York, NY, USA. http://www.bigstockphoto.com/image-33299180/stock-photo-mahi-mahi-swimming-underwater-in-blue-ocean. Accessed 5 December 2016
Furnestin J, Dardignac J (1962) Le thon rouge du maroc atlantique (Thunnus thynnus Linn‚). Rev Trav Inst Marit 26:382–398
Futch C (1966) Lisa—The Florida black mullet. Florida Board of Conservation, Marine Research Laboratory, Leaflet Series 6, St. Petersburg, FL, USA, 6 p
Futch C (1976) Biology of striped mullet. In: Cato JC, McCullough WE (eds) Economics, biology, and food technology of mullet. Florida Sea Grant Program Report 15, Gainesville, FL, USA, pp 63–69
Futch RB, Burger GE (1976) Age, growth, and production of red snapper in Florida waters. In: Bullis HR Jr, Jones AC (eds) Colloquium on Snapper-Grouper fishery resources of the Western Central Atlantic Ocean. Florida Sea Grant Program Report 17, Gainesville, FL, USA, pp 165–184
Gallaway BJ (1981) An ecosystem analysis of oil and gas development on the Texas-Louisiana Continental Shelf. FWS/OBS-81/27, U.S. Fish and Wildlife Service, Office of Biological Services, Washington, DC, USA, 89 p
Gallaway BJ, Lewbel GS (1982) The ecology of petroleum platforms in the northwestern Gulf of Mexico: A community profile. FWS/OBS 82/27, U.S. Fish and Wildlife Service, 92 p
Gallaway BJ, Cole JG, Meyer R, Roscigno P (1999) Delineation of essential habitat for juvenile red snapper in the northwestern Gulf of Mexico. Trans Am Fish Soc 128:713–726
Gallaway BJ, Gazey WJ, Cole JG, Fechhelm RG (2007) Estimation of potential impacts from offshore liquefied natural gas terminals on red snapper and red drum fisheries in the Gulf of Mexico: an alternative approach. Trans Am Fish Soc 136:655–677
Gallaway BJ, Szedlmayer ST, Gazey WJ (2009) A life history review for red snapper in the Gulf of Mexico with an evaluation of the importance of offshore petroleum platforms and other artificial reefs. Rev Fish Sci 17:48–67
Garcia A, Alemany F, de la Serna JM, Oray I, Karakulak S, Rollandi L, Arigo A, Mazzola S (2005) Preliminary results of the 2004 bluefin tuna larval surveys off different Mediterranean sites (Balearic Archipelago, Levantine Sea, and the Sicilian Channel). Collect Vol Sci Pap ICCAT 58:1420–1428
García-Cortés B, Mejuto J, Quintans M (2003) Summary of swordfish (Xiphias gladius) recaptures carried out by the Spanish surface longline fleet in the Atlantic Ocean: 1984–2002. Collect Vol Sci Pap ICCAT 55:1476–1484
Gesteira TCV, Mesquita ALL (1976) Epoca de reproducao tamanho e idade na primeira desova da cavala e da serra, na costa do Estado do Ceara (Brasil). Arq Cienc Mar 16:83–86
Gibbs RH, Collette BB (1959) On the identification, distribution, and biology of the dolphins, Coryphaena hippurus and C. equiselis. Bull Mar Sci Gulf Caribb 9:117–152
GMFMC (Gulf of Mexico Fishery Management Council) (2004a) Amendment 22 of the Reef Fish Fishery Management Plan to Set Red Snapper Sustainable Fisheries Act Targets and Thresholds, Set a Rebuilding Plan, and Establish Bycatch Reporting Methodologies for the Reef Fish Fishery, Tampa, FL, USA, 76 p
GMFMC (2004b) EFH map for reef fish in the final environmental impact statement for the generic amendment to the fishery management plans of the Gulf of Mexico. http://www.habitat.noaa.gov/protection/efh/newInv/index.html/. Accessed 31 May 2013
GMFMC (2004c) EFH Map for red drum in the final environmental impact statement for the generic amendment to the fishery management plans of the Gulf of Mexico. http://www.habitat.noaa.gov/protection/efh/newInv/index.html/. Accessed 31 May 2013
GMFMC (2004d) EFH map for coastal migratory pelagics in the final environmental impact statement for the generic amendment to the fishery management plans of the Gulf of Mexico. http://www.habitat.noaa.gov/protection/efh/newInv/index.html/. Accessed 31 May 2013
GMFMC (2005) Generic Amendment Number 3 for Addressing Essential Fish Habitat Requirements, Habitat Areas of Particular Concern, and Adverse Effects of Fishing in the Following Fishery Management Plans of the Gulf of Mexico: Shrimp Fishery of the Gulf of Mexico, United States Waters; Red Drum Fishery of the Gulf of Mexico; Reef Fish Fishery of the Gulf of Mexico; Coastal Migratory Pelagic Resources (Mackerels) in the Gulf of Mexico and South Atlantic; Stone Crab Fishery of the Gulf of Mexico; Spiny Lobster in the Gulf of Mexico and South Atlantic; Coral and Coral Reefs of the Gulf of Mexico. Tampa, FL, USA, 104 p
GMFMC (2011) Final regulatory amendment to set 2011–2015 total allowable catch and adjust bag limit for red grouper. Tampa, FL, USA, 46 p
GMFMC and SAFMC (South Atlantic Fishery Management Council) (2011) Final Amendment 18 to the Fishery Management Plan for Coastal Migratory Pelagic Resources in the Gulf of Mexico and Atlantic Region Including Environmental Assessment, Regulatory Impact Review, and Regulatory Flexibility Act Analysis. GMFMC, Tampa, FL, USA and South Atlantic Fishery Management Council, North Charleston, SC, USA, 373 p
Gold JR, Richardson LR (1991) Genetic studies in marine fishes. IV. An analysis of population structure in the red drum (Sciaenops ocellatus) using mitochondrial DNA. Fish Res 12:213–241
Gold JR, Richardson LR (1998) Population structure in greater amberjack, Seriola dumerili, from the Gulf of Mexico and the western Atlantic Ocean. Fish Bull 96:767–778
Gold JR, Turner T (2002) Population structure of red drum (Sciaenops ocellatus) in the northern Gulf of Mexico, as inferred from variation in nuclear-encoded microsatellites. Mar Biol 140:249–265
Gold JR, Richardson LR, Furman C, King TL (1993) Mitochondrial DNA differentiation and population structure in red drum (Sciaenops ocellatus) from the Gulf of Mexico and Atlantic Ocean. Mar Biol 116:175–185
Gold JR, Sun F, Richardson LR (1997) Population structure of red snapper from the Gulf of Mexico as inferred from analysis of mitochondrial DNA. Trans Am Fish Soc 126:386–396
Gold JR, Richardson LR, Turner TF (1999) Temporal stability and spatial divergence of mitochondrial DNA haplotype frequencies in red drum (Sciaenops ocellatus) from coastal regions of the western Atlantic Ocean and Gulf of Mexico. Mar Biol 133:593–602
Goldstein RM, Simon PT (1999) Toward a united definition of guild structure for feeding ecology of North American freshwater fishes. In: Simon TP (ed) Assessing the sustainability and biological integrity of water resources using fish communities. CRC Press, Boca Raton, FL, USA, pp 123–202
Goodyear CP (1989) Status of the red drum stocks of the Gulf of Mexico report for 1989. CRD 88/89-14, Southeast Fisheries Science Center, Miami Laboratory, Coastal Resources Division, Miami, FL, USA, 64 p
Goodyear CP (1995) Mean size at age: An evaluation of sampling strategies using simulated red grouper data. Trans Am Fish Soc 124:746–755
Govoni JJ, Grimes CB (1992) The surface accumulation of larval fishes by hydrodynamic convergence within the Mississippi River plume front. Cont Shelf Res 12:1265–1276
Govoni JJ, Hoss DE, Chester AJ (1983) Comparative feeding of three species of larval fishes in the northern Gulf of Mexico: Brevoortia patronus, Leiostomus xamhurus, and Micropogonius undulates. Mar Ecol Prog Ser 13:189–199
Govoni JJ, Hoss DE, Colby DR (1989) The spatial distribution of larval fishes about the Mississippi River plume. Limnol Oceanogr 34:178–187
Govoni JJ, Laban EH, Hare JA (2003) The early life history of swordfish (Xiphias gladius) in the western North Atlantic. Fish Bull 101:778–789
Grall C, de Sylva DP, Houde ED (1983) Distribution, relative abundance, and seasonality of swordfish larvae. Trans Am Fish Soc 112:235–246
Greeley MS Jr, Calder DR, Wallace RA (1987) Oocyte growth and development in the striped mullet, Mugil cephalus, during seasonal ovarian recrudescence: Relationship to fecundity and size at maturity. Fish Bull 85:187–200
Grimes CB, Finucane JH (1991) Spatial distribution and abundance of larval and juvenile fish, chlorophyll and macrozooplankton around the Mississippi River discharge plume, and the role of the plume in fish recruitment. Mar Ecol Prog Ser 75:109–119
Grimes CB, Turner SC (1999) The complex life history of tilefish, Lopholatilus chamaeleonticeps. Am Film 23:17–26
Grimes CB, Turner SC, Able KW (1983) A technique for tagging deepwater fish. Fish Bull 81:663–666
Grimes CB, Johnson AG, Fable WA Jr (1987) Delineation of King Mackerel (Scomberomorus cavalla) stocks along the U.S. East Coast and in the Gulf of Mexico. In: Proceedings, Stock Identification Workshop, Panama City Beach, FL, USA, November, pp 186–187
Grimes CB, Idelberger CF, Able KW, Turner SC (1988) The reproductive biology of tilefish, Lopholatilus chamaeleonticeps Goode and Bean, from the United States Mid-Atlantic Bight, and the effects of fishing on the breeding system. Fish Bull 86:745–762
Grimes CB, Finucane JH, Collins LA, DeVries DA (1990) Young king mackerel, Scomberomorus cavallas, in the Gulf of Mexico, a summary of the distribution and occurrence of larvae and juveniles, and spawning dates for Mexican juveniles. Bull Mar Sci 46:640–654
Gudger EW (1929) On the morphology, coloration and behavior of seventy teleostean fishes of Tortugas, Florida. Carnegie Inst Wash Pap Tortugas Lab 26:149–204
Gunter G (1945) Studies on marine fishes of Texas. Publ Inst Mar Sci Univ Texas 1:1–190
Hampton J (2000) Natural mortality rates in tropical tunas: Size really does matter. Can J Fish Aquat Sci 57:1002–1010
Harris MJ, Grossman GD (1985) Growth, mortality, and age composition of a lightly exploited tilefish stock off Georgia. Trans Am Fish Soc 114:837–846
Harris PJ, Wyanski DM, White DB, Mikell PP, Eyo PB (2007) Age, growth, and reproduction of greater amberjack off the southeastern U.S. Atlantic coast. Trans Am Fish Soc 136:1534–1545
Hassler WW, Hogarth WT (1977) The growth and culture of dolphin (Coryphaena hippurus) in North Carolina. Aquaculture 12:115–122
Heath SR, Eckmayer WJ, Wade CW, Trimble WC, Tatum WM (1979) Research and management of Alabama coastal fisheries. Annual Progress Report PL 88-309, Project 2-330-R-1. Alabama Marine Resources Division, Gulf Shores, AL, USA, 70 p
Heemstra PC, Randall JE (1993) FAO Species catalogue: An annotated and illustrated catalogue of the grouper, Rockcod, Hind, Coral Grouper, and Lyretail Species known to date. FAO Fisheries Synopsis No. 125, vol 16. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, 382 p
Heist EJ, Gold JR (2000) DNA microsatellite loci and genetic structure of red snapper in the Gulf of Mexico. Trans Am Fish Soc 129:469–475
Heist EJ, Musick JA, Graves JE (1996) Genetic population structure of the shortfin mako (Isurus oxyrinchus) inferred from restriction fragment length polymorphism analysis of mitochondrial DNA. Can J Fish Aquat Sci 53:583–588
Hellier WF Jr (1968) Artificial fertilization among yellowfin and Gulf menhaden (Brevoortia) and their hybrid. Trans Am Fish Soc 97:119–123
Heupel MR, Simpfendorfer CA (2005) Quantitative analysis of aggregation behavior in juvenile blacktip sharks. Mar Biol 147:1239–1249
Hildebrand SF (1948) A review of the American menhaden, genus Brevoortia, with a description of a new species. Smithson Misc Coll 107:1–39
Hill KT, Cailliet GM, Radtke RL (1989) A comparative analysis of growth zones in four calcified structures of Pacific blue marlin, Makaira nigricans. Fish Bull 87:829–843
Hoenig JM (1983) Empirical use of longevity data to estimate mortality rates. Fish Bull 8:898–903
Hoese HD, Moore RH (1998) Fishes of the Gulf of Mexico-Texas, Louisiana, and adjacent waters, 2nd edn. Texas A&M University Press, College Station, TX, USA, 422 p
Hoffmayer ER, Franks JS, Driggers WB, Oswald KJ, Quattro JM (2007) Observations of a feeding aggregation of whale sharks, Rhincodon typus, in the North Central Gulf of Mexico. Gulf Caribb Res 19:69–74
Holliday M (1978) Food of Atlantic Bluefin Tuna, Thunnus thynnus (L.), from the coastal waters of North Carolina to Massachusetts. Long Island University, Long Island, NY, USA, 31 p
Holt J, Godbout R, Arnold CR (1981) Effects of temperature and salinity on egg hatching and larval survival of red drum, Sciaenops ocellatus. Fish Bull 79:569–573
Homma K, Maruyama T, Itoh T, Ishihara H, Uchida S (1999) Biology of the manta ray, Manta birostris Walbaum, in the Indo-Pacific. In: Proceedings, 5th Indo-Pacific Fisheries Conference, Noumea, New Caledonia, November, pp 209–216
Houde E, Swanson LJ Jr (1975) Description of eggs and larvae of yellowfin menhaden, Brevoortia smithi. Fish Bull 73:660–673
Ibañez Aguirre AL, Gallardo-Cabello M, Chiappa CX (1999) Growth analysis of striped mullet, Mugil cephalus, and white mullet, M. curema (Pisces: Mugilidae), in the Gulf of Mexico. Fish Bull 97:861–872
ICCAT (International Commission for the Conservation of Atlantic Tunas) (1997) Report of the ICCAT SCRS bluefin tuna stock assessment session. Collect Vol Sci Pap ICCAT 46:1–186
ICCAT (2001) Report of the fourth ICCAT billfish workshop. Collect Vol Sci Pap ICCAT 53:1–130
ICCAT (2010) Report of the 2009 sailfish stock assessment. Collect Vol Sci Pap ICCAT 65:1507–1632
ICCAT (2011a) ICCAT geographical delimitations (2011 version). http://www.iccat.int/Data/ICCATMaps2011.pdf. Accessed 31 May 2013
ICCAT (2011b) Report of the 2010 Atlantic bluefin tuna stock assessment session. Collect Vol Sci Pap ICCAT 66:505–714
ICCAT (2012a) Report of the 2012 Atlantic bluefin tuna stock assessment session. Document No. SCI-033/2012. International Commission for the Conservation of Atlantic Tunas, Madrid, Spain, 124 p
ICCAT (2012b) Report of the 2011 blue marlin stock assessment and white marlin data preparatory meeting. Collect Vol Sci Pap ICCAT 68:1273–1386
Ingram GW Jr (2006) Data summary of gray triggerfish (Balistes capriscus), vermilion snapper (Rhomboplites aurorubens), and greater amberjack (Seriola dumerili) collected during small pelagic trawl surveys, 1988–1996. SEDAR 9-DW-22, Southeast Data, Assessment and Review, North Charleston, SC, USA, 13 p
Jannke TE (1971) Abundance of young sciaenid fishes in Everglades National Park, Florida, in relation to season and other variables. University of Miami, Coral Gables, FL, USA, 128 p
Jenkins GP, Davis TLO (1990) Age, growth rate, and growth trajectory determined from otolith microstructure of southern bluefin tuna Thunnus maccoyii larvae. Mar Ecol Prog Ser 63:93–104
Jenkins GP, Young JW, Davis TL (1991) Density dependence of larval growth of a marine fish, the southern bluefin tuna, Thunnus maccoyii. Can J Fish Aquat Sci 48:1358–1363
Jensen AL (1996) Beverton and Holt Life history invariants result from optimal trade-off of reproduction and survival. Can J Fish Aquat Sci 53:820–822
Johnson GD (1978) Development of fishes of the Mid-Atlantic Bight: An atlas of egg, larval and juvenile stages, Part IV: Carangidae through Ephippidae. FWS/OB5-78/12, U.S. Fish and Wildlife Service, Office of Biological Sciences, Washington, DC, USA, 314 p
Johnson AG, Fable WA Jr, Williams ML, Barger LE (1983) Age, growth, and mortality of king mackerel, Scomberomorus cavalla, from the southeastern United States. Fish Bull 81:97–106
Johnson AG, Fable WA Jr, Grimes CB, Trent L, Perez JV (1994) Evidence for distinct stocks of king mackerel, Scomberomorus cavallas, in the Gulf of Mexico. Fish Bull 92:91–101
Johnson AK, Thomas P, Wilson RR Jr (1998) Seasonal cycles of gonadal development and plasma sex steroid levels in Epinephelus morio, a protogynous grouper in the eastern Gulf of Mexico. J Fish Biol 52:502–518
Jory DE, Iverson ES (1989) Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (South Florida): Black, red, and Nassau Groupers. U.S. Fish and Wildlife Service Biology Report 82(11.110), TR EL-82-4. U.S. Army Corps of Engineers, Coastal Ecology Group, Waterways Experiment Station, Vicksburg, MS, USA, 21 p
June FC, Carlson FT (1971) Food of young Atlantic menhaden, Brevoortia tyrannus, in relation to metamorphosis. Fish Bull 68:493–512
June FC, Chamberlin JL (1959) The role of the estuary in the life history and biology of Atlantic menhaden. In: Proceedings, 11th Annual Meeting of the Gulf and Caribbean Fisheries Institute, Coral Gables, FL, USA, November, pp 41–45
Karakulak S, Oray I, Corriero A, Deflorio M, Santamaria N, Desantis S, De Metrio G (2004a) Evidence of a spawning area for the bluefin tuna (Thunnus thynnus L.) in the eastern Mediterranean. J Appl Ichthyol 20:318–320
Karakulak S, Oray I, Corriero A, Aprea A, Spedicato D, Zubani D, Santamaria N, De Metrio G (2004b) First information on the reproductive biology of the bluefin tuna (Thunnus thynnus) in the eastern Mediterranean. Collect Vol Sci Pap ICCAT 56:1158–1162
Karnauskas M, Schirripa MJ, Kelble CR, Cook GS, Craig JK (2013) Ecosystem status report for the Gulf of Mexico. NOAA Technical Memorandum NMFS-SEFSC-653. NOAA, Washington, DC, USA, 52 p
Kasapidis P, Mejuto J, Tserpes G, Antoniou A, Garcia-Cortes B, Peristeraki P, Magoulas A (2007) Genetic structure of the swordfish (Xiphias gladius) stocks in the Atlantic using microsatellite DNA analysis. Collect Vol Sci Pap ICCAT 61:89–98
Katz SJ, Grimes CB, Able KW (1983) Delineation of tilefish, Lopholatilus chamaeleonticeps, stocks along the United States East Coast and in the Gulf of Mexico. Fish Bull 81:41–50
Keeney DB, Heupel M, Hueter RE, Heist EJ (2003) Genetic heterogeneity among blacktip shark, Brevoortia gunteri, continental nurseries along the U.S. Atlantic and Gulf of Mexico. Mar Biol 143:1039–1046
Keeney DB, Heupel MR, Hueter RE, Heist EJ (2005) Microsatellite and mitochondrial DNA analyses of the genetic structure of blacktip shark (Brevoortia gunteri) nurseries in the northwestern Atlantic, Gulf of Mexico, and Caribbean Sea. Mol Ecol 14:1911–1923
Kerstetter DW, Bayse SM, Graves JE (2010) Sailfish (Istiophorus platypterus) habitat utilization in the southern Gulf of Mexico and Florida Straits with Implications on vulnerability to shallow-set pelagic longline gear. Collect Vol Sci Pap ICCAT 65:1701–1712
Kilby JD (1955) The fishes of two Gulf coastal marsh areas of Florida. Tulane Stud Zool 2:175–247
Kitagawa T, Kimura S, Nakata H, Yamada H (2006) Thermal adaptation of Pacific bluefin tuna Thunnus orientalis to temperate waters. Fish Sci 72:149–156
Kleisner KM, Walter JF, Diamond SL, Die DJ (2010) Modeling the spatial autocorrelation of pelagic fish abundance. Mar Ecol Prog Ser 411:203–213
Klimley AP (1985) The areal distribution and autoecology of the white shark, Carcharodon carcharias, off the west coast of North America. Mem South Cali Acad Sci 9:15–40
Kraus RT, Rooker JR (2007) Patterns of vertical habitat use by Atlantic blue marlin (Makaira nigricans) in the Gulf of Mexico. Gulf Caribb Res 19:89–97
Kraus RT, Wells RJD, Rooker JR (2011) Horizontal movements of Atlantic blue marlin (Makaira nigricans) in the Gulf of Mexico. Mar Biol 158:699–713
Kroger RL, Pristas PJ (1975) Movements of tagged juvenile menhaden (Brevoortia patronus) in the Gulf of Mexico. Tex J Sci 26:473–477
Lang KL, Grimes CB, Shaw RF (1994) Variations in the age and growth of yellowfin tuna larvae, Thunnus albacares, collected about the Mississippi River plume. Environ Biol Fish 39:259–270
Lassuy DR (1983) Species profiles: Life histories and environmental requirements (Gulf of Mexico) Gulf Menhaden. U.S. Fish and Wildlife Service Biology Report FWS/OBS-82(11.2), TR EL-82-4, U.S. Army Corps of Engineers, Coastal Ecology Group, Waterways Experiment Station, Vicksburg, MS, USA, 13 p
Leard R, Mahmoudi B, Blanchet H, Lazauski H, Spiller K, Buchanan M, Dyer C, Keithly W (1995) The striped mullet fishery of the Gulf of Mexico, United States: A regional management plan. Report number 33, Gulf States Marine Fisheries Commission, Ocean Springs, MS, USA, 196 p
Lee TN, Williams E (1999) Mean distribution and seasonal variability of coastal currents and temperature in the Florida Keys with implications for larval recruitment. Bull Mar Sci 64:35–56
Lessa RP, Monteiro A, Duarte‐Neto PJ, Vieira AC (2009) Multidimensional analysis of fishery production systems in the state of Pernambuco, Brazil. J Appl Ichthyol 25:256–268
Lewis RM, Roithmayer CM (1981) Spawning and sexual maturity of Gulf menhaden Brevoortia patronus. Fish Bull 78:947–951
Lewis MA, Dantin DD, Chancy CA, Abel KC, Lewis KG (2007) Florida seagrass habitat evaluation: A comparative survey for chemical quality. Environ Pollut 146:206–218
Lindeman KC, Pugliese R, Waugh GT, Ault JS (2000) Developmental patterns within a multispecies reef fishery: Management applications for essential fish habitats and protected areas. Bull Mar Sci 66:929–956
Linton E (1901) Fish parasites collected at Woods Hole in 1898. Bull US Fish Comm 19:267–304
Loefer JK, Sedberry GR (2003) Life history of the Atlantic sharpnose shark (Rhizoprionodon terraenovae) (Richardson, 1836) off the southeastern United States. Fish Bull 101:57–88
Logerwell EA, Smith PE (2001) Mesoscale eddies and survival of late stage Pacific sardine (Sardinops sagax) larvae. Fish Oceanogr 10:13–25
Loman M (1978) Other finfish. In: Christmas JY (ed) Fisheries assessment and monitoring—Mississippi. Completion report, PL 88-309, 2-215-R. Gulf Coast Research Laboratory, Ocean Springs, MD, USA, pp 143–147
Lombardi LA, Fitzhugh G, Lyon H (2010) Golden tilefish (Lopholatilus chamaeleonticeps) age, growth, and reproduction from the Northeastern Gulf of Mexico: 1985, 1997–2009. SEDAR 22-DW-01, Southeast Data, Assessment and Review, North Charleston, SC, USA, 35 p
Lombardi-Carlson LA, Fitzhugh GR, Mikulas JJ (2002) Red grouper (Epinephelus morio) age-length structure and description of growth from the Eastern Gulf of Mexico: 1992–2001. Panama City Laboratory Contribution Series 2002-06. National Marine Fisheries Service, Southeastern Fisheries Science Center, Panama City Beach, FL, USA, 42 p
Lombardi-Carlson LA, Cort E, Parsons GR, Manire CA (2003) Latitudinal variation in life-history traits of bonnethead sharks, Sphyrna tiburo (Carcharhiniformes: Sphyrnidae), from the eastern Gulf of Mexico. Mar Freshw Res 54:875–883
Lombardi-Carlson LA, Palmer C, Gardner C, Farsky B (2006) Temporal and spatial trends in red grouper (Epinephelus morio) age and growth from the northeastern Gulf of Mexico: 1979–2005. SEDAR 12-DW-03, Panama City Laboratory Contribution Series 2006-09. National Marine Fisheries Service, Southeastern Fisheries Science Center, Panama City Beach, FL, USA, 43 p
Lombardi-Carlson LA, Fitzhugh GR, Palmer C, Gardner C, Farsky R, Ortiz M (2008) Regional size, age and growth differences of red grouper (Epinephelus morio) along the West Coast of Florida. Fish Res 91:239–251
Longley WH, Hildebrand SF (1941) Systematic catalogue of the fishes of Tortugas, Florida, with observations on colour, habits and local distributions. Carnegie Inst Wash Pap Tortugas Lab 34:1–331
Lorenzen K (1996) A simple von Bertalanffy model for density-dependent growth in extensive aquaculture, with an application to common carp (Cyprinus carpio). Aquaculture 142:191–205
Luckhurst BE, Prince ED, Llopiz JK, Snodgrass D, Brothers EB (2006) Evidence of blue marlin (Makaira nigricans) spawning in Bermuda waters and elevated mercury levels in large specimens. Bull Mar Sci 79:691–704
Luthy SA, Cowen RK, Serafy JE, McDowell JR (2005) Toward identification of larval sailfish (Istiophorus platypterus), white marlin (Tetrapturus albidus), and blue marlin (Makaira nigricans) in the western North Atlantic Ocean. Fish Bull 103:588–600
Lux FE, Mahoney JV (1969) First record of the channel bass, Sciaenops ocellata (Linnaeus), in the Gulf of Maine. Copeia 1969:632–633
Lyczkowski-Shultz J, Hanisko DS (2007) A time series of observations on red snapper larvae from SEAMAP surveys, 1982–2003: Seasonal occurrence, distribution, abundance, and size. In: Patterson WF, Gowan JH Jr, Fitzhugh GR, Nieland DL (eds) Red Snapper ecology and fisheries in the U.S. Gulf of Mexico, vol 60, American Fisheries Society Symposium Series. American Fisheries Society, Bethesda, MD, USA, pp 3–24
Lyczkowski-Shultz J, Hanisko DS, Ingram GW (2005) The potential for incorporating a larval index of abundance for stock assessment of red snapper, Lutjanus campechanus. SEDAR 7-DW-14. National Marine Fisheries Service, Miami, FL, USA, 10 p
Magnuson JJ, Block BA, Deriso RB, Gold JR, Grant WS, Quinn TJ, Saila SB, Shapiro L, Stevens ED (eds) (1994) An assessment of Atlantic Bluefin Tuna. National Academy Press, Washington, DC, USA, 148 p
Maguire JJ, Sissenwine M, Csirke J, Grainger R, Garcia S (2006) The state of world highly migratory, straddling and other high seas fishery resources and associated species. FAO fisheries technical paper, no. 495, Rome, LZ, Italy, 84 p
Mahmoudi B (1991) Population assessment of black mullet (Mugil cephalus) in the Eastern Gulf of Mexico. Final report of cooperative agreement (MARFIN) NA86-WC-H-06138. Florida Department of Environmental Protection, St. Petersburg, FL, USA, 78 p
Mahmoudi B (1993) Update on black mullet stock assessment. Final report submitted to the Florida Marine Fisheries Commission, Tallahassee, FL, USA, 38 p
Mahmoudi B (2000) Status and trends in the Florida Mullet Fishery and an updated stock assessment. Florida Fish and Wildlife Commission. Florida Marine Research Institute, St. Petersburg, FL, USA, 48 p
Mahmoudi B (2005) The 2005 update of the stock assessment for striped mullet, Mugil cephalus, in Florida. Florida Fish and Wildlife Conservation Commission. Fish and Wildlife Research Institute, St. Petersburg, FL, USA, 43 p
Mahmoudi B (2008) The 2008 update of the stock assessment for striped mullet, Mugil cephalus, in Florida. In-House Report IHR2008. Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, St. Petersburg, FL, USA, 114 p
Manooch CS III (1984) Fisherman’s guide to the fishes of the Southeastern United States. North Carolina Museum of Natural History, Raleigh, NC, USA, 362 p
Manooch CS III, Laws ST (1979) Survey of the charter boat troll fishery in North Carolina, 1977. Mar Fish Rev 41:1–11
Manooch CS III, Potts JC (1997a) Age, growth, and mortality estimates of greater amberjack, Seriola dumerili, from the U.S. Gulf of Mexico headboat fishery. Bull Mar Sci 61:671–683
Manooch CS III, Potts JC (1997b) Age, growth, and mortality estimates of greater amberjack from the southeastern United States. Fish Res 30:229–240
Manooch CS III, Mason DL, Nelson RS (1984) Food and gastrointestinal parasites of dolphin Coryphaena hippurus collected along the southeastern and Gulf Coasts of the United States. Bull Jap Soc Sci Fish 50:1511–1525
NMFS (National Marine Fisheries Service) (1993) Fishery management plan for sharks of the Atlantic Ocean. NOAA, Office of Sustainable Fisheries, Highly Migratory Species Management Division, Silver Spring, MD, USA, 287 p
NMS (National Marine Sanctuaries) (2013) Fish photos. National Oceanic and Atmospheric Administration, Silver Spring, MD, USA. https://marinelife.noaa.gov. Accessed 31 May 2013
Martin FD, Drewry GE (1978) Development of fishes of the Mid-Atlantic Bight, an Atlas of egg, larval, and juvenile stages, vol 6. U.S. Fish and Wildlife Service, Washington, DC, USA, 416 p
Martinez-Andrade F (2003) A comparison of life histories and ecological aspects among snappers (Pisces: Lutjanidae). PhD Thesis, Louisiana State University, Baton Rouge, LA, USA, 201 p
Mason JM (1976) Food of Small, Northwestern Atlantic Bluefin Tuna, Thunnus thynnus (L.). MS Thesis, University of Rhode Island, Kingston, RI, USA, 31 p
Mather FJ III, Schuck HA (1960) Growth of bluefin tuna of the western North Atlantic. Fish Bull 179:39–52
Mather FJ, Mason JM Jr, Jones A (1995) Historical document: Life history and fisheries of Atlantic bluefin tuna. NMFS-SEFSC-370, NOAA technical memorandum. National Marine Fisheries Service, Southeast Fisheries Science Center, Miami, FL, USA, 165 p
Matlock GC (1987) The role of hurricanes in determining year class strength. Contrib Mar Sci 30:39–47
Matlock GC, Weaver JE (1979) Fish tagging in Texas Bays during November 1975–September 1976. Management data series no. 1. Texas Parks and Wildlife Department, Coastal Fisheries Branch, Austin, TX, USA, 136 p
Matthews FD, Damaker DM, Knapp LW, Collette BB (1977) Food of Western North Atlantic tunas (Thunnus) and lancetfish (Alepisaurus). NOAA technical report 706. National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Special Scientific Report on Fisheries, Washington, DC, USA, 26 p
McCawley JR, Cowan JH (2007) Seasonal and size specific diet and prey demand of red snapper on artificial reefs. In: Patterson WF, Cowan JH Jr, Fitzhugh GR, Nieland DL (eds) Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society symposium series 60. American Fisheries Society, Bethesda, MD, USA, pp 77–104
McClellan DB, Cummings NJ (1997) Preliminary analysis of tag and recapture data of the greater amberjack, Seriola dumerili, in the Southeastern United States. In: Proceedings, 49th Annual Meeting of the Gulf and Caribbean Fisheries Institute, Dover, Christ Church, New Zealand, November, pp 25–45
McCosker JE (1985) White shark attack behavior: Observations of and speculations about predator and prey strategies. Mem South Calif Acad Sci 9:123–135
McEachran JD (2009) Fishes (Vertebrata: Pisces) of the Gulf of Mexico. In: Felder DL, Camp DK (eds) Gulf of Mexico: Origin, waters, and biota, vol 1, Biodiversity. Texas A&M University Press, College Station, TX, USA, pp 1223–1317
McEachran JD, de Carvalho MR (2002) Batoid fishes. In: Carpenter KE (ed) FAO species identification guide for fishery purposes: The living marine resources of the Western Central Atlantic, introduction, molluscs, crustaceans, hagfishes, sharks, batoid fishes, and chimaeras, vol 1. American Society of Ichthyologists and Herpetologists Special Publication No. 5. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, pp 507–586
McEachran JD, Fechhelm JD (2005) Fishes of the Gulf of Mexico, vol 2, Scorpaeniformes to Tetraodontiformes. University of Texas Press, Austin, TX, USA, 1004 p
McEachran JD, Finucane JH, Hall LS (1980) Distribution, seasonality and abundance of king and Spanish mackerel larvae in the northwestern Gulf of Mexico (Pisces: Scombridae). Northeast Gulf Sci 4:1–16
McEachron LW, Matlock GC, Bryan CE, Unger P, Cody TJ, Martin JH (1994) Winter mass mortality of animals in Texas bays. Northeast Gulf Sci 13:121–138
McGovern JC, Burgos JM, Harris PJ, Sedberry GR, Loefer JK, Pashuk O, Russ D (2002) Aspects of the life history of Red Grouper, Epinephelus morio, along the Southeastern United States. MARFIN final report NA97FF0347, South Carolina Department of Natural Resources, Charleston, SC, USA, 59 p
McGowan MF, Richards WJ (1986) Distribution and abundance of bluefin tuna (Thunnus thynnus) larvae in the Gulf of Mexico in 1982 and 1983 with estimates of the biomass and population size of the spawning stock for 1977, 1978, and 1981–1983. Collect Vol Sci Pap ICCAT 24:182–195
McGowan MF, Richards WJ (1989) Bluefin tuna, Thunnus thynnus, larvae in the Gulf Stream off the southeastern United States: Satellite and shipboard observations of their environment. Fish Bull 87:615–631
McHugh JL (1967) Estuarine nekton. In: Lauff GH (ed) Estuaries. American Association for the Advancement of Science Publication No. 83. Washington, DC, USA, pp 581–620
Miles P (1971) The mystery of the great white shark. Oceans 4:51–59
Minello TJ, Webb JW Jr (1997) Use of natural and created Spartina alterniflora salt marshes by fishery species and other aquatic fauna in Galveston Bay, Texas, USA. Mar Ecol Prog Ser 151:165–179
Minton RV, Hawke JP, Tatum WM (1983) Hormone induced spawning of red snapper, Lutjanus campechanus. Aquaculture 30:363–368
Mitchell KM, Henwood T, Fitzhugh GR, Allman RJ (2004) Distribution, abundance, and age structure of red snapper (Lutjanus campechanus) caught on research longlines in U.S. Gulf of Mexico. Gulf Mex Sci 22:164–172
Miyashita S, Sawada Y, Okada T, Murata O, Kumai H (2001) Morphological development and growth of laboratory-reared larval and juvenile Thunnus thynnus (Pisces: Scombridae). Fish Bull 99:601–616
Moe MA Jr (1966) Tagging fishes in Florida offshore waters. Florida Board of Conservation, Marine Research Lab, Technical Series No. 49. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 40 p
Moe MA Jr (1969) Biology of the red grouper Epinephelus morio (Valenciennes) from the Eastern Gulf of Mexico. Florida Board of Conservation, Marine Research Lab, Technical Series No. 10. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 95 p
Moe MA Jr (1972) Movement and migration of South Florida fishes. Florida Board of Conservation, Marine Research Lab, Technical Series No. 69. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 25 p
Mohn R (1999) The retrospective problem in sequential population analysis: An investigation using cod fishery and simulated data. ICES J Mar Sci 56:473–488
Moore RH (1974) General ecology, distribution, and relative abundance of Mugil cephalus and Mugil curema on the South Texas Coast. Contrib Mar Sci 18:241–255
Moriarity DJW (1976) Quantitative studies on bacteria and algae in the food of the mullet Mugil cephalus L. and the prawn Metapenaeus bennettae (Racek and Dall). J Exp Mar Biol Ecol 22:131–143
Murie DJ, Parkyn DC (2010) Age, growth and sex maturity of greater amberjack (Seriola dumerili) in the Gulf of Mexico, MARFIN Grant No. NA05NMF4331071. In: Proceedings, 18th Annual Marine Fisheries Research Initiative Program (MARFIN) Conference, St. Petersburg, FL, USA, April, pp 28–29
Murphy MD, Munyandorero J (2009) An assessment of the status of red drum in Florida waters through 2007. IHR 2008-008, Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, St. Petersburg, FL, USA, April, 106 p
Murphy MD, Taylor RG (1990) Reproduction, growth, and mortality of red drum Sciaenops ocellatus, in Florida waters. Fish Bull 88:531–542
Musyl MK, Brill RW, Boggs CH, Curran DS, Kazama TK, Seki MP (2003) Vertical movements of bigeye tuna (Thunnus obesus) associated with islands, buoys, and seamounts near the main Hawaiian Islands from archival tagging data. Fish Oceanogr 12:152–169
Nakamura I (1985) FAO species catalogue: An annotated and illustrated catalogue of marlins, sailfishes, spearfishes and swordfishes known to date. FAO Fisheries Synopsis No. 125, vol 5. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, 65 p
NaluPhoto (2012) Sailfish with bait ball. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/photo/sailfish-with-bait-ball-gm138186926-16170177. Accessed December 5, 2016
Neer JA (2005) Aspects of the life history, ecophysiology, bioenergetics, and population dynamics of the cownose ray, Rhinoptera bonasus, in the Northern Gulf of Mexico. PhD Thesis, Louisiana State University, Baton Rouge, LA, USA, 124 p
Neer JA, Thompson BA (2004) Aspects of the biology of the finetooth shark, Carcharhinus isodon, in Louisiana waters. Gulf Mex Sci 22:108–113
Neilson JD, Paul SD, Smith SC (2007) Stock structure of swordfish (Xiphias gladius) in the Atlantic: A review of the non-genetic evidence. Collect Vol Sci Pap ICCAT 61:25–60
Neilson JD, Smith S, Royer F, Paul SD, Porter JM, Lutcavage M (2009) Investigations of horizontal movements of Atlantic swordfish using pop-up satellite archival tags. Rev Methods Technol Fish 9 Biol Fish 9 (Part 1, Part 3):145–159
Nelson JS (2006) Fishes of the world, 4th edn. John Wiley and Sons, New York, NY, USA, 601 p
Nelson WR, Ahrenholz DW (1986) Population and fishery characteristics of Gulf menhaden, Brevoortia patronus. Fish Bull 84:311–325
Nelson WR, Carpenter JS (1968) Bottom longline explorations in the Gulf of Mexico. Comp Fish Rev 30:57–62
Nelson RS, Manooch CS III (1982) Growth and mortality of red snappers in the West-Central Atlantic Ocean and northern Gulf of Mexico. Trans Am Fish Soc 111:465–475
Nelson WR, Ingham MC, Schaaf WE (1977) Larval transport and year-class strength of Atlantic menhaden, Brevoortia tyrannus. Fish Bull 75:23–41
Nichols JT (1922) Color of the tuna. Copeia 1922:74–75
Nieland DL, Wilson CA (2003) Red snapper recruitment to and disappearance from oil and gas platforms in the northern Gulf of Mexico. In: Stanberg DR, Scarborough-Bull A (eds) Fisheries, reefs, and offshore development, vol 36, American Fisheries Society Symposium Series. American Fisheries Society, Bethesda, MD, USA, pp 73–81
Nishida T, Tsuji S, Segawa K (1998) Spatial data analyses of Atlantic bluefin tuna larval surveys in the 1994 ICCAT BYP. Collect Vol Sci Pap ICCAT 48:107–110
NMFS (National Marine Fisheries Service) (1993) Fishery management plan for sharks of the Atlantic Ocean. NOAA, Office of Sustainable Fisheries, Highly Migratory Species Management Division, Silver Spring, MD, USA, 287 p
NMFS (1999) Amendment 1 to the Atlantic Billfish fishery management plan including: Revised final supplemental environmental impact statement, regulatory impact review final regulatory flexibility analysis, and social impact assessment/fishery impact statement. NOAA, Office of Sustainable Fisheries, Highly Migratory Species Management Division, Silver Spring, MD, USA, 387 p
NMFS (2001) Annual report to congress on the status of U.S. Fisheries-2000. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 122 p
NMFS (2002a) Status of red grouper in United States Waters of the Gulf of Mexico During 1986–2001. Sustainable Fisheries Division Contribution No. SFD-01/02-175. NOAA, Southeast Fisheries Science Center, Miami, FL, USA
NMFS (2002b) Annual report to congress on the status of U.S. Fisheries-2001. U.S. Department of Commerce, NOAA, NMFS
,Silver Spring, MD, USA, 142 pNMFS (2003) Annual report to congress on the status of U.S. Fisheries-2002. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 156 p
NMFS (2004) Annual report to congress on the status of U.S. Fisheries-2003. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 24 p
NMFS (2005) Annual report to congress on the status of U.S. Fisheries-2004. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 20 p
NMFS (2006a) Annual report to congress on the status of U.S. Fisheries-2005. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 20 p
NMFS (2006b) Final consolidated atlantic highly migratory species fishery management plan including: Final environmental impact statement, final regulatory impact review, final regulatory flexibility analysis, final social impact assessment, framework actions, and the 2006 stock assessment and fishery evaluation report. NOAA, Office of Sustainable Fisheries, Highly Migratory Species Management Division, Silver Spring, MD, USA, 629 p
NMFS (2007) Annual report to congress on the status of U.S. Fisheries-2006. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 28 p
NMFS (2008) Annual report to congress on the status of U.S. Fisheries-2007. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 23 p
NMFS (2009a) Annual report to congress on the status of U.S. Fisheries-2008. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 23 p
NMFS (2009b). Final amendment 1 to the 2006 consolidated atlantic highly migratory species fishery management plan, essential fish habitat. U.S. Department of Commerce, NOAA, Office of Sustainable Fisheries, Highly Migratory Species Management Division, Silver Spring, MD, USA, 395 p
NMFS (2009c) Our living oceans, report on the status of U.S. living marine resources. 6th ed. NOAA Technical Memorandum NMFS-F/SPO-80. U.S. Department of Commerce, NOAA, Washington, DC, USA, 369 p
NMFS (2009d) Recovery plan for smalltooth sawfish (Pristis pectinata). Prepared by the Smalltooth Sawfish Recovery Team for the National Marine Fisheries Service, Silver Spring, MD, USA, 102 p
NMFS (2010) Annual report to congress on the status of U.S. Fisheries-2009. U.S. Department of Commerce, NOAA, NMFS, Silver Spring, MD, USA, 20 p
NMFS (2011) Annual report to congress on the status of U.S. Fisheries-2010. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 21 p
NMFS (2012a) Annual report to congress on the status of U.S. Fisheries-2011. U.S. Department of Commerce, NOAA, Silver Spring, MD, USA, 20 p
NMFS (2012b) Species information system public portal, NOAA Fisheries Service. https://www.st.nmfs.noaa.gov/sisPortal/sisPortalMain.jsp. Accessed 31 May 2013
NMFS, SERO (Southeast Regional Office) (1986) Final secretarial fishery management plan regulatory impact review regulatory flexibility analysis for the red drum fishery of the Gulf of Mexico. NMFS Miami, FL, USA. December
NOAA (National Oceanic and Atmospheric Administration) (2003) Tilefish distributions in the Gulf of Mexico. http://www.ncddc.noaa.gov/website/CHP. Accessed 31 May 2013
NOAA Fisheries Office of Sustainable Fisheries (2009a) HMS EFH 2009 Bluefin Tuna. http://www.nmfs.noaa.gov/sfa/hms/EFH/shapefiles.htm/. Accessed 31 May 2013
NOAA Fisheries Office of Sustainable Fisheries (2009b) HMS EFH 2009 Blue Marlin. http://www.nmfs.noaa.gov/sfa/hms/EFH/shapefiles.htm/. Accessed 31 May 2013
NOAA Fisheries Office of Sustainable Fisheries (2009c) HMS EFH 2009 Swordfish. http://www.nmfs.noaa.gov/sfa/hms/EFH/shapefiles.htm/. Accessed 31 May 2013
NOAA Fisheries Office of Sustainable Fisheries (2009d) HMS EFH 2009 Sailfish. http://www.nmfs.noaa.gov/sfa/hms/EFH/shapefiles.htm/. Accessed 31 May 2013
NOAA (2013a) Red grouper distributions in the Gulf of Mexico. http://www.ncddc.noaa.gov /website/CHP. Accessed 31 May 2013
NOAA (2013b) Dolphin distributions in the Gulf of Mexico. http://www.ncddc.noaa.gov /website/CHP. Accessed 31 May 2013
NOAA (2013c) Greater amberjack distributions in the Gulf of Mexico. http://www.ncddc.noaa.gov/website/CHP. Accessed 31 May 2013
Nordlie FG, Szelistowshi WA, Nordlie WC (1982) Ontogenesis of osmoregulation in the striped mullet Mugil cephalus Linnaeus. J Fish Biol 20:79–86
O’Connell MT, Cashner RC, Schieble CS (2004) Fish assemblage stability over fifty years in the Lake Pontchartrain estuary: Comparisons among habitats using canonical correspondence analysis. Estuaries 27:807–817
Odum WE (1970) Utilization of the direct grazing and plant detritus food chains by the striped mullet, Mugil cephalus. In: Steele JJ (ed) Marine food chains. Oliver and Boyd, Edinburgh, Scotland, UK, pp 222–240
Orbesen ES, Snodgrass D, Hoolihan JP, Prince ED (2010) Updated U.S. conventional tagging data base for Atlantic sailfish (1956–2009), with comments on potential stock structure. Collect Vol Sci Pap ICCAT 65:1692–1700
Ortiz M, Palmer C (2008) Review and estimates of von Bertalanffy growth curves for the King Mackerel Atlantic and Gulf of Mexico stock units. SEDAR 16-DW-12. Southeast Data, Assessment and Review, North Charleston, SC, USA, 20 p
Ortiz M, Methot R, Cass-Calay SL, Linton B (2008) Preliminary report King Mackerel stock assessment results 2008. SEDAR 16-AW-08. Southeast Data, Assessment and Review, North Charleston, SC, USA, 75 p
Osburn HR, Matlock GC, Green AW (1982) Red drum (Sciaenops ocellatus) movement in Texas bays. Contrib Mar Sci 25:85–97
Overstreet RM (1983) Aspects of the biology of the red drum, Sciaenops ocellatus, in Mississippi. Gulf Res Rep Suppl 1:1–43
Overstreet RM, Heard RW (1982) Food contents of six commercial fishes from Mississippi Sound. Gulf Res Rep 7:137–149
Oxenford HA (1985) Biology of the Dolphin, Coryphaena hippurus, and its implications for the Barbadian Fishery. University of the West Indies, Cave Hill, St. Michael, 366 p
Oxenford HA (1999) Biology of the dolphinfish (Coryphaena hippurus) in the western Central Atlantic: A review. Sci Mar 63:277–301
Oxenford HA, Hunt W (1986) A preliminary investigation of the stock structure of the dolphin, Coryphaena hippurus, in the western Central Atlantic. Fish Bull 84:451–460
Palko BJ, Beardsley GL, Richards WJ (1981) Synopsis of the biology of the Swordfish, Xiphias gladius Linnaeus. FAO Fisheries Synopsis No. 127. NOAA Technical Report, NMFS Circular 441. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Washington, DC, USA, 21 p
Palmer SM, Harris PJ, Powers PT (1998) Age, growth, and reproduction of tilefish, Lopholatilus chamaeleonticeps, along the southeast coast of the United States 1980–87 and 1996–98. SEDAR 4-DW-18, Southeast Data, Assessment and Review, North Charleston, SC, USA, 34 p
Parsons GR (1983) The reproductive biology of the Atlantic sharpnose shark, Rhizoprionodon terraenovae (Richardson). Fish Bull 81:61–74
Parsons GR (1993) Geographic variation in reproduction between two populations of the bonnethead shark, Sphyrna tiburo. Environ Biol Fish 38:25–35
Parsons GR (2006) Sharks, skates, and rays of the Gulf of Mexico, A field guide. University Press of Mississippi, Jackson, MS, USA, 165 p
Patterson WF, Watterson JC, Shipp RL, Cowan JH Jr (2001) Movement of tagged red snapper in the northern Gulf of Mexico. Trans Am Fish Soc 130:533–545
Pattillo ME, Czapla TE, Nelson DM, Monaco ME (1997) Distribution and abundance of fishes and invertebrates in Gulf of Mexico Estuaries, vol II: Species life history summaries. ELMR Report No. 11. NOAA, National Ocean Service, Strategic Environmental Assessments Division, Estuarine Living Marine Resources Program, Silver Spring, MD, USA, 377 p
Pearson JC (1929) Natural history and conservation of the redfish and other commercial sciaenids on the Texas Coast. Bull US Bur Fish 44:129–214
Peck J (1893) On the food of the menhaden. Bull US Fish Comm 13:113–126
Perera PAB, De Silva SS (1978) Studies on the biology of the young grey mullet (Mugil cephalus) digestion. Mar Biol 44:383–387
Perret WS, Latapie WR, Pollard IF, Mock WR, Adkins BG, Gaidry W, White CJ (1971) Fishes and invertebrates collected in trawl and seine samples in Louisiana estuaries. In: Section I, Cooperative Gulf of Mexico Estuarine Inventory and Study, Louisiana, Phase I, Area Description and Phase IV, Biology, Louisiana Wildlife and Fisheries Commission, Baton Rouge, LA, USA, pp 39–105
Peters KM, McMichael RG Jr (1987) Early life history of Sciaenops ocellatus (Pisces: Sciaenidae) in Tampa Bay, Florida. Estuaries 10:92–107
Piko AA, Szedlmayer ST (2007) Effects of habitat complexity and predator exclusion on the abundance of juvenile red snapper. J Fish Biol 70:758–769
Podestá GP, Browder JA, Hoey JJ (1993) Exploring the association between swordfish catch rates and thermal fronts on U.S. longline grounds in the western North Atlantic. Cont Shelf Res 13:253–277
Porch CE (2000) Status of the red drum stocks of the Gulf of Mexico, Version 2.1. SFD-99/00-85, Southeast Fisheries Science Center, Miami Laboratory, Miami, FL, USA, 43 p
Pough HF, Janis CM, Heiser JB (2008) Vertebrate life, 8th edn. Benjamin Cummings Publishing Company, San Francisco, CA, USA, 752 p
Powels H, Stender BW (1976) Observations on composition, seasonality and distribution of ichthyoplankton from MARMAP cruises in the South Atlantic Bight in 1973. South Carolina Marine Research Center Technical Report Series No. 11, Charleston, SC, USA, May
Powers JE, Eldridge P (1983) A preliminary Assessment of king mackerel resources of the Southeast United States. Unpublished report, NOAA, NMFS, Southeast Fisheries Science Center, Miami, FL, USA, 38 p
Powers SP, Hightower CL, Drymon JM, Johnson MW (2012) Age composition and distribution of red drum (Sciaenops ocellatus) in offshore waters of the North Central Gulf of Mexico: An evaluation of a stock under a federal harvest moratorium. Fish Bull 110:283–292
Prager MH (2000) Exploratory assessment of dolphinfish, Coryphaena hippurus, based on U.S. landings from the Atlantic Ocean and the Gulf of Mexico. NOAA, NMFS, Southeast Fisheries Science Center, Beaufort, NC, USA, 18 p
Pristas PJ, Levi EJ, Dryfoos RL (1976) Analysis of returns of tagged Gulf menhaden. Fish Bull 74:112–117
Pritchard ES (ed) (2005) Fisheries of the United States 2004. National Marine Fisheries Service, Office of Science and Technology, Silver Spring, MD, USA, 19 p
Puntel LF (2016) Goliath Grouper and shipwreck. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/photo/goliath-grouper-and-shipwreck-gm518386616-90009669?st=_p_goliath%20grouper. Accessed 13 December 2016
Quinn TJ II, Deriso RB (1999) Quantitative fish dynamics. Oxford University Press, New York, NY, USA, 546 p
Reintjes JW (1970) The Gulf menhaden and our changing estuaries. In: Proceedings, 22nd Annual Meeting of the Gulf and Caribbean Fisheries Institute, Coral Gables, FL, USA, May, pp 87–90
Reintjes JW, Pacheco AL (1966) The relation of menhaden to estuaries. In: Smith RF, Swartz AH, Massmann WH (eds) A Symposium on Estuarine Fisheries. American Fisheries Society Special Publication No. 3. American Fisheries Society, Bethesda, MD, USA, pp 50–58
Render JH (1995) The life history (age, growth, and reproduction) of red snapper (Lutjanus campechanus) and its affinity for oil and gas platforms. PhD Thesis, Louisiana State University, Baton Rouge, LA, USA, 76 p
Richards WJ (1976) Spawning of bluefin tuna (Thunnus thynnus) in the Atlantic Ocean and adjacent seas. Collect Vol Sci Pap ICCAT 5:267–278
Richards WJ (1997) Report on U.S. collections from the Gulf of Mexico, 1994. Collect Vol Sci Pap ICCAT 46:186–188
Richards WJ, Lindeman KC (1987) Recruitment dynamics of reef fishes: Planktonic processes, settlement and demersal ecologies, and fishery analysis. Bull Mar Sci 41:392–410
Richards WJ, Potthoff T (1980) Distribution and abundance of bluefin tuna larvae in the Gulf of Mexico in 1977 and 1978. Collect Vol Sci Pap ICCAT 9:433–441
Richards WJ, Leming T, McGowan MF, Lamkin JT, Kelley-Fraga S (1989) Distribution of fish larvae in relation to hydrographic features of the Loop Current boundary in the Gulf of Mexico. ICES Mar Sci Symp 191:169–176
Richardson LR, Gold JR (1997) Mitochondrial DNA diversity in and population structure of red grouper, Epinephelus morio, from the Gulf of Mexico. Fish Bull 95:174–179
Richardson DE, Cowen RK, Prince ED, Sponaugle S (2009) Importance of the Straits of Florida spawning ground to Atlantic sailfish (Istiophorus platypterus) and blue marlin (Makaira nigricans). Fish Oceanogr 18:402–418
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Fish Res Board Can Bull 191:382
Riley CM, Holt SA, Holt GJ, Buskey EJ, Arnold CR (1989) Mortality of larval red drum (Sciaenops ocellatus) associated with a Ptychodiscus brevis red tide. Publ Inst Mar Sci Univ Texas 31:137–146
Rivas LR (1975) Synopsis of biological data on blue marlin, Makaira nigricans Lacepede, 1802. In: Shomura RS, Williams F (eds) Proceedings of the International Billfish Symposium, Kailua-Kona, HI, USA, August, Part 3, pp 1–16
Rivas LR (1980) Synopsis of knowledge on the taxonomy, biology, distribution and fishery of the Gulf of Mexico mullets (Pisces: Mugilidae). In: Proceedings, Workshop for Potential Fishery Resources of the Northern Gulf of Mexico, New Orleans, LA, USA, pp 34–53
Rivera GA, Appeldoorn RS (2000) Age and growth of dolphin, Coryphaena hippurus, off Puerto Rico. Fish Bull 98:345–352
Roberts DE Jr, Harpster BV, Henderson GE (1978) Conditioning and spawning of the red drum (Sciaenops ocellatus) under varied conditions of photoperiod and temperature. In: Proceedings, 9th Annual Meeting of the World Mariculture Society, Atlanta, GA, USA, January, pp 311–332
Roe RB (1976) Distributions of snappers and groupers in the Gulf of Mexico as determined from exploratory fishing data. In: Proceedings, Colloquium on Snapper-Grouper Fishery Resources of the Western Central Atlantic Ocean, Pensacola Beach, FL, USA, October, pp 129–164
Roelke LA, Cifuentes LA (1997) Use of stable isotopes to assess groups of king mackerel, Scomberomorus cavallas, in the Gulf of Mexico and southeastern Florida. Fish Bull 95:540–551
Rooker JR, Landry AM, Geary BW, Harper JA (2004) Assessment of a shell bank and associated substrates as nursery habitat of post settlement red snapper. Estuar Coast Shelf Sci 59:653–661
Rooker JR, Alvarado Bremer JR, Block BA, De Metrio G, Corriero A, Kraus RT, Prince ED, Rodriguez-Marin E, Secor DH (2007) Life history and stock structure of Atlantic bluefin tuna (Thunnus thynnus). Rev Fish Sci 15:265–310
Rooker JR, Simms JR, Wells RJD, Holt SA, Holt GJ, Graves JE, Furey NB (2012) Distribution and habitat associations of billfish and swordfish larvae across mesoscale features in the Gulf of Mexico. PLoS One 7(4):e34180. doi:10.1371/journal.pone.0034180
Rose CD, Hassler WW (1974) Food habits and sex ratios of dolphin captured in the western Atlantic Ocean off Hatteras, North Carolina. Trans Am Fish Soc 103:94–100
Ross ST (2001) The inland fishes of Mississippi. University Press of Mississippi, Jackson, MS, USA, 624 p
Ross JL, Stevens TM, Vaughan DS (1995) Age, growth, mortality, and reproductive biology of red drums in North Carolina waters. Trans Am Fish Soc 124:37–54
Rowe GT, Kennicutt MC II (2009) Northern Gulf of Mexico Continental Slope habitats and benthic ecology study: Final report. OCS Study MMS 2009-039. U.S. Department of the Interior, Minerals Management Service, Gulf of Mexico OCS Region, New Orleans, LA, USA, 456 p
SAFMC (South Atlantic Fishery Management Council) (1998) Dolphin/Wahoo workshop report. Charleston, SC, USA, May
Saloman CH, Naughton SP (1983) Food of king mackerel, Scomberomorus cavallas, from the Southeastern United States including the Gulf of Mexico. NOAA Technical Memorandum NMFS-SEFC-126. U.S. Department of Commerce, NOAA, NMFS, Washington, DC, USA, 25 p
Sarà G, Sarà R (2007) Feeding habits and trophic levels of bluefin tuna (Thunnus thynnus) of different size classes in the Mediterranean Sea. J Appl Ichthyol 23:122–127
Schaefer KM (2001) Reproductive biology of tunas. Fish Physiol 19:225–270
Schaefer HC, Fable WA Jr (1994) King mackerel, Scomberomorus cavallas, mark-recapture studies off Florida’s East Coast. Mar Fish Rev 56:13–23
Schirripa MJ, Burns KM (1997) Growth estimates for three species of reef fish in the eastern Gulf of Mexico. Bull Mar Sci 61:581–591
Schirripa MJ, Legault CM, Ortiz M (1999) The red grouper fishery of the Gulf of Mexico: Assessment 3.0. SEDAR 12-RD05, 98/99-56. Sustainable Fisheries Division, Southeast Fisheries Science Center, Miami, FL, USA, 58 p
Scott G, Porch C (2007) Additional VPA analysis of northern Atlantic swordfish. Collect Vol Sci Pap ICCAT 60:2069–2076
Scott GP, Turner SC, Churchill GB, Richards WJ, Brothers EB (1993) Indices of larval bluefin tuna, Thunnus thynnus, abundance in the Gulf of Mexico: Modelling variability in growth, mortality, and gear selectivity. Bull Mar Sci 53:912–929
Scubaguys (2016) Manta flying thru water. Bigstockphoto, New York, New York, USA. http://www.bigstockphoto.com/image-5985799/stock-photo-manta-flying-thru-water. Accessed 5 December 2016
semet (2013) School of amberjacks with a shipwreck behind. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/photo/school-of-amberjacks-with-a-shipwreck-behind-gm182746082-12690060. Accessed December 5, 2016
SEDAR 7 (Southeast Data, Assessment and Review) (2005) SEDAR 7, stock assessment report, Gulf of Mexico Red Snapper. North Charleston, SC, USA, 480 p
SEDAR 9 (2006) SEDAR 9, stock assessment report, Gulf of Mexico Greater Amberjack. North Charleston, SC, USA, 178 p
SEDAR 11 (2006) SEDAR 11, stock assessment report, large coastal shark complex, blacktip and sandbar shark. Panama City, FL, USA, 387 p
SEDAR 12 (2006) SEDAR 12, Stock assessment report, Gulf of Mexico red grouper. North Charleston, SC, USA, 358 p
SEDAR 13 (2007) SEDAR 13, stock assessment report, small coastal complex, Atlantic sharpnose, blacknose, bonnethead, and finetooth shark. Panama City, FL, USA, 395 p
SEDAR 7 (2009) SEDAR 7 update, stock assessment report, Gulf of Mexico Red Snapper. Miami, FL, USA, 224 p
SEDAR 12 (2009) SEDAR 12 update, stock assessment report, Gulf of Mexico Red Grouper. North Charleston, SC, USA, 143 p
SEDAR 16 (2009) SEDAR 16, stock assessment report, South Atlantic and Gulf of Mexico King Mackerel. North Charleston, SC, USA, 484 p
SEDAR 31 (2009) SEDAR 31, stock assessment report, Gulf of Mexico red snapper. Pensacola, FL, USA, 224 p
SEDAR 9 Update (2011) SEDAR 9 update, stock assessment report, Gulf of Mexico greater amberjack. North Charleston, SC, USA, 190 p
SEDAR 21 (2011) SEDAR 21, stock assessment report, HMS sandbar shark stock assessment report. Annapolis, MD, USA, 459 p
SEDAR 22 (2011) SEDAR 22, stock assessment report, Gulf of Mexico tilefish. North Charleston, SC, USA, 467 p
SEDAR 27 (2011) SEDAR 27, stock assessment report, Gulf of Mexico Menhaden. North Charleston, SC, USA, 492 p
SEDAR 29 (2012) SEDAR 29, stock assessment report, HMS Gulf of Mexico blacktip shark. North Charleston, SC, USA, 197 p
SEDAR 12 Addendum 1 (2007) SEDAR 12, stock assessment report, Gulf of Mexico Red Grouper. Miami, FL, USA, 64 p
Sedberry GR, Loefer J (2001) Satellite telemetry tracking of swordfish, Xiphias gladius, off the eastern United States. Mar Biol 139:355–360
Sedberry GR, Pashuk O, Wyanski DM, Stephen JA, Weinbach P (2006) Spawning locations for Atlantic reef fishes off the Southeastern U.S. In: Proceedings, 57th Annual Meeting of the Gulf and Caribbean Fisheries Institute, St. Petersburg, FL, USA, November, pp 463–514
SEFSC (Southeast Fisheries Science Center) (1996) Report on the shark evaluation workshop. NMFSC, Sustainable Fisheries Division, Miami, FL, USA, 80 p
SEFSC (1998) Report on the shark evaluation workshop. NMFSC, SEFSC, Southeast Fisheries Science Center, Sustainable Fisheries Division, Miami, FL, USA, 109 p
Seki MP, Polovina JJ, Kobayashi DR, Bidigare RR, Mitchum GT (2002) An oceanographic characterization of swordfish (Xiphias gladius) longline fishing grounds in the springtime subtropical North Pacific. Fish Oceanogr 11:251–266
Serafy JE, Cowen RK, Paris CB, Capo TR, Luthy SA (2003) Evidence of blue marlin, Makaira nigricans, spawning in the vicinity of Exuma Sound, Bahamas. Mar Freshw Res 54:299–306
Seyoum S, Tringali MD, Bert TM, McElroy D, Stokes R (2000) An analysis of genetic population structure in red drum, Sciaenops ocellatus, based on mtDNA control region sequences. Fish Bull 98:127–138
Shaw RF, Cowan JH Jr, Tillman TL (1985a) Distribution and density of Brevoortia patronus (Gulf menhaden) eggs and larvae in the continental shelf waters of Western Louisiana. Bull Mar Sci 36:96–103
Shaw RF, Wiseman WJ Jr, Turner RE, Rouse LJ Jr, Condrey RE, Kelly FJ Jr (1985b) Transport of larval Gulf menhaden Brevoortia patronus in continental shelf waters of western Louisiana: a hypothesis. Trans Am Fish Soc 114:452–460
Shepard KE, Patterson WF, DeVries DA, Ortiz M (2010) Contemporary versus historical estimates of king mackerel (Scomberomorus cavallas) age and growth in the U.S. Atlantic Ocean and Gulf of Mexico. Bull Mar Sci 86:515–532
Sheperd TD, Myers RA (2005) Direct and indirect fishery effects on small coastal elasmobranchs in the northern Gulf of Mexico. Ecol Lett 8:1095–1104
Shipp RL (1992) Introduction: biogeography of Alabama’s marine fishes. In: Boschung HT Jr (ed) Catalog of freshwater and marine fishes of Alabama. Alabama Museum of Natural History Bulletin No. 14. University of Alabama, Tuscaloosa, AL, USA, pp 7–9
Simmons EG (1957) Ecological survey of the Upper Laguna Madre of Texas. Publ Inst Mar Sci Univ Texas 4:156–200
Simmons EG, Breuer JP (1962) A study of redfish (Sciaenops ocellatus Linnaeus) and black drum (Pogonias cromis Linnaeus). Publ Inst Mar Sci Univ Texas 8:184–211
Simms J (2009). Early life ecology of sailfish, Istiophorus platypterus, in the Northern Gulf of Mexico. MS thesis, Texas A&M University, College Station, TX, USA, 49 p
Simpfendorfer CA, Wiley TR (2005) Determination of the distribution of Florida’s remnant sawfish population and identification of areas critical to their conservation, final report. Florida Fish and Wildlife Conservation Commission, Tallahassee, FL, USA, 40 p
Smith CL (1961) Synopsis of biological data on groupers (Epinephelus and Allied Genera) of the Western North Atlantic. FAO Fisheries Biology Synopsis No. 23. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, 61 p
Smith ML, Carpenter KE, Waller RW (2002) An introduction to the oceanography, geology, biogeography, and fisheries of the tropical and subtropical Western Central Atlantic. In: Carpenter KE (ed) FAO species identification guide for fishery purposes: The living marine resources of the Western Central Atlantic, introduction, molluscs, crustaceans, hagfishes, sharks, batoid fishes, and chimaeras, vol 1. American Society of Ichthyologists and Herpetologists Special Publication No. 5. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, pp 1375–1398
Spitzer PR (1989) Osprey. In: Proceedings, Northeast Raptor Management Symposium Workshop, National Wildlife Federation, Washington, DC, USA, March, pp 22–29
Springer VG (1960) Icthyological surveys of the lower St. Lucie and Indian Rivers, Florida East Coast. Mimeo file report no. 60-19. Florida State Board of Conservation Marine Laboratory, St. Petersburg, FL, USA, 22 p
Springer VG (1964) A revision of the carcharhinid shark genera Scoliodon, Loxodon, and Rhizoprionodon. Proc US Nat Mus 115:559–632
Stanley DR (1994) Seasonal and spatial abundance and size distribution of fishes associated with a petroleum platform in the Northern Gulf of Mexico. PhD Thesis, Louisiana State University, Baton Rouge, LA, USA, 123 p
Stokesbury MJW, Teo SLH, Seitz A, O’Dor RK, Block BA (2004) Movement of Atlantic bluefin tuna (Thunnus thynnus) as determined by satellite tagging experiments initiated off New England. Can J Fish Aquat Sci 61:1976–1987
Strong WR Jr, Murphy RC, Bruce BD, Nelson DR (1992) Movements and associated observations of bait-attracted white sharks, Carcharodon carcharias: a preliminary report. Aust J Mar Freshw Res 43:13–20
Sturges W, Leben R (2000) Frequency of ring separations from the Loop Current in the Gulf of Mexico: A revised estimate. J Phys Oceanogr 30:1814–1819
Sutter FC, Williams RO, Godcharles MF (1991) Movement patterns and stock affinities of king mackerel in the southeastern United States. Fish Bull 89:315–324
Suttkus RD (1956) Early life history of the Gulf menhaden, Brevoortia patronus, in Louisiana. Trans N Amer Wildl Conf 21:290–307
Szedlmayer ST, Conti J (1999) Nursery habitats, growth rates, and seasonality of age-0 red snapper, Lutjanus campechanus, in the Northeast Gulf of Mexico. Fish Bull 97:626–635
Szedlmayer ST, Furman C (2000) Estimation of abundance, mortality, fecundity, age frequency, and growth rates of red snapper, Lutjanus campechanus, from a fishery independent, stratified random survey, final report for 1999. Gulf and South Atlantic Fisheries Development Foundation, Tampa, FL, USA, 43 p
Szedlmayer ST, Howe JC (1997) Substrate preference in age-0 red snapper, Lutjanus campechanus. Environ Biol Fish 50:203–207
Szedlmayer ST, Lee JD (2004) Diet shifts of juvenile red snapper (Lutjanus campechanus) with changes in habitat and fish size. Fish Bull 102:366–375
Szedlmayer ST, Shipp RL (1994) Movement and growth of red snapper, Lutjanus campechanus, from an artificial reef area in the northeastern Gulf of Mexico. Bull Mar Sci 55:2–3
Takahashi M, Okamura H, Yokawa K, Okazaki M (2003) Swimming behaviour and migration of a swordfish recorded by an archival tag. Mar Freshw Res 54:527–534
Taylor HF (1951) Survey of marine fisheries of North Carolina. University of North Carolina Press, Chapel Hill, NC, USA, 555 p
Teo SLH, Boustany A, Block BA (2007a) Oceanographic preferences of Atlantic bluefin tuna, Thunnus thynnus, on their Gulf of Mexico breeding grounds. Mar Biol 152:1105–1119
Teo SLH, Boustany A, Dewar H, Stokesbury M, Weng K, Beemer S, Seitz A, Farwell C, Prince ED, Block BA (2007b) Annual migrations, diving behavior and thermal biology of Atlantic bluefin tuna, Thunnus thynnus, on their Gulf of Mexico breeding grounds. Mar Biol 151:1–18
Thompson BA, Render JH, Allen KL, Nieland DL (1989) Life history and population dynamics of commercially harvested striped mullet Mugil cephalus in Louisiana. Final report to the Louisiana Board of Regents. LSU-CFI-89-01. Coastal Fisheries Institute, Louisiana State University, Baton Rouge, LA, USA, 80 p
Thompson BA, Wilson CA, Render JH, Beasley M, Cauthron C (1991) Age, growth, and reproductive biology of greater amberjack and cobia from Louisiana waters. MARFIN final report NA90AAH-MF722. Marine Fisheries Research Initiative Program, National Marine Fisheries Service, St. Petersburg, FL, USA, 77 p
Thompson BA, Beasley M, Wilson CA (1999) Age distribution and growth of greater amberjack, Seriola dumerili, from the North-Central Gulf of Mexico. Fish Bull 97:362–371
Thomson JM (1963) Synopsis of biological data on the grey mullet Mugil cephalus, Linnaeus 1758. Australia CSIRO Division of Fisheries and Oceanography, Fishery Synopsis No. 1. Hobart, Tasmania, Australia, 68 p
Tiews K (1963). Synopsis of biological data on the bluefin tuna Thunnus thynnus (Linnaeus) 1758 (Atlantic and Mediterranean). In: Rosa H Jr (ed) Proceedings of the World Scientific Meeting on the Biology of Tunas and Related Species. FAO Fisheries Synopsis No. 6. Food and Agriculture Organization of the United Nations, Rome, LZ, Italy, pp 422–481
Toll RB, Hess SC (1981) Cephalopods in the diet of the swordfish, Xiphias gladius, from the Florida Straits. Fish Bull 79:765–774
Tserpes G, Tsimenides N (1995) Determination of age and growth of swordfish, Xiphias gladius L., 1758, in the eastern Mediterranean using anal-fin spines. Fish Bull 93:594–602
Tserpes G, Peristeraki P, Valavanis VD (2008) Distribution of swordfish in the eastern Mediterranean, in relation to environmental factors and the species biology. Hydrobiologia 612:241–250
Turner WR (1969) Life history of menhadens in the Eastern Gulf of Mexico. Trans Am Fish Soc 98:216–224
Turner SC, Restrepo VR (1994) A review of the growth rate of West Atlantic bluefin tuna, Thunnus thynnus, estimated from marked and recaptured fish. Collect Vol Sci Pap ICCAT 42:170–172
Turner SC, Grimes CB, Able KW (1983) Growth, mortality, and age/size structure of the fisheries for tilefish, Lopholatilus chamaeleonticeps, in the middle Atlantic-Southern New England region. Fish Bull 81:751–763
Turner SC, Arocha F, Scott GP (1996) U.S. swordfish catch at age by sex. Collect Vol Sci Pap ICCAT 45:373–378
Turner SC, Cummings NJ, Porch CE (2000) Stock assessments of Gulf of Mexico greater amberjack using data through 1998. SFD 99/00-100. Southeast Fisheries Science Center, National Marine Fisheries Service, Miami, FL, USA, 27 p
Uchida S, Toda M, Teshima K, Yano K (1996) Pregnant white sharks and full-term embryos from Japan. In: Klimley AP, Ainley DG (eds) Great White Sharks: The biology of Carcharodon carcharias. Academic, Academic Press, San Diego, CA, USA, pp 139–155
Uotani I, Matsuzaki K, Makino Y, Noda K, Inamura O, Horikawa M (1981) Food habits of larvae of tunas and their related species in the area northwest of Australia. Bull Jap Soc Sci Fish 47:1165–1172
Uotani I, Saito T, Hiranuma K, Nishikawa Y (1990) Feeding habit of bluefin tuna Thunnus thynnus larvae in the western North Pacific Ocean. Nippon Suisan Gakkaishi 56:713–717
USEPA (U.S. Environmental Protection Agency) (1994) Living aquatic resources action agenda for the Gulf of Mexico, first generation—management committee report. EPA 800-B-94-007. U.S. Environmental Protection Agency, Office of Water, Gulf of Mexico Program, Stennis, MS, USA, 168 p
USEPA (2004) National coastal condition report II. EPA 620/R-03/002, U.S. Environmental Protection Agency, Office of Research and Development, Washington, DC, USA, 286 p
USGS (U.S. Geological Survey) (2010a). Gulf Coast wildlife habitat ranges: Scamp grouper, mangrove snapper and red snapper. http://www.usgs.gov/oilspill/sesc/maps.asp#ranges/. Accessed 31 May 2013
USGS (2010b) Gulf Coast wildlife habitat ranges: Bay anchovy, menhaden and striped mullet. http://www.usgs.gov/oilspill/sesc/maps.asp#ranges/. Accessed 31 May 2013
USGS (2010c) Gulf Coast Wildlife habitat ranges: Red drum, black drum, sand seatrout, spotted seatrout, Atlantic croaker and southern kingfish. http://www.usgs.gov/oilspill/sesc/maps.asp#ranges/. Accessed 31 May 2013
USGS (2010d) Gulf Coast Wildlife habitat ranges: King mackerel, Spanish mackerel and sailfish. http://www.usgs.gov/oilspill/sesc/maps.asp#ranges/. Accessed 31 May 2013
VanderKooy SJ, Smith JW (eds) (2002) The Menhaden Fishery of the Gulf of Mexico, United States: A regional management plan, 2002 revision. Report No. 99. Gulf States Marine Fisheries Commission, Ocean Springs, MS, USA, 143 p
Vaughan DS, Smith JW, Prager MH (2000) Population characteristics of gulf menhaden, Brevoortia patronus. NOAA technical report NMFS 149. National Marine Fisheries Service, Seattle, WA, USA, 19 p
Vaughan DS, Shertzer KW, Smith JW (2007) Gulf menhaden (Brevoortia patronus) in the U.S. Gulf of Mexico: Fishery characteristics and biological reference points for management. Fish Res 83:263–275
Vinnichenko VI (1996) New data on the distribution of some species of tuna (Scombridae) in the North Atlantic. J Ichthyol 36:679–681
von Brandis R (2013) Red snapper profile. iStockphoto, Calgary, Alberta, Canada. http://www.istockphoto.com/stock-photo-20240311-red-snapper-profile.php. Accessed 4 December 2016
Walter J, Ingram W (2009) Exploration of the NMFS bottom longline survey for potential deepwater cryptic biomass. National Marine Fisheries Service, Southeast Fisheries Science Center, 18 p. http://ftp.gulfcouncil.org/. Accessed 31 May 2013
Walter JF, Cook M, Linton B, Lombardi L, Quinlan JA (2011) Explorations of habitat associations of yellowedge grouper and golden tilefish. SEDAR 22-DW-05. Southeast Data, Assessment and Review, North Charleston, SC, USA, 26 p
Wells RJD (2007) The effects of trawling and habitat use on red snapper and the associated community. PhD Thesis, Louisiana State University, Baton Rouge, LA, USA, 179 p
Wells RJD, Rooker JR (2003) Distribution and abundance of fishes associated with Sargassum mats in the NW Gulf of Mexico. In: Proceedings, 54th Annual Meeting of the Gulf and Caribbean Fisheries Institute, Providenciales, Turks & Caicos Islands, November, pp 609–621
Wells RJD, Rooker JR (2004a) Distribution, age, and growth of young-of-the-year greater amberjack (Seriola dumerili) associated with pelagic Sargassum. Fish Bull 102:545–554
Wells RJD, Rooker JR (2004b) Spatial and temporal patterns of habitat use by fishes associated with Sargassum mats in the northwestern Gulf of Mexico. Bull Mar Sci 74:81–99
Wells RJD, Rooker JR, Prince ED (2010) Regional variation in the otolith chemistry of blue marlin (Makaira nigricans) and white marlin (Tetrapturus albidus) from the western North Atlantic Ocean. Fish Res 106:430–435
Wenner C (1999) Red drum: Natural history and fishing techniques in South Carolina. Educational report no. 17. Marine Resources Research Institute, Marine Resources Division, South Carolina Department of Natural Resources, Charleston, SC, USA, 40 p
Wilson CA, Nieland DL (1994) Reproductive biology of red drum, Sciaenops ocellatus, from the neritic waters of the northern Gulf of Mexico. Fish Bull 92:841–850
Wilson CA, Nieland DL (2001) Age and growth of red snapper, Lutjanus campechanus, from the northern Gulf of Mexico off Louisiana. Fish Bull 99:653–664
Wilson CA, Dean JM, Prince ED, Lee DW (1991) An examination of sexual dimorphism in Atlantic and Pacific blue marlin using body weight, sagittae weight, and age estimates. J Exp Mar Biol Ecol 151:209–225
Wollam MB (1970) Description and distribution of larvae and early juveniles of king mackerel, Scomberomorus cavallas (Cuvier), and Spanish mackerel, Scomberomorus maculatus (Mitchill); (Pisces: Scombridae); in the Western North Atlantic. Florida Board of Conservation, Marine Research Lab, Technical Series No. 61. Florida Department of Natural Resources, Division of Marine Resources, St. Petersburg, FL, USA, 35 p
Wood M (2016) A school of striped mullet swim along the bottom of Fanning Springs, Florida. http://www.gettyimages.com/photos/a-school-of-striped-mullet-swim?excludenudity=true&family=creative&page=1&phrase=a%20school%20of%20striped%20mullet%20swim&sort=best#license. Accessed 13 December 2016.
Woods MK (2003) Demographic differences in reproductive biology of female red snapper (Lutjanus campechanus) in the Northern Gulf of Mexico. MS Thesis, University of South Alabama, Mobile, AL, USA, 129 p
Woods MK, Cowan JH Jr, Nieland DL (2007) Demographic difference in northern Gulf of Mexico red snapper reproduction maturation: Implication for the unit stock hypothesis. In: Patterson WF, III, Cowan JH Jr, Fitzhugh GR, Nieland DL (eds) Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society symposium series 60. Bethesda, MD, USA, pp 217–277
Workman IK, Foster DG (1994) Occurrence and behavior of juvenile red snapper, Lutjanus campechanus, on commercial shrimp fishing grounds in the northeastern Gulf of Mexico. Mar Fish Rev 56:9–11
Workman IK, Shah A, Foster D, Hataway B (2002) Habitat preferences and site fidelity of juvenile red snapper (Lutjanus campechanus). ICES J Mar Sci 59(Suppl):S43–S50
Yáñez E, Silva C, Barbieri MA, Órdenes A, Vega R (2009) Environmental conditions associated with swordfish size compositions and catches off the Chilean coast. Latin Am J Aquat Res 37:71–81
Yokel BJ (1966) A contribution to the biology and distribution of the red drum, Sciaenops ocellatus. University of Miami, Coral Gables, FL, USA, 160 p
Acknowledgments
BP sponsored the preparation of this chapter. This chapter has been peer reviewed by anonymous and independent reviewers with substantial experience in the subject matter. I thank the peer reviewers, as well as others, who provided assistance with research and the compilation of information. Completing this chapter would not have been possible without the tireless work of Kym Rouse Holzwart, Jonathan Ipock, and Paul Irvin, ENVIRON International Corporation, in obtaining documents, compiling data and information, and preparing maps, graphs, and lists of references.
Small fish images used throughout Chapter 9 are from GulfFINFO (http://gulffishinfo.org/) with the exception of the following: (1) Yellowfin Menhaden (from DM Nelson and ME Pattillo (1992) Distribution and abundance of fishes and invertebrates in Gulf of Mexico estuaries. NOAA National Ocean Service, Rockville, MD, USA. Image available at https://www.flickr.com/photos/internetarchivebookimages/20786380240/in/photolist-xXtgv2-xV8FtW-xEPCEd, accessed 14 December 2016); (2) Finescale Menhaden (from http://txmarspecies.tamug.edu/fishdetails.cfm?scinameID=Brevoortia%20gunteri, accessed 13 December 2016); (3) Atlantic Bluefin Tuna (NOAA FishWatch, http://www.fishwatch.gov/profiles/western-atlantic-bluefin-tuna, accessed 14 December 2016); (4) Blue Marlin (Oceloti, 2014, iStock image at http://www.istockphoto.com/vector/blue-marlin-fish-gm505255597-44750310?clarity=false, accessed 5 December 2016); and (5) Atlantic Sailfish (Szabo D, 2012, iStock image at http://www.istockphoto.com/vector/atlantic-sailfish-gm156019592-13474335?clarity=false; accessed 13 December 2016).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial 2.5 International License (http://creativecommons.org/licenses/by-nc/2.5/), which permits any noncommercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2017 The Author(s)
About this chapter
Cite this chapter
Chen, Y. (2017). Fish Resources of the Gulf of Mexico. In: Ward, C. (eds) Habitats and Biota of the Gulf of Mexico: Before the Deepwater Horizon Oil Spill. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3456-0_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3456-0_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3454-6
Online ISBN: 978-1-4939-3456-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)