Abstract
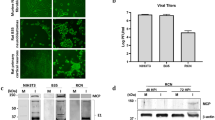
Coronavirus JHM (JHMV) shows specific tropism for oligodendrocytes of the CNS.1 Primary oligodendrocytes induced to differentiate using agents like dibutyryl cyclic AMP (dbcAMP) develop resistance to JHMV infection.1,2 Several studies suggest that the virus enters the host cell through receptor mediated endocytosis.3,4 Earlier studies also found that the nucleocapsid protein (NC) of JHMV is reduced in molecular weight (MW) from a 56K to a 50K component during the early stages of infection.5 A change in the molecular weight of this magnitude with phosphorylated proteins can be accounted for by dephosphorylation.6 The reversible phosphorylation-dephosphorylation of a capsid polypeptide-nucleic acid binding protein has been shown to influence the binding of nucleic acid in a retrovirus.7 This information suggests that a phosphoprotein phosphatase (PPPase) dephosphorylating the NC of JHMV effects the uncoating of the RNA genome during the early stages of infection. Here we present evidence for such a dephosphorylating activity in neural and other cells which are hosts for JHMV.
Chapter PDF
Similar content being viewed by others
Keywords
- Demyelinating Disease
- Nucleocapsid Protein
- Murine Fibroblast
- Phosphoprotein Phosphatase
- Coronavirus Infection
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
References
S. Beushausen, and S. Dales, In vivo and in vitro models of demyelinating disease XI. Tropism and differentiation regulate the infectious process of coronaviruses in primary explants of the rat CNS, Virology 141:89 (1985).
S. Beushausen, S. Narindrasorasak, B.D. Sanwal, and S. Dales, In vivo and in vitro models of demyelinating disease: Activation of the adenylate cyclase system influences JHM virus expression in explanted rat oligodendrocytes, J. Virol. 61:3795 (1987).
K. Krzystyniak, and J.M. Dupuy, Entry of mouse hepatitis 3 into cells, J. Gen. Virol. 65:227 (1984).
L. Mizzen, S. Hilton, S. Cheley, and R. Anderson, Attenuation of murine Coronavirus infection by ammonium chloride, Virology 142:378 (1985).
M. Coulter-Mackie, R. Adler, G. Wilson, and S. Dales, In vivo and in vitro models of demyelinating diseases. XII. Persistence and expression of corona JHM virus functions in RN2–2 schwannoma cells during latency, Virus Res. 3:245 (1985).
C.L. Smith, C. Debouck, M. Rosenberg, and J.S. Culp, Phosphorylation of serine residue 89 of human adenovirus E1A proteins is responsible for their characteristic electrophoretic mobility shifts, and its mutation affects biological function, J. Virol. 63:1569 (1989).
J. Leis, S. Johnson, L.S. Collins, and J.A. Traugh, Effects of phosphorylation of avian retroviruses nucleocapsid protein ppl2 on binding of viral RNA, J. Biol. Chem. 259:7726 (1984).
H. Maeno, and P. Greengard, Phosphoprotein phosphatases from rat cerebral cortex. Subcellular distribution and characterization, J. Biol. Chem. 247:3269 (1972).
U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature (London) 227:680 (1970).
M. Merion, and R.D. Poretz, The resolution of two populations of lysosomal organelles containing endocytosed Wistario floribunda agglutinin from murine fibroblasts, J. Supramol. Struct. Cell. Biochem. 17:337 (1981).
T.S. Ingebristen, and P. Cohen, Protein phosphatases: properties and role in cellular regulation, Science 221:331 (1983).
R. Fuchs, S. Schmid, and I. Mellman, A possible role for Na+, K+-ATPase in regulating ATP-dependent endosome acidification, Proc. Natl. Acad. Sci. U.S.A. 86:539 (1989).
D.L. Brantigan, and C.L. Shriner, Methods to distinguish various types of protein phosphatase activity, Methods in Enzymoloqy 159:339 (1988).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1990 Plenum Press, New York
About this paper
Cite this paper
Mohandas, D.V., Dales, S. (1990). In Vivo and In Vitro Models of Demyelinating Disease: A Phosphoprotein Phosphatase in Host Cell Endosomes Dephosphorylating the Nucleocapsid Protein of Coronavirus JHM. In: Cavanagh, D., Brown, T.D.K. (eds) Coronaviruses and their Diseases. Advances in Experimental Medicine and Biology, vol 276. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-5823-7_35
Download citation
DOI: https://doi.org/10.1007/978-1-4684-5823-7_35
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-5825-1
Online ISBN: 978-1-4684-5823-7
eBook Packages: Springer Book Archive