Abstract
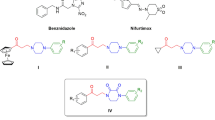
Pneumocystis carinii and Toxoplasma gondii remain the major cause of morbidity and mortality in patients with AIDS. We have recently reported the synthesis of a series of 2,4-diamino-5-methyl-6-substituted pyrido[2,3-d]pyrimidines of general structure 1 1,2 and their selective and potent inhibitory activity against dihydrofolate reductases (DHFR) from P. carinii and T. gondii. These compounds, like the clinically used nonclassical antifolates, trimethoprim, pyrimethamine and trimetrexate (TMQ), bypass the folate uptake mechanisms necessary for classical antifolates such as methotrexate and thus are able to penetrate P. carinii and T. gondii organisms which appear to lack the folate uptake systems. Compounds 1 were designed as hybrid molecules of the nonselective DHFR inhibitors TMQ and piritrexim (PTX) to provide more potent and selective inhibitors of P. carinii DHFR and/or T. gondii DHFR. The most potent and selective of the series of compounds 1 was that with R1 = CH3 and R2 = 3’,4’,5’-trimethoxy which was several times as potent and selective as the analog with R1 = H as well as TMQ and PTX against both P. carinii DHFR and T. gondii DHFR. Larger R1 substituents such as Et, i-Pr and propargyl decrease potency (except R1 = i-Pr for P. carinii DHFR) with some loss of selectivity towards both P. carinii DHFR (except R1 = i-Pr) and T. gondii DHFR. In an attempt to study the effect of conformational restrictions of τ3 of 1 to potency and selectivity we have synthesized the methoxy indoline analogs 2–4, the corresponding indole analogs 5–7 and the 6,7-dimethoxytetrahydroisoquinoline analog 8. In a previous report,3 we had shown that restriction of τ3, by means of an indoline, in the 2,4-diaminotetrahydroquinazoline series, does indeed provide increased potency and selectivity for T. gondii DHFR compared to the unrestricted analog.
Chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
References
Gangjee, A; Shi, J.; Queener, S.F. 5-Deaza Nonclassical Folates as Potent and Selective Inhibitors of Dihydrofolate Reductase From Pneumocystis carinii and Toxoplasma gondii ,Presented at the 23rd National Medicinal Chemistry Symposium. Buffalo, N.Y. June 1448, 1992. Abstr.:61.
Vasudevan, A.; Gangjee, A.; Queener, S.F. Nonclassical 2,4-Diamino-5-methyl-5-deaza Antifolates: Synthesis and Biological Acitvity. Presented at the 204th American Chemical Society National Meeting, Washington, D.C. August 23–28, 1992. Abstr: MEDI 148.
Zaveri, N.; Gangjee, A.; Queener, S.F. A Series of Nonclassical 2,4-Diamino-6-(aminomethyl)tetrahydroquinazoline Antifolates: Synthesis and Biological Activity. Presented at the 204th American Chemical Society National Meeting, Washington, D.C. August 23–38, 1992, Abstr.:MEDI 130.
Piper, J.R.; McCaleb, G.S.; Montgomery, J.A.; Kisliuk, R.L.; Gaumont, Y.;Sirotnak, F.M. J. Med. Chem. ,29:1080–87, (1986).
Piper, J.R.; Montgomery, J.A.; Sirotnak, F.M.; Kisliuk, R.L. J. Med. Chem.,31:264, (1988).
Broughton, M.C.; Queener, S.F. Pneumocystis carinii Dihydrofolate reductase used to Screen Potential Antipneumocystis Drugs. Antimicrob. Agents Chemother ,35:1348–1355,(1991).
Queener, S.F. Identification of Potent and Selective Inhibitors of Toxoplasma gondii Dihydrofolate Reductase (DHFR) and Dihydropteroate Synthase (DHPS). Thirty-first Interscience Conference on Antimicrobial Agents and Chemotherapy, Abstr. 385, (1991).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1993 Springer Science+Business Media New York
About this chapter
Cite this chapter
Gangjee, A., Vasudevan, A., Queener, S.F. (1993). Bicyclic Conformationally Restricted Analogs of Nonclassical Pyrido[2,3-d] Pyrevddines as Potential Inhibitors of Dihydrofolate Reductases. In: Ayling, J.E., Nair, M.G., Baugh, C.M. (eds) Chemistry and Biology of Pteridines and Folates. Advances in Experimental Medicine and Biology, vol 338. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-2960-6_90
Download citation
DOI: https://doi.org/10.1007/978-1-4615-2960-6_90
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-6287-6
Online ISBN: 978-1-4615-2960-6
eBook Packages: Springer Book Archive