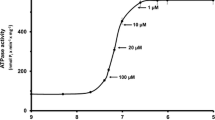
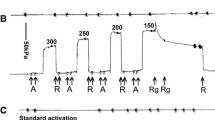
Abstract
Increased ionic strength decreases maximal calcium-activated force (Fmax) of skinned muscle fibers via mechanisms that are incompletely understood. In detergent-skinned fibers from either rabbit (psoas) or lobster (leg or abdomen), Fmax in KCl-containing solutions was less than in potassium methanesulfonate (KMeSO3), which we showed previously was the least deleterious salt for adjusting ionic strength. In either salt, lobster fibers were considerably less sensitive to elevated ionic strength than rabbit fibers. Trimethylamine N-oxide (TMAO, a zwitterionic osmolyte found in high concentration in cells of salt-tolerant animals) increased Fmax, especially in high KC1 solutions. In this regard, TMAO was more effective than a variety of other natural or synthetic zwitterions. In rabbit fibers, increasing ionic strength decreases Fmax but has little effect on contractile ATPase rate measured simultaneously using a linked-enzyme assay. Thus high salt increases the tension-cost of contraction (i.e. ratio ATPase/Fmax). At both high and low salt, TMAO decreases tension-cost. Given a simple two-state model of the cross-bridge cycle, these data indicate that ionic strength and TMAO affect the apparent detachment rate constant. High ionic strength KC1 solutions extract myosin heavy and light-chains, and troponin C from rabbit fibers. This extraction is virtually abolished by TMAO. Natural zwitterions, such as TMAO, have been shown to protect proteins against destabilization by high salt or other denaturatants. Our data indicate that, even in the best of salts, destabilization of the actomyosin complex may play a role in the effect of high ionic strength on the contractile process.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Brenner, B., Schoenberg, M., Chalovich, J.M. & Greene, L.E. Proc. Natl. Acad. Sci. USA 79, 7288–7291 (1982).
Hodgkin, A.L. & Horowicz, P. J. Physiol. (Lond.) 136, 17p (1957).
Gordon, A.M. & Godt, R.E. J. Gen. Physiol. 55, 254–275 (1970).
Howarth, J.V. J. Physiol. (Lond.) 144, 167–175 (1958).
Gordon, A.M., Godt, R.E., Donaldson, S.K.B. & Harris, C.E. J. Gen. Physiol. 62, 550–574 (1973).
Yancey, P.H., Clark, M.E., Hand, S.C., Bowlus, R.D. & Somero, G.N. Science 217, 1214–1222 (1982).
Hochachka, P.W. & Somero, G.N. Biochemical Adaptation pp. 305–354 (Princeton University Press, Princeton, 1984).
Andrews, M.A., Martyn, D.A., Fogaca, R.T.H. & Godt, R.E. Biophys. J. 59, 45a (1991).(Abstract)
Fogaca, R.T.H., Andrews, M.A. & Godt, R.E. Biophys. J. 57, 546a (1990).(Abstract)
Andrews, M.A.W., Maughan, D.W., Nosek, T.M. & Godt, R.E. J. Gen. Physiol. 98, 1–21 (1991).
Godt, R E. & Lindley, B.D. J. Gen. Physiol. 80, 279–297 (1982).
Giith, K. & Wojciechowski, R. Pflügers Arch. 407, 552–557 (1986).
Manchester, K.L. Biochem. Biophys. Acta 630, 225–231 (1980).
Wold, F., & Ballou, C.E. J. Biol. Chem. 227, 301–328 (1957).
Switzer, R.C. III, Merril, C.R. & Shifrin, S. Analyt. Biochem. 98, 231–237 (1979).
April, E.W. & Brandt, P.W. J. Gen. Physiol. 61, 490–508 (1973).
Thames, M.D., Teichholz, L.E. & Podolsky, R.J. J. Gen. Physiol. 63, 509–530 (1974).
Gulati, J. & Podolsky, R.J. J. Gen. Physiol. 72, 701–716 (1978).
Kawai, M. in Basic Biology of Muscles: A Comparative Approach (eds. Twarog, B.M., Levine, R.J.C. & Dewey, M.M.) 109–130 (Raven Press, New York, 1982).
Schoenberg, M. Biophys. J. 54, 135–148 (1988).
Kawai, M., Wray, J.S. & Güth, K. J. Muscle. Res. Cell Motility. 11, 392–402 (1990).
Brenner, B. in Molecular Mechanisms in Muscular Contraction (ed. Squire, J.M.), pp. 77–149 (CRC Press, Inc., Boca Raton, 1990).
Geeves, M.A. & Goldmann, W.H. Biochem. Soc. Trans. 18, 584–585 (1990).
Geeves, M.A. Biochem. J. 274, 1–14 (1991).
Ashley, C.C. & Moisescu, D.G. J. Physiol. (Lond.) 270, 627–652 (1977).
April, E.W., Brandt, P.W. & Elliott, G.F. J. Cell Biol. 53, 53–65 (1972).
Godt, R.E. & Maughan, D.W. Biophys. J. 19, 103–116 (1977).
Matsubara, I., Umazume, Y. & Yagi, N. J. Physiol. (Lond.) 360, 135–184 (1985).
Highsmith, S. Arch. Biochem. Biophys. 180, 404–408 (1977).
Moos, C. Cold Spring Harbor Symposium of Quantitative Biology 37, 137–143 (1973).
Jacobs, H.K. & Guthe, K.F. Arch. Biochem. Biophys. 136, 36–40 (1970).
Altringham, J.D., Yancey, P.H. & Johnston, I.A. J. Exp. Biol. 96, 443–445 (1982).
Timasheff, S. & Arakawa, T. in Protein Structure & Function: A Practical Approach (ed. Creighton, T.E.) 331–345 (IRL Press, Oxford, 1989).
Arakawa, T. & Timasheff, S.N. Biophys. J. 47, 411–414 (1985).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1993 Springer Science+Business Media New York
About this chapter
Cite this chapter
Godt, R.E., Fogaça, R.T.H., Andrews, M.A.W., Nosek, T.M. (1993). Influence of Ionic Strength on Contractile Force and Energy Consumption of Skinned Fibers From Mammalian and Crustacean Striated Muscle. In: Sugi, H., Pollack, G.H. (eds) Mechanism of Myofilament Sliding in Muscle Contraction. Advances in Experimental Medicine and Biology, vol 332. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-2872-2_67
Download citation
DOI: https://doi.org/10.1007/978-1-4615-2872-2_67
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-6245-6
Online ISBN: 978-1-4615-2872-2
eBook Packages: Springer Book Archive