Abstract
Infectious diseases continue to be the major causes of illness, disability, and death. Moreover, in recent years, new infectious agents and diseases are being identified, and some diseases that were previously considered under control have reemerged. Furthermore, antimicrobial resistance has grown rapidly in a variety of hospital as well as community acquired infections. Thus, humanity still faces big challenges in the prevention and control of infectious diseases. Vaccination, generally considered to be the most effective method of preventing infectious diseases, works by presenting a foreign antigen to the immune system to evoke an immune response. The administered antigen can either be a live, but weakened, form of a pathogen (bacteria or virus), a killed or inactivated form of the pathogen, or a purified material such as a protein. However, no vaccine is completely safe; therefore, vaccine safety research and monitoring are necessary to minimize vaccine related harms. From the formulation point of view, the goal continues to be to improve the quality and global availability of vaccine delivery systems. This chapter provides an introduction to vaccine formulation, describes the delivery routes that are utilized, and discusses the factors that affect the safety and stability of a vaccine formulation.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Human Papilloma Virus
- West Nile Virus
- Severe Acute Respiratory Syndrome
- Vaccine Formulation
- Vaccine Delivery
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Despite the outstanding successes in control of diseases provided by improved sanitation, immunization, and antimicrobial therapy, infectious diseases continue to be a common and significant medical problem. Infectious diseases take many manifestations. The most common disease of mankind, the common cold is an infectious disease, just as the fearsome modern disease acquired immune deficiency syndrome (AIDS) is. Some chronic neurological diseases that were previously thought to be degenerative have now been proven to be infectious as well.
Infectious diseases are caused by infective agents, pathogens (such as bacteria, viruses, and fungi) or parasites. Some infectious diseases can be passed from person to person. Some, however, are transmitted via animals. Others are acquired by ingesting contaminated food or water or other exposures in the environment. These microbes, especially viruses, are unstable and evolve rapidly.
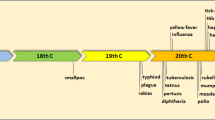
During the past 30 years, more than 30 new organisms have been identified worldwide [1]. The emergence of the new or newly recognized pathogens, such as the viruses causing novel viral hepatitis, severe acute respiratory syndrome (SARS), Ebola, and highly pathogenic modified strains of influenza virus, has altered the entire insight of public health. There is also increasing awareness of the potential for novel or established infections of animals to cross the species barrier and affect man, such as the avian flu and swine flu. Many old infectious diseases, such as tuberculosis, have become renascent due to relaxation of control measures as a result of satisfaction, increasing resistance to antimicrobial agents, and social factors that include increased travel, social displacement and poverty [2]. Some long established infections have expanded to previously unaffected regions, for example, West Nile virus in North America. The threat of bioterrorism has raised the spectra of new outbreaks of highly infectious and deadly diseases, such as smallpox, anthrax, prion diseases such as variant Creutzfeldt–Jakob disease (vCJD) and plague. There is now some evidence that global climate change might be contributing to the spread of infectious disease [3]. Unless controlled effectively, emerging infectious diseases will take a heavy toll of human life regardless of age, gender, lifestyle, ethnic background, and socioeconomic status.
Infectious diseases may not only cause suffering and death but also impose an enormous financial burden on society hence efforts continue for prevention, care, and treatment of the infectious diseases. Both the conventional and the novel formulation technologies for various drugs, which are described in elsewhere in this book, are also applicable for delivery of the chemotherapeutic agents used to treat infectious diseases via the oral, parenteral, transdermal, and transmucosal routes. However, the focus of this chapter is on vaccines that are used for the prevention and/or therapy of infectious diseases, and that require quite different formulation approaches when compared to those used to deliver conventional drugs.
2 Vaccines
Vaccines for the prevention of infectious diseases have made a major contribution to the improvement in the health of people world-wide during the past century. Vaccines work by presenting an antigen to the immune system to evoke an immune response that improves the body resistance to the effects of an infectious pathogen or a disease process [4]. Receiving a vaccination (antigen) activates the immune system’s “memory” allowing the body to react quickly by releasing antibodies to future exposures and thereby destroying the pathogen before it can cause illness (Fig. 16.1). Antibodies, which are also known as immunoglobulins (Ig), are gamma globulin proteins found in blood and are used by the immune system to identify and neutralize bacteria and viruses.
Most of the vaccines used today involve killed or attenuated microorganisms (bacteria, viruses, fungi, etc.) or chemically detoxified toxins (toxoids) from bacteria. However, despite the efficacy of killed and attenuated vaccines, there is concern over their safety. Killed bacterial or viral vaccines often have residual toxicity following inactivation and might contain toxic components, such as lipopolysaccharide [5]. To overcome this problem, although often less immunogenic, subunit vaccines composed of purified antigenic components of the microorganism have been developed. Extensive research has been carried out to also identify the antigens expressed on microorganisms that evoke a protective immune response. Recombinant vaccines have been developed, in which genes for desired antigens are inserted into a vector, usually a virus, which has a very low virulence. The vector expressing the antigen may be used as the vaccine, or the antigen may be purified and injected as a subunit vaccine [6]. Among the subunit recombinant vaccines currently available on the market are the Hepatitis B Virus (HBV) Vaccine (Engerix-B®, GSK; Recombivax Hb®, Merck and Co. Inc) and Human Papilloma Virus (HPV) (Cervarix®, GSK; Gardasil®, Merck and Co. Inc) based on the ability of the viral L1 capsid protein to form virus-like particles (VLP), which are self assembling particles composed of one or more viral proteins.
DNA vaccines based on the injection of plasmid DNA encoding the protective antigenic protein have also been investigated [7–9]. The USDA (US Department of Agriculture) recently granted full licensure for a therapeutic DNA vaccine to help extend survival time of dogs with oral melanoma. The first and only USDA approved vaccine, Oncept™, developed by Merial in partnership with the Memorial Sloan-Kettering Cancer Center and the Animal Medical Center of New York, uses a DNA plasmid containing a gene for the human version of tyrosinase, a protein present on melanoma cancer cells in humans and dogs [10]. For the subunit and DNA vaccines to reach their full potential, it is important to get them to the right place, at the right time, in the right condition [11]. Therefore, delivery systems for vaccines need to be advanced and innovative as well. A successful vaccine formulation must be effective; in other words, it should be capable of inducing appropriate immune response; it should be safe to administer and should be stable, reproducible, and easily affordable.
2.1 Formulation of Vaccines
Traditional live vaccines based on attenuated pathogens typically do not require the addition of any other agents into the formulation but are generally dispersed in buffer solution, whereas vaccines based on inactivated viruses or bacteria may require adjuvants to enhance their immunogenicity. While subunit vaccines, such as purified protective proteins or carbohydrates, provide a much cleaner, safer, and more immunologically defined alternative to live or killed whole cell vaccines, these vaccines are not sufficiently immunogenic on their own; thus, the use of an adjuvant is required to enhance their ability to evoke effective immune responses [12–14].
2.1.1 Adjuvants
Adjuvants are defined as molecules, compounds, or macromolecular complexes that boost the potency and longevity of specific immune response to antigens, causing only minimal toxicity or long lasting immune effects on their own [15]. Adjuvants act by a diverse series of pathways that may involve changing the properties of the antigen, providing a slow release antigen depot, targeting innate immune pathways to selectively activate specific pathways of immunity [16–20]. In regard to their mechanism of action, the adjuvants can be classified into two groups as follows [15, 21]:
-
1.
Immunostimulants: these act directly on the immune system to increase the response to antigens that stimulate immune responses. Examples include TLR (Toll-like receptor) ligands, MPL®, cytokines (GM-CSF, IL-2, IFN-γ, and Flt-3), saponins, and bacterial exotoxins (CT cholera toxin, LT heat labile enterotoxin of Escherichia coli).
-
2.
Vehicles (delivery systems): these present vaccine antigens to the immune system in an optimal manner, including controlled release and depot delivery systems, to increase the specific immune response to the antigen, and can also serve to deliver the immunostimulants. Examples include the following: mineral salts (aluminum) [22]; emulsions (montanide®) [23], MF59™ [24, 25]; virosomes [26, 27]; liposomes; biodegradable polymeric microparticles; and immune stimulating complexes – ISCOMs.
Currently, there are very few adjuvants and delivery systems licensed for human use. These include Alum, MF59™ (an oil-in-water emulsion containing nonionic surfactants and squalene incorporated in influenza vaccines, Fluad® and Focetria®, Novartis), AS03 (10% oil-in-water emulsion containing squalene incorporated in pandemic H1N1 influenza vaccine, Pandemrix®, GSK), MPL® (monophosphoryl lipid A), AS04 [Alum + MPL®, incorporated in Human papilloma virus vaccine, Cervarix®, and hepatitis B virus (HBV) vaccine, Fendrix®, GSK], virus-like particles (VLP) (self-assembling particles composed of one or more viral proteins); immunopotentiating reconstituted influenza virosomes (IRIV) (Epaxal®, hepatitis A virus particles adsorbed on the surface of the IRIV, and Inflexal® V, influenza, Berna, a Crucell Company), and cholera toxin. Safety of adjuvants still remains an important issue, as many of the adjuvants are reported to show some undesired effects [28, 29].
2.1.2 Preservatives
Preservatives such as phenol, benzethonium chloride, and 2-phenoxyethanol are also added into formulations to prevent bacterial and fungal growth in some vaccines during storage, and particularly during the use of opened multidose vials. Thiomersal (also known as thimerosal; mercurothiolate and sodium 2-ethylmercuriothio-benzoate), which is approximately 50% mercury by weight, is one of the most commonly used preservatives in vaccine formulations. It has also been used during vaccine production both to inactivate certain organisms and toxins and to maintain a sterile production line. Recently, there have been some concerns about the safety of this compound due to its mercury content. Such safety concerns have led to initiatives in some countries to eliminate, reduce, or replace thiomersal in vaccines, both in single dose and multidose presentations. However, the WHO Global Advisory Committee on Vaccine Safety [30] continues to recommend the use of vaccines containing thiomersal for global immunization programmes because the benefits of using such products far outweigh any theoretical risk of toxicity.
2.1.3 Stabilizers and Solubilizers
Stabilizers and solubilizers such as polyoxyethylene sorbitan monooleate (Tween® 80), t-octylphenoxypolyethoxyethanol (octoxynol 9, Triton® X-100) are added into vaccine formulations to improve formulation characteristics such as dispersibility. Sugars such as sucrose and lactose, amino acids such as glycine or the monosodium salt of glutamic acid, and proteins such as human serum albumin or gelatin are also added as stabilizers. They are sometimes added to help protect the vaccine from the effects of adverse conditions such as are encountered in the freeze drying process, for those vaccines that are freeze dried.
Most vaccine preparations have to be stored within a specific temperature range to maintain potency. The “cold chain” system (often 2 to 8°C) is a means for storing and transporting vaccines in a potent state from the manufacturer to the person being immunized (Fig. 16.2). This approach is very important since all vaccines lose potency over time if exposed to heat and/or when frozen [31]. However, for innovative technology based vaccines, the cold chain system may be very costly due to the requirements for significantly more space in transportation and storage. Therefore, development of thermostable vaccine formulations becomes an important consideration.
2.2 Vaccine Delivery Routes
The vast majority of vaccines are delivered intramuscularly (im) or subcutaneously (sc) using a needle and syringe. Traditionally, the intradermal (id) route for delivery has been used as the route of choice for only a very limited number of vaccines, such as Bacille Calmette Guérin (BCG) for tuberculosis (TB), and in some countries, for postexposure rabies vaccination. However, over the past few years, the use of the intradermal administration as an alternative delivery route for several other vaccines including hepatitis B (HBV), measles, and influenza has attracted attention due to the possible advantages it offers compared to the intramuscular and subcutaneous routes. These advantages include reduced dose (therefore reduced cost and improved access to vaccines with limited supply), improved safety, and improved logistics [32].
However, there are concerns over inadequate safe injection practices such as reuse of equipment, unsafe collection, and unsafe disposal (resulting in risk of infections and disease transmission, e.g., HIV via contaminated syringes) as well as the availability of trained personnel to administer injections safely. These problems are more critical during mass campaigns when millions of doses of vaccine are administered. Furthermore, patient compliance is restricted with injection. All of these concerns lead to the search for alternate, noninvasive means of vaccine delivery that do not require a needle and syringe. These studies have been accelerated by recent concerns regarding pandemic disease, bioterrorism, and disease eradication campaigns. Noninvasive vaccine delivery would allow large mass vaccinations to be possible by increasing the ease and speed of delivery, and by providing improved efficacy, safety and compliance, decreasing costs, and reducing pain associated with vaccinations. Methods currently in use and under development are focused on needle free injection devices, transcutaneous immunization, and mucosal immunization.
2.2.1 Transcutaneous Immunization
Transcutaneuos immunization (TCI) is a novel vaccination route involving the topical application of vaccine antigens onto the skin surface [33]. It has been shown that skin has an effective immune system, and its physical barrier is not as impermeable as previously thought; hence, it became an attractive route for noninvasive delivery of vaccines. Studies in several animal species and clinical trials in humans have established the proof of principle [34].
2.2.2 Mucosal Immunization
Mucosal immunization has focused on oral, nasal, and aerosol vaccines. Vaccines that induce protective mucosal immunity are attractive since most infectious agents come into contact with the host at mucosal surfaces. Mucosal delivery of vaccines allows concerted action against diseases caused by pathogens that either invade through, or cause disease at, mucosal surfaces [35]. The general approach is to combine systemic response and local mucosal immune response to induce specific protection in distant mucosal sites [36, 37].
2.3 Vaccine Delivery Systems
In response to fears of injection (needle and syringe) mentioned above, alternative delivery technologies have been developed and some of them have already become available on the market. The World Health Organization (WHO) has undertaken a prioritization exercise to determine which delivery technologies (such as jet injector, nasal, aerosol, transcutaneous, ballistic) will be the most feasible and have the greatest impact for existing and future vaccines that will set the global vaccine delivery agenda for the forthcoming years [38]. New immunization supportive technologies anticipated by 2015 are jet injectors, vaccine patches, vaccine nasal sprays, vaccine aerosols, and thermostable vaccines [38].
2.3.1 Needle Free Injection
Jet injectors are needle free devices that deliver vaccine through a nozzle orifice via a high pressure, high speed narrow stream that penetrates the skin [39]. They generate improved or equivalent immune responses compared to needle and syringe. A vaccine can be delivered to intradermal, subcutaneous, or intramuscular tissue depending on the mechanical properties of the fluid stream [40]. Multidose jet injectors were used widely in the 1960s–1980s, with billions of immunizations given with these devices. However, concerns about the transmission of blood borne diseases via these devices led to their withdrawal. New generation multidose jet injectors with disposable caps on the nozzle have been developed in an attempt to overcome this risk; however, they were not found to be adequately safe [46]. The older injectors that used the same nozzle on consecutive patients have been superseded by a new generation of disposable cartridge jet injectors (DCJIs), which are currently being used in the USA for needle free immunization in adults and pediatric populations with several vaccines (Fig. 16.3). The devices currently in use are relatively expensive and not suited for use in developing countries; hence, the development of smaller, lighter, and cheaper designs, which could be applicable for both routine and campaign immunization, is required.
2.3.2 Vaccine Patches
Travelers’ Diarrhea (TD) Vaccine Patch (Intercell) has been reported to have entered clinical Phase III development [41]. The TD vaccine system consists of a self-adhesive patch containing the vaccine antigen and a single use device used to prepare the skin at the site of patch administration (the Skin Preparation System), which partially disrupts the stratum corneum of the skin. The dry patch contains the antigen in a stabilizing excipient formulation and delivers the antigen to the skin. Activated Langerhans cells take up the antigen and deliver it to the draining lymph nodes. If approved, it will be the first vaccine delivered with a patch and to prevent TD, which is caused by enterotoxigenic E. coli. (Note added in proof: Development of this product was halted following failure in Phase III.)
Similarly, a patch containing a trivalent inactivated influenza vaccine (TIV) has been developed in a dried, stabilized formulation for transcutaneous delivery [42]. The dry TIV patch has been described as a major advance for needle free influenza vaccination due to its effectiveness in vaccine delivery and its superior thermostable characteristics.
2.3.3 Vaccine Nasal Sprays
The nasal route offer several benefits to the administration of vaccines, such as its highly vascular mucous membranes, low enzymatic degradation compared to oral vaccines, and greater acceptability to patients. Nasal vaccines, however, have to overcome several limitations, including mucociliary clearance and inefficient uptake of soluble antigens. Therefore, nasal vaccines require potent adjuvants and delivery systems to enhance their immunogenicity and to protect their antigens. Among the various bioadhesive polymers studied for nasal vaccine delivery, chitosan has been shown to exhibit advantages as a vaccine carrier due to its immune stimulating activity and bioadhesive properties that enhance cellular uptake, permeation and antigen protection, as well as being well tolerated by humans [21, 43, 44].
FluMist® (MedImmune, LLC, the USA) is the first FDA approved needle free influenza vaccine made from a weakened live virus (Live, attenuated influenza vaccine – LAIV). It is given as a gentle mist, with a quick spray in each nostril. FluMist® is engineered to not cause disease, and replicates efficiently only in the cooler temperatures of the nasopharynx, but not in the warmer temperatures of the lower respiratory tract. Influenza A (H1N1) 2009 Monovalent Vaccine, Live (MedImmune, LLC, the USA) is the second nasal vaccine that has been approved recently by the FDA for pandemic influenza. Neither of these vaccine formulations contain any adjuvants.
2.3.4 Vaccine Aerosols
The Measles Aerosol Project was established in 2002 by WHO, in collaboration with the US Centers for Disease Control and Prevention and the American Red Cross, with the purpose of conducting the necessary studies to achieve the licensure of a product (device and vaccine) administered through this route [45]. It has long been recognized that new delivery systems may facilitate measles immunization efforts, especially mass campaigns, and that such approaches may facilitate the long-term sustainability of measles mortality reduction and measles elimination goals. Studies are to be undertaken for at least three devices for aerosol administration of reconstituted vaccine and, if feasible in the time frame, a dry powder device.
2.3.5 Particulate Delivery Systems
Vaccine delivery systems are generally particulate systems, e.g., emulsions, polymeric micro/nanoparticles [47–53], ISCOMs [54, 55], and liposomes [56, 57]. They mainly function to target associated antigens into antigen presenting cells (APC), including macrophages and dendritic cells, which facilitate the immune response by holding antigens on its surface and presenting them to lymphocytes. Significantly enhanced immune responses have been reported with encapsulation or adsorption of antigens onto particles. Polyanhydrides, polyorthoesters, hyaluronic acid, and poly(lactic-co-glycolic acid) (PLGA) are the most commonly investigated polymers for the preparation of particulate systems [58–60]. Preparation methods employed to obtain such systems have been reported to be an important parameter for the stability of the antigen. In many cases, an organic solvent and high temperatures are needed for preparation of the particles, which may result in degradation and denaturation of the antigen during processing or after loading [61, 62]. The use of alginates [63, 64] and chitosan offers advantages over other polymers by avoiding use of organic solvents and requiring mild conditions for preparation [43, 52, 65–67].
Particulate systems with a particle size smaller than 10 μm were reported to significantly improve the immune response [61]. However, a variety of systems within a wide range of particle size (between 5 nm and 10 μm) have been investigated for antigen delivery [51, 68, 69]. These investigations showed that there was no clear confirmation that decreasing the particle size of the delivery system improved the immune response. Also there is still an inconsistency in the literature about the differential uptake of nanoparticles by antigen presenting cells (APCs), which changes according to the particle and antigen properties as well as the application route. Currently, there are no vaccine products available on the market based on polymeric particulate systems.
2.3.6 Oral Mucosal Vaccines
Oral immunization with vaccines against intestinal infectious diseases has been extensively explored for several decades. Despite the immunologic and economic rationale behind oral immunization, only a few mucosal vaccines are available for the prevention of mucosal infections due to the limited absorption from the intestinal tract and sensitivity to degradation. “Oral” polio vaccine (OPV) contains live but weakened poliovirus and is the WHO-recommended vaccine for polio eradication. However, because of the risk of a rare, but serious, condition called vaccine-associated paralytic poliomyelitis, the use of the oral polio vaccine in the USA was discontinued in 2000.
Currently, two oral vaccines have approval from the FDA. These are Typhoid Vaccine Live Oral Ty21a (Vivotif®, enteric coated tablet, Berna, Ltd), and Rotavirus Vaccine, Live, Oral (Rotateq®, ready-to-use liquid doses, MSD; Rotarix®, a lyophilized vaccine that is reconstituted with a liquid diluent in a prefilled oral applicator, GSK) [70].
2.3.7 Edible Plant Derived Vaccines
Recently, new live-attenuated bacterial and viral or edible plant derived vaccines have been introduced for oral vaccination [39, 71]. Plants are used as recombinant biofactories to express a number of vaccines [72]. Plant derived vaccine antigens have been found to be safe and induce a sufficiently high immune response in humans. Hence transgenic plants, including edible plant parts, have been suggested as excellent alternatives for the production of vaccines and economic scaleup through cultivation [73]. Such edible encapsulation protects the antigen through the mucosal and gut systems to allow its uptake. Furthermore, oral plant based vaccines are reported to be stable during storage at ambient temperatures, thereby eliminating the need for a cold chain. In addition, they do not require syringes, needles, or trained personnel for administration [74]. These features also favor use of these vaccines for large scale immunization programmes, particularly in developing countries where resources to provide a cold chain and the equipment and personnel needed for injections are limited. Initially the transgenic fruit or vegetable expressing an antigen from a virus or bacteria as the edible vaccine was intended to be eaten raw without any processing to evoke the protective immune response against a particular disease. However, recently the approach to “edible vaccines” has been replaced by “plant-derived vaccine antigens.” This approach results in the antigen in a pure form and at standardized concentrations [73]. In general plants with high food value are being chosen as expression systems such as apple, banana, tomato, and guava (fruits), peanut, corn, soybean, and chickpea (seeds), cabbage, lettuce, potato, and spinach (vegetables) [73]. Recently, a rice based mucosal vaccine expressing CT-B (MucoRice CT-B) has been reported as a new possible form of oral cholera vaccine [75]. Transgenic rice has been shown to be stable in the harsh environment of the gastrointestinal tract, also eliminating the need for syringe/needle administration as well as the cold chain storage process, and providing physicochemical stability. However, it has been pointed out that a highly sophisticated and a closed soil-less farming facility with artificial sunlight would be required for technical advancement of the rice based transgenic vaccine system.
3 Therapeutic Vaccines
Vaccines are by definition prophylactic, but in recent years therapeutic vaccines have been developed for chronic viral infections such as those caused by hepatitis B virus, human papilloma virus (HPV), herpes simplex virus, and HIV [76]. Therapeutic vaccines are intended to treat persistent, recurrent, or chronic infections, where drug intervention is either ineffective or suboptimal, and where intracellular pathogens have established mechanisms to escape from the immune system [77]. Unlike the traditional vaccines, which are administered to healthy individuals to prevent infection, therapeutic vaccines are designed to stimulate immune defences in patient populations after they have been infected/colonized with a pathogen, or even after they developed a disease. Therapeutic vaccination is proposed as an satisfactory replacement for, or as an adjunct to, existing therapies [78].
4 Concluding Remarks
There are always risks and benefits associated with the use of vaccines. The side effects of vaccines are often reported; however, considering the fact that a person is at risk from most infections, and that more and more reports are confirming the safety of vaccines, immunization should be considered the first line of defense against infectious diseases. In response to challenges in global immunization, WHO and UNICEF have developed the Global Immunization Vision and Strategy (GIVS), which covers the period 2006–2015 [79]. This strategy aims to assist countries to immunize more people, from infants to seniors, with a greater range of vaccines, and introduce a range of newly available vaccines and technologies to fulfill their main mission of “a world in which all people at risk are protected against vaccine-preventable diseases.”
As a formulator, we also have a mission in the immunization of people, i.e., “to develop safe and more potent vaccine delivery systems, which are stable at all temperatures, self-administrable, with suitable packaging for storage and transportation, and which are affordable.”
References
WHO-combating emerging infectious diseases in the South-East Asia region. Accessed 7 Nov 2009
Girard MP, Fruth U, Kieny MP (2005) A review of vaccine research and development: tuberculosis. Vaccine 23:5725–5731
Snell NJ (2003) Examining unmet needs in infectious disease. Drug Discov Today 8:22–30
Ogra PL, Faden H, Welliver RC (2001) Vaccination strategies for mucosal immune responses. Clin Microbiol Rev 14:430–445
Ryan EJ, Daly LM, Mills KH (2001) Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol 19:293–304
Soler E, Houdebine LM (2007) Preparation of recombinant vaccines. Biotechnol Annu Rev 13:65–94
Brennan FR, Dougan G (2005) Non-clinical safety evaluation of novel vaccines and adjuvants: new products, new strategies. Vaccine 23:3210–3222
Garmory HS, Perkins SD, Phillpotts RJ, Titball RW (2005) DNA vaccines for biodefence. Adv Drug Deliv Rev 57:1343–1361
Greenland JR, Letvin NL (2007) Chemical adjuvants for plasmid DNA vaccines. Vaccine 25:3731–3741
ONCEPT consumer release. http://us.merial.com/merial_corporate/news/press_releases/02-16-2010_ONCEPT_consumer_release.asp. Accessed 1 Apr 2010
Eriksson K, Holmgren J (2002) Recent advances in mucosal vaccines and adjuvants. Curr Opin Immunol 14:666–672
Griffin JF (2002) A strategic approach to vaccine development: animal models, monitoring vaccine efficacy, formulation and delivery. Adv Drug Deliv Rev 54:851–861
Mills KH (2009) Designer adjuvants for enhancing the efficacy of infectious disease and cancer vaccines based on suppression of regulatory T cell induction. Immunol Lett 122:108–111
Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW (2008) Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm 364:272–280
Reed SG, Bertholet S, Coler RN, Friede M (2009) New horizons in adjuvants for vaccine development. Trends Immunol 30:23–32
Cox E, Verdonck F, Vanrompay D, Goddeeris B (2006) Adjuvants modulating mucosal immune responses or directing systemic responses towards the mucosa. Vet Res 37:511–539
Liang MT, Davies NM, Blanchfield JT, Toth I (2006) Particulate systems as adjuvants and carriers for peptide and protein antigens. Curr Drug Deliv 3:379–388
Petrovsky, N. 2006. Novel human polysaccharide adjuvants with dual Th1 and Th2 potentiating activity. Vaccine 24 Suppl 2: S2-26–29
Trujillo-Vargas CM, Mayer KD, Bickert T, Palmetshofer A, Grunewald S, Ramirez-Pineda JR, Polte T, Hansen G, Wohlleben G, Erb KJ (2005) Vaccinations with T-helper type 1 directing adjuvants have different suppressive effects on the development of allergen-induced T-helper type 2 responses. Clin Exp Allergy 35:1003–1013
Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B (2009) Vaccine adjuvants: current challenges and future approaches. J Pharm Sci 98:1278–1316
Arca HC, Gunbeyaz M, Şenel S (2009) Chitosan-based systems for the delivery of vaccine antigens. Expert Rev Vaccines 8:937–953
Lin X, Hudock H, Arumugham R, Loun B (2008) Identification of particulates in vaccine formulations containing aluminum phosphate. Vaccine 26:6814–6817
Miles AP, McClellan HA, Rausch KM, Zhu D, Whitmore MD, Singh S, Martin LB, Wu Y, Giersing BK, Stowers AW, Long CA, Saul A (2005) Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 23:2530–2539
de Bruijn I, Meyer I, Gerez L, Nauta J, Giezeman K, Palache B (2007) Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. Vaccine 26:119–127
Schultze V, D'Agosto V, Wack A, Novicki D, Zorn J, Hennig R (2008) Safety of MF59 adjuvant. Vaccine 26:3209–3222
Cavanagh DR, Remarque EJ, Sauerwein RW, Hermsen CC, Luty AJ (2008) Influenza virosomes: a flu jab for malaria? Trends Parasitol 24:382–385
Li Q, Gao JQ, Qiu LY, Cui FD, Jin Y (2007) Enhanced immune responses induced by vaccine using Sendai virosomes as carrier. Int J Pharm 329:117–121
Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R (2004) Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med 350:896–903
O'Hagan DT, MacKichan ML, Singh M (2001) Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng 18:69–85
WHO-Global Advisory Committee on Vaccine Safety. http://www.who.int/vaccine_safety/en/. Accessed 23 Nov 2009
WHO-safe vaccine handling, cold chain and immunizations. http://www.who.int/vaccines-documents/docsPDF/WWW9825.pdf. Accessed 23 November 2009
WHO-intradermal delivery of vaccines. http://www.who.int/immunization_delivery/systems_policy/Intradermal-delivery-vaccines_report_2009-Sept.pdf. Accessed 23 Nov 2009
Skountzou I, Kang SM (2009) Transcutaneous immunization with influenza vaccines. Curr Top Microbiol Immunol 333:347–368
Partidos CD, Beignon AS, Mawas F, Belliard G, Briand JP, Muller S (2003) Immunity under the skin: potential application for topical delivery of vaccines. Vaccine 21:776–780
Del Giudice G, Pizza M, Rappuoli R (1999) Mucosal delivery of vaccines. Methods 19:148–155
Holmgren J, Czerkinsky C (2005) Mucosal immunity and vaccines. Nat Med 11:S45–53
Neutra MR, Kozlowski PA (2006) Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 6:148–158
WHO-immunization service delivery and accelerated disease control, new vaccines and technologies. http://www.who.int/immunization_delivery/new_vaccines/technologies/en/index.html. Accessed 23 Nov 2009
Giudice EL, Campbell JD (2006) Needle-free vaccine delivery. Adv Drug Deliv Rev 58:68–89
Jackson LA, Austin G, Chen RT, Stout R, DeStefano F, Gorse GJ, Newman FK, Yu O, Weniger BG (2001) Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine 19:4703–4709
Intercell smart vaccines. http://www.intercell.com/main/forbeginners/news/not-in-menu/news-full/back_to/travelers-diarrhea-vaccine-patch/article/intercell-starts-european-pivotal-phase-iii-clinical-trial-for-the-patch-based-travelers-diarrhea-v/. Accessed 23 Nov 2009
Frolov VG, Seid RC Jr, Odutayo O, Al-Khalili M, Yu J, Frolova OY, Vu H, Butler BA, Look JL, Ellingsworth LR, Glenn GM (2008) Transcutaneous delivery and thermostability of a dry trivalent inactivated influenza vaccine patch. Influenza Other Respi Viruses 2:53–60
Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS (2001) Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev 51:81–96
Klas SD, Petrie CR, Warwood SJ, Williams MS, Olds CL, Stenz JP, Cheff AM, Hinchcliffe M, Richardson C, Wimer S (2008) A single immunization with a dry powder anthrax vaccine protects rabbits against lethal aerosol challenge. Vaccine 26:5494–5502
WHO-Immunization Service Delivery and Accelerated Disease Control, New Vaccines and Technologies, Measles Aerosol Project. http://www.who.int/immunization_delivery/new_vaccines/technologies_aerosol/en/index.html. Accessed 23 Nov 2009
WHO-immunization service delivery and accelerated disease control. Disposable cartridge jet injectors. http://www.who.int/immunization_delivery/new_vaccines/technologies_disposable/en/index.html. Accessed 23 Nov 2009
Borges O, Borchard G, Verhoef JC, de Sousa A, Junginger HE (2005) Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int J Pharm 299:155–166
Estevan M, Gamazo C, Grillo MJ, Del Barrio GG, Blasco JM, Irache JM (2006) Experiments on a sub-unit vaccine encapsulated in microparticles and its efficacy against Brucella melitensis in mice. Vaccine 24:4179–4187
He X, Jiang L, Wang F, Xiao Z, Li J, Liu LS, Li D, Ren D, Jin X, Li K, He Y, Shi K, Guo Y, Zhang Y, Sun S (2005) Augmented humoral and cellular immune responses to hepatitis B DNA vaccine adsorbed onto cationic microparticles. J Control Release 107:357–372
O'Hagan DT, Singh M, Ulmer JB (2006) Microparticle-based technologies for vaccines. Methods 40:10–19
Peek LJ, Middaugh CR, Berkland C (2008) Nanotechnology in vaccine delivery. Adv Drug Deliv Rev 60:915–928
Sayın B, Şenel S (2008) Chitosan and its derivatives for mucosal immunization. In: Jayakumar R, Prabaharan M (eds) Current research and development on chitin in biomaterial science. Research Signpost, Kerala, India, pp 145–165
Zhao A, Rodgers VG (2006) Using TEM to couple transient protein distribution and release for PLGA microparticles for potential use as vaccine delivery vehicles. J Control Release 113:15–22
Pearse MJ, Drane D (2005) ISCOMATRIX adjuvant for antigen delivery. Adv Drug Deliv Rev 57:465–474
Wee JL, Scheerlinck JP, Snibson KJ, Edwards S, Pearse M, Quinn C, Sutton P (2008) Pulmonary delivery of ISCOMATRIX influenza vaccine induces both systemic and mucosal immunity with antigen dose sparing. Mucosal Immunol 1:489–496
Jaafari MR, Badiee A, Khamesipour A, Samiei A, Soroush D, Kheiri MT, Barkhordari F, McMaster WR, Mahboudi F (2007) The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine 25:6107–6117
Yan W, Huang L (2009) The effects of salt on the physicochemical properties and immunogenicity of protein based vaccine formulated in cationic liposome. Int J Pharm 368:56–62
Alpar HO, Somavarapu S, Atuah KN, Bramwell VW (2005) Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev 57:411–430
Florindo HF, Pandit S, Goncalves LMD, Alpar HO, Almeida AJ (2008) Streptococcus equi antigens adsorbed onto surface modified poly-epsilon-caprolactone microspheres induce humoral and cellular specific immune responses. Vaccine 26:4168–4177
Kipper MJ, Wilson JH, Wannemuehler MJ, Narasimhan B (2006) Single dose vaccine based on biodegradable polyanhydride microspheres can modulate immune response mechanism. J Biomed Mater Res A 76:798–810
O'Hagan DT, Singh M (2003) Microparticles as vaccine adjuvants and delivery systems. Expert Rev Vaccines 2:269–283
Singh M, Fang JH, Kazzaz J, Ugozzoli M, Chesko J, Malyala P, Dhaliwal R, Wei R, Hora M, O'Hagan D (2006) A modified process for preparing cationic polylactide-co-glycolide microparticles with adsorbed DNA. Int J Pharm 327:1–5
Mutwiri G, Bowersock T, Kidane A, Sanchez M, Gerdts V, Babiuk LA, Griebel P (2002) Induction of mucosal immune responses following enteric immunization with antigen delivered in alginate microspheres. Vet Immunol Immunopathol 87:269–276
Wee S, Gombotz WR (1998) Protein release from alginate matrices. Adv Drug Deliv Rev 31:267–285
Borges O, Tavares J, de Sousa A, Borchard G, Junginger HE, Cordeiro-da-Silva A (2007) Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. Eur J Pharm Sci 32:278–290
Sayın B, Somavarapu S, Li XW, Sesardic D, Şenel S, Alpar OH (2009) TMC-MCC (N-trimethyl chitosan-mono-N-carboxymethyl chitosan) nanocomplexes for mucosal delivery of vaccines. Eur J Pharm Sci 38:362–369
Sayın B, Somavarapu S, Li XW, Thanou M, Sesardic D, Alpar HO, Şenel S (2008) Mono-N-carboxymethyl chitosan (MCC) and N-trimethyl chitosan (TMC) nanoparticles for non-invasive vaccine delivery. Int J Pharm 363:139–148
Allaker RP, Ren G (2008) Potential impact of nanotechnology on the control of infectious diseases. Trans R Soc Trop Med Hyg 102:1–2
McGeary RP, Olive C, Toth I (2003) Lipid and carbohydrate based adjuvant/carriers in immunology. J Pept Sci 9:405–418
FDA-list of approved products. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm. Accessed 23 Nov 2009
Mestecky J, Nguyen H, Czerkinsky C, Kiyono H (2008) Oral immunization: an update. Curr Opin Gastroenterol 24:713–719
Green BA, Baker SM (2002) Recent advances and novel strategies in vaccine development. Curr Opin Microbiol 5:483–488
Tiwari S, Verma PC, Singh PK, Tuli R (2009) Plants as bioreactors for the production of vaccine antigens. Biotechnol Adv 27:449–467
Streatfield SJ, Howard JA (2003) Plant-based vaccines. Int J Parasitol 33:479–493
Takahashi I, Nochi T, Yuki Y, Kiyono H (2009) New horizon of mucosal immunity and vaccines. Curr Opin Immunol 21:352–358
Sela M, Arnon R, Schechter B (2002) Therapeutic vaccines: realities of today and hopes for the future. Drug Discov Today 7:664–673
Moingeon P, Almond J, de Wilde M (2003) Therapeutic vaccines against infectious diseases. Curr Opin Microbiol 6:462–471
Vandepapeliere P (2002) Therapeutic vaccination against chronic viral infections. Lancet Infect Dis 2:353–367
Global immunization vision and strategy. http://www.who.int/immunization/givs/en/index.html. Accessed 1 Apr 2010
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer US
About this chapter
Cite this chapter
Şenel, S. (2012). Fundamentals of Vaccine Delivery in Infectious Diseases. In: Siepmann, J., Siegel, R., Rathbone, M. (eds) Fundamentals and Applications of Controlled Release Drug Delivery. Advances in Delivery Science and Technology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-0881-9_16
Download citation
DOI: https://doi.org/10.1007/978-1-4614-0881-9_16
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-0880-2
Online ISBN: 978-1-4614-0881-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)