Abstract
Critical illness has consequences for the nervous system. Patients experiencing critical illness are at risk for common global neurologic disturbances, such as delirium, long-term cognitive dysfunction, ICU-acquired weakness, sleep disturbances, recurrent seizures, and coma. In addition, complications related to specific organ dysfunction may be anticipated. Cardiovascular disease presents the possibility for CNS injury after cardiac arrest, sequelae of endocarditis, aberrancies of blood flow autoregulation, and malperfusion. Respiratory disease is known to cause short-term effects of hypoxia and long-term effects after ARDS. Sepsis encephalopathy and sickness behavior syndrome are early signs of infection in patients. In addition, commonly encountered organ dysfunction including uremia, hepatic failure, endocrine, and metabolic disturbances present with neurologic findings which may manifest in the critically ill patient as well.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Delirium
- Cognitive dysfunction
- Coma
- Posterior reversible encephalopathy syndrome
- PRES
- ICU-acquired weakness
- ICU-AW
- Critical illness polyneuropathy
- Critical illness myopathy
- Critical illness polyneuromyopathy
- CIPNM
- Sleep deprivation
- Cerebral autoregulation
- Hypertensive crisis
- ARDS
- Hepatic encephalopathy
- Uremia
- Dialysis disequilibrium syndrome
- Septic encephalopathy
- Sickness behavior syndrome
Introduction
Life-threatening alterations in central and peripheral nerve function are a central manifestation of systemic critical illness. Neurologic failure is an important sign which may herald a treatable underlying disease. It is related to many factors: changes in inflammatory and immune signaling, hypoxia, circulatory shock, infection, endocrine changes, metabolic changes, and medications. Acquired neurologic disorders such as delirium and ICU-acquired weakness are independently associated with adverse short-term outcome. Cognitive impairment and neuromuscular weakness are prevalent in survivors of critical illnesses in particular ARDS and sepsis. Given this chronicity, neurologic expressions of critical illness may be viewed as distinct disorders with self-sustaining biological mechanisms rather than dependent processes which resolve with remission of the inciting illness. Management should be directed to underlying mechanisms as well as symptoms.
Neurologic Disorders Acquired During Critical Illness
Altered Mental Status
The term “altered mental status” refers broadly to any change in the overall level of conscious awareness. The level of consciousness is described as a clinical spectrum ranging from hyperalert to unresponsive, with intermediate states that include delirium, lethargy, obtundation, stupor, and coma. Severe brain injury may evolve toward chronic disorders such as the vegetative and minimally conscious states. Consciousness may be viewed in terms of two separate dimensions: the level of wakefulness and the level of awareness. Wakefulness and awareness are often covariable but may be unlinked as in the vegetative state.
Delirium
Delirium is an acute confusional state developing in the setting of systemic disease. Cardinal features are an acute alteration in mental status with inattention, disorganized thinking, and a fluctuating course. Delirium presents in two motoric subtypes, the more prevalent hypoactive delirium and the more easily recognized hyperactive form. Hyperactive delirium is readily identified, while the hypoactive form may be overlooked and untreated. The incidence of delirium is very high, up to 80 % of mechanically ventilated patients [1] and 30–40 % of less severe patients in the ICU [2]. In recent years, the significance of delirium has been recognized beyond the immediate safety of the patient, as delirium in the ICU has been shown to be predictive of mortality [1, 3, 4], prolonged ICU stay [5], increased cost [6], and long-term cognitive impairment [7, 8].
Different screening and assessment tools have been developed for identifying and rating patients with delirium in the ICU. The Confusion Assessment Method for the ICU (CAM-ICU) [9, 10] generates a binary result with patients categorized as either having or not having delirium; the tool may be implemented by the bedside nurse along with other routine clinical assessments. When compared with standard identifiers, the sensitivity and specificity of CAM-ICU is good (81 and 96 %), and the inter-rater reliability is high (kappa 0.79) [11]. CAM-ICU does not assess the type or severity of delirium; however, the burden of delirium can be quantified by estimating the time a patient is in delirium (Fig. 21.1) [12]. The Intensive Care Delirium Screening Checklist (ICDSC) [13] assigns a numerical score based on the presence or absence of eight characteristics. The ICSDC can be used to identify patients with incomplete presentations who may be at risk for delirium (subsyndromal delirium).
The pathogenesis of delirium in the ICU is believed to reflect a multifactorial process. Patients receive sedative and pain medication and may have sepsis or fever, sleep deprivation, weakness, lethargy, and a host of metabolic derangements [14]. Risk factors for delirium in the ICU include hypertension [2, 15], alcoholism [2, 15], dementia, isolation from social contact [14], and environmental factors such as the absence of a window [15]. Dementia is both a predisposing factor [14, 16] and a differential diagnosis. Age, a risk factor in the general medical population, is not associated with delirium in the ICU [2, 14]. Delirium must also be differentiated from alcohol and substance withdrawal states which have distinct biological mechanisms and treatment implications.
The management of delirium should be driven by a methodical consideration of inciting mechanisms. Specific strategies include pharmacologic and non-pharmacologic interventions and should target all psychomotor types of delirium. Medications that could worsen delirium should be minimized. Benzodiazepines should be avoided whenever possible as they have been shown to increase the likelihood of delirium [17, 18]. Medications with anticholinergic side effects, and especially any anticholinergic drug known to cross the blood–brain barrier such as atropine, should be avoided. Sedation protocols and daily interruptions of sedation should be implemented to decrease the exposure to deliriogenic medications and lessen the impact of delirium. Pharmacologic treatment centers on the use of antipsychotic medication both for confusional states and as a mild sedative. Neuroleptic medications such as haloperidol and more recently atypical antipsychotics such as quetiapine and olanzapine have found frequent use in ICU-associated delirium [19–23]. These medications work particularly well against agitated symptoms of delirium, but may also be theoretically beneficial in hypoactive delirium analogously to their benefits against the negative symptoms of schizophrenia. A randomized controlled trial may help determine if antipsychotics are in fact efficacious for hypoactive delirium [20].
Non-pharmacologic management includes removal of unnecessary catheters and devices, noise reduction, measures to promote sleep [24] (see section on sleep disorders in this chapter), and reorientation strategies. Patients benefit from the presence of calendars and clocks and from reassuring contact with family and ICU staff. Familiar items from home and pictures may also be beneficial. Continuing routines from daily life such as reading newspapers, morning and evening routines, engaging in conversation, and being able to see outside a window are other examples of non-pharmacologic interventions.
Coma
Coma is characterized by loss of alertness and awareness and is demonstrated by unresponsiveness to stimuli. Historical gradations of decreasing arousal include hypersomnolence, lethargy (patient is difficult to arouse), obtundation (incomplete arousal), stupor (no sustained arousal from sleeplike state), and finally coma (lack of arousal). Coma may occur secondary to any number of neurologic injuries, but it may also develop in the setting of a severe metabolic or physiologic disturbance.
The biological origin of coma is understood by the study of (1) ascending brainstem arousal systems and their projections in the diencephalon, basal forebrain, and neocortex (Fig. 21.2) [25], and (2) thalamocortical integrative systems responsible for higher-order awareness and cognition. The arousal system maintains alertness (wakefulness, vigilance) and serves as a gating mechanism for sensory inputs. It originates from the tegmental sections of the rostral pons and the midbrain. Two large branches ascend: one through the lateral hypothalamus and another through the thalamus. Neurotransmitters involved in these pathways are predominantly:
Ascending arousal centers. Injuries to the ascending arousal system, from the rostral pons through the thalamus and hypothalamus (purple region), can cause loss of consciousness (Reprinted with permission of The McGraw-Hill Company from Saper [25])
-
1.
Noradrenergic neurons from the locus coeruleus with diffuse cortical projections
-
2.
Histaminergic neurons prominent in the lateral hypothalamus
-
3.
Cholinergic neurons in the dorsal pons with diffuse ascending and descending projections, but notably connections to the thalamus that are thought to regulate sleep and wakefulness
-
4.
A more recently characterized orexin system located in the hypothalamus and responsible for modulating arousal pathways
Coma may result from injury or impairment at all levels of the arousal/awareness system (Table 21.1) [26]. Discrete lesions in the dorsal and paramedian midbrain or pons may cause coma. Injury to either of the main branches through the lateral hypothalamus or through the thalamus will independently cause coma, just as a bihemispheric or diffuse cortical process. Drugs, toxins, or metabolic factors that interfere with these pathways may cause coma (Table 21.2) [27].
Coma caused by brainstem lesions are generally neurologic emergencies requiring swift decisive intervention (Table 21.3) [26]. Diffuse cortical causes of coma may be more slowly evolving, although they should be met with prompt initiation of treatment as well. Localization is aided by the testing of cranial nerves whose nuclei are in proximity to arousal systems. Pupillary findings may be characteristic, such as the pinpoint pupils seen in pontine lesions. Extraocular movements are controlled by pathways adjacent to the ascending arousal system, and vestibuloocular and oculocephalic reflexes may be helpful. Well-defined respiratory patterns may be associated with injury at different levels of the brainstem. Cheyne–Stokes respirations are linked with lesions above the midbrain, tachypnea with midbrain lesions, apneusis (breath holding at full inspiration) with rostral pontine lesions, and irregular ataxic breathing with lesions in the lower pons and upper medulla.
Coma is assessed by evaluating the response to graded stimulus. The Glasgow Coma Scale (GCS) assesses motor, verbal, and eye responses and is a powerful predictor of outcome in critically ill patients. The Full Outline of Unresponsiveness (FOUR) score [28] provides a quantitative measure of breathing patterns, pupillary responses, and the response to stimulus by motor response and eye opening (Table 21.4). The FOUR score provides more detailed information on brainstem function than the GCS; however, its prognostic value has not been shown to be superior.
Resolution of coma does not always result in return to consciousness. The persistent vegetative state is seen when signs of arousal (e.g., spontaneous eye opening) return, but there is no awareness of self or of the environment. The minimally conscious state is a state of severely impaired consciousness characterized by inconsistently appearing, but unequivocal, signs of conscious awareness (e.g., tracking of objects, simple phonation or speech, but without evidence of reliable communication).
Seizures and Status Epilepticus
New-onset seizures are uncommon but can occur due to alcohol or substance withdrawal or severe metabolic disturbance (Table 21.5) [29]. Nonconvulsive status seizures or status epilepticus may produce alteration of mental status and coma. Studies indicate that as many as 20 % of critically ill patients with coma have nonconvulsive status epilepticus (NCSE) [30]. Nonconvulsive status most often follows a clearly defined seizure and usually occurs in the setting of anoxic–ischemic damage, traumatic brain injury, withdrawal from sedatives or antiepileptics, or sepsis. In patients at risk (i.e., history of seizures, known primary brain injury, sepsis), continuous EEG monitoring for at least 24 h should be considered. Treatment is the same as for convulsive seizures or status.
Posterior Reversible Encephalopathy Syndrome
Posterior reversible encephalopathy syndrome (PRES), also known as posterior reversible leukoencephalopathy syndrome, is characterized by headache, altered mental status, seizures, and visual disturbances (even cortical blindness) associated with CT or MRI evidence of vasogenic edema usually in the parietal and occipital lobes. This condition is associated with severe hypertension, immunosuppressive drugs, and hypertensive disorders of pregnancy (eclampsia or preeclampsia). Though the exact pathophysiology is unknown, prevailing theories point toward a disturbance of autoregulatory mechanisms in the cerebral vasculature leading to endothelial injury, blood–brain barrier breakdown, and vasogenic edema [31, 32]. MRI is more sensitive and specific for making this diagnosis than CT. Treatment should be targeted toward the underlying cause, blood pressure management, and seizure prophylaxis. Long-term prognosis with this condition is generally very good. Resolution of clinical and radiologic findings occurs over days to weeks in the majority of cases.
Sleep Disorders and Sleep Deprivation
Normal patterns of sleep and wakefulness are compromised in the ICU setting. The sickness behavior linked to acute infection (discussion to come) induces daytime hypersomnolence and disruptions in sleep. Pain is common among various critical illness states and may prevent rest at night. Delirium is common in the critically ill, and agitated delirium may keep patients in an animated state, initiating a vicious cycle of sleep deprivation and agitated delirium. Critical illness and the ICU environment are associated with a high likelihood of sleep disruption and deprivation [33–38]. Patients are subject to procedures and assessments around the clock, and persistent lighting and noise may remove important cues driving circadian regulation. Noise is a major contributor to sleep impairment in the ICU due to physiologic alarms, overhead paging systems, staff conversations, and radio or television. Mechanical ventilation and patient-ventilator dyssynchrony in particular may disrupt sleep. Assist-control ventilation has been shown to be superior to pressure-support modes if nocturnal hyperventilation results in central apneas and arousals [39]. Further, proportional assist ventilation (PAV) may be superior to pressure support at night, an effect attributed to improved ventilator tolerance and synchrony [40]; additionally, PAV also avoids nocturnal hyperventilation. Medications, including stimulants and catecholamine agents, may decrease sleep. Benzodiazepine sedation may decrease REM sleep and the restorative effect of sleep.
Continuous polysomnography [41] studies have documented the marked fragmentation of sleep in the intensive care setting; patients with fragmented sleep spend less time in deeper stages of sleep. With frequent interruptions, the efficiency of sleep decreases, resulting in lower quality of sleep, although the total time spent in sleep over a 24-h period may be similar to controls.
Sleep deprivation has many detrimental consequences. Cognitive function declines, and eventually delirium is induced. Agitation and irritability are common, eventually hallucinosis may occur. Animal studies of prolonged sleep deprivation and of selective REM deprivation seem to indicate that, if carried out indefinitely; total sleep deprivation will be uniformly fatal. The cause for the mortality related to sleep deprivation is not clear. Sleep deprivation alters immune function, but not in a predictable way. Animals may be more likely to survive influenza when sleep deprived, but bacterial sepsis becomes increasingly fatal. Sleep deprivation alters metabolism, animals markedly increase caloric intake, but still experience cachexia over time. There is negative nitrogen balance and an overall catabolic state. Sleep deprivation is a stressor, but the effects of sleep deprivation can be distinguished from stress [42].
Management of sleep disturbance in the ICU should target environmental modification. Sedative agents may be helpful in the short term but are unlikely to have the restorative properties of natural sleep [43–47].
Long-Term Cognitive Impairment Following Critical Illness
Long-term impairments in cognition occur with significant frequency among the survivors of critical illness [48–50]. Memory and executive function, attention, processing speed, intellectual function, and visual-spatial testing are frequently affected [49]. Reported rates of cognitive dysfunction at the time of discharge are similar to the rates of ICU delirium. One recent cohort by Girard showed evidence of cognitive impairment in 79 % of ICU survivors at 3 months and 71 % at 1 year, with severe cognitive impairment at 1 year in 36 % of subjects [8]. The rate of cognitive impairment was higher than in other reports perhaps because it included an older sample (median age 61 years), which is consistent with the adult ICU population in the United States.
Acute delirium in hospitalized patients is linked to long-term neurocognitive dysfunction [51]. It has been observed that the natural history of dementia may be accelerated by an intervening period of critical illness [52]. Surprisingly, the severity of critical illness does not appear to correlate with the likelihood of long-term cognitive dysfunction [53–55], although length of stay did correlate with cognitive dysfunction in non-delirious patients immediately following the acute illness [56]. The normal decline with aging may not have been fully accounted for in all longitudinal cohort studies involving the elderly after ICU admission [57].
Cognitive assessments may be confounded by covariables present in survivors of critical illness: sleep deprivation and recovery can have persisting effects months after ICU discharge. Generalized weakness and fatigue are common in the survivors of critical illness, and the methods of testing in these studies were often modified in order to accommodate the fatigue commonly present in the ICU survivors. Depression and post-traumatic stress disorder are common in ICU survivors and can confound neuropsychological performance.
Long-term cognitive outcomes are important in that they can predict functional outcome after the illness. Older, previously high-functioning patients may need institutionalization or other costly care [49]. Younger patients have a low return to full employment rate (49 and 65 % of previously employed returned to their previous employment at 1 and 2 years, respectively) and lower quality-of-life scores after critical illness [58]. These factors represent an increased personal and societal burden of critical illness whose magnitude is only beginning to be appreciated.
ICU-Acquired Weakness
There are many causes of severe muscle weakness that can occur in critically ill patients (Table 21.6) [59]. Preexisting conditions that cause weakness may be exacerbated in the critically ill. The etiologies may be broken down anatomically: cortical lesions, brainstem lesions, myelopathies, anterior horn disease, polyneuropathies, neuromuscular junction disorders, and intrinsic myopathies.
If there is no plausible etiology for severe weakness other than the underlying critical illness, then a diagnosis of ICU-acquired weakness (ICU-AW) should be considered [60–75]. ICU-AW is an umbrella term which includes critical illness polyneuropathy (CIP), critical illness myopathy (CIM), and the overlap condition critical illness neuromyopathy (CINM). As many as 50 % of patients with sepsis, multiorgan failure, or prolonged mechanical ventilation demonstrate evidence of ICU-AW [76]. Factors associated with ICU-AW include sepsis, systemic inflammatory response syndrome (SIRS), multiple-system organ failure, renal replacement therapy, mechanical ventilation, catecholamine administration, and poor glycemic control. Exposure to glucocorticoids and the use of neuromuscular blockers (NMBA) in the ICU were implicated in ICU-AW, but have shown inconsistent association with ICU-AW in recent systematic review [75].
The weakness of ICU-AW is generalized and symmetric. Distal extremity strength such as grip strength may be affected earlier than proximal muscles. Decreased tone is common, and deep tendon reflexes are generally normal to decreased. Weakness may affect the diaphragm leading to prolonged respiratory failure, but facial involvement is rare, as facial grimace is generally preserved. Quantification of the degree of impairment should be done for initial diagnosis and following the progress of disease. The recommended measure of global weakness is the Medical Research Council (MRC) sum score (Table 21.7). The MRC score grades strength in a functional muscle group from 0 to 5. The sum score is generated when three muscle groups are tested from each extremity giving a maximum score of 60. ICU-AW is considered with MRC sum score < 48, severe weakness < 36. The test is repeatable and not costly to perform. Patients must be awake and cooperative for this assessment, and distal extremity strength is not tested.
ICU-AW was initially described by comparison to Guillain–Barré syndrome. Guillain-Barré remains an important differential diagnosis because it can be treated with plasma exchange and intravenous immunoglobulin. Distinguishing features of GBS, in addition to a consistent history and clinical onset, are the greater involvement of cranial nerves (generally absent in ICU-AW), the existence of dysautonomia, and elevated cerebrospinal fluid protein.
EMG and nerve conduction studies are recommended in cases of diagnostic uncertainty, if weakness is severe or if there is no improvement in neuromuscular function over the course of hospitalization. In cooperative patients capable of voluntary muscle contraction, nerve conduction studies and electromyography can distinguish CIP from CIM. When voluntary contraction cannot be obtained, direct muscle stimulation and muscle biopsy may be helpful if a severe underlying myopathy is suspected. Prolonged paralysis after neuromuscular-blocking agents (NMBA) may exist as another subcategory of ICU-AW, generally occurring in the setting of concurrent multiorgan failure [76].
ICU-AW is associated with prolongation of mechanical ventilation [62, 65] and higher in-hospital mortality [64, 69], longer ICU stays, longer hospital stays, and greater associated costs. Long-term effects of ICU-AW include persistent weakness up to 1 year after ICU discharge [67]. While specific therapies for ICU-AW do not exist, efforts to mitigate or prevent it are centered on early physical therapy and occupational therapy [74]. The solution often involves culture change in the intensive care environment to allow early mobilization of patients.
Neurobiologic Effects of Medications Commonly Used in the ICU
Sedatives
Benzodiazepines and other GABA-ergic agents such as propofol are commonly used in critically ill patients needing sedation for mechanical ventilation or other procedures. Benzodiazepines are, however, associated with a significant risk of developing delirium, and there is interest in alternative sedative regimens. Dexmedetomidine in particular has been associated with a lower likelihood of delirium than benzodiazepines [77, 78].
Antibiotics
Several broad-spectrum antibiotics including fluoroquinolones, cefepime, and piperacillin have been linked with encephalopathy, while imipenem has been associated with seizures and metronidazole with peripheral neuropathy. The aminoglycosides induce ototoxicity and impair synaptic transmission at the neuromuscular junction. Vancomycin also has an association with ototoxicity. The determination of whether an encephalopathy is related to an infection or the drug used to treat it may be difficult to make and may complicate management of CNS infections. Ascribing neurologic effects to antibiotic therapy should be a diagnosis of exclusion.
Immune Suppressants
The deleterious effects of steroids on central and peripheral neurologic function are well known and include agitation, delirium and psychosis, and ICU-AW. There is a sizable body of information on the neurologic side effects of immunosuppressive medications used in the setting of solid organ or hematological transplantation [79–86]. Neurologic complications after liver transplantation are particularly common [79, 86], exacerbated by the requirement of many metabolites to be cleared via hepatic metabolism. Following liver transplantation, encephalopathy has been noted in up to 47 % of patients and seizures in 10 % [84].
The calcineurin inhibitors are a significant cause of neurotoxicity. The choice of calcineurin inhibitor (cyclosporine versus tacrolimus) has no significant impact on neurologic complication rates (17 versus 19 %) [84]. Cyclosporine neurotoxicity may appear in up to 60 % of patients receiving the drug and includes headache, amnesia, paresthesias, agitation, anxiety, insomnia, and tremor; more significant findings include decreased responsiveness, hallucinations, delusions, ataxia, aphasia, stroke-like findings, cortical blindness, and seizures. Tacrolimus-associated neurologic manifestations are similarly varied. Symptoms include headache, confusion, myoclonus, seizures, visual disturbances, encephalopathy, and memory loss; hypertension is often induced. Toxicity can occur even with low trough levels, and the time to onset averages 15 days. Drug levels must be monitored closely immediately posttransplant as changes in renal function or volume of distribution can increase drug levels into neurotoxic range. The development of nephrotoxicity further elevates levels. Mycophenolate mofetil has a neurologic side effect profile that is milder than the calcineurin inhibitors.
Neurological Implications of Organ Dysfunction
Cardiovascular
Cardiac and vascular causes of encephalopathy characteristically have an abrupt onset. Intracranial hemorrhages (aneurysmal subarachnoid hemorrhage or primary intracerebral hemorrhage) or ischemic stroke may result in coma if lesions involve bilateral cerebral hemispheres, bilateral thalami, or if they are located in reticular activating system in the rostral brainstem. Focal strokes affecting the right parietal lobe and the basal ganglia have been associated with delirium. When a cerebrovascular etiology is likely, brain CT and/or MRI should be obtained emergently; consultation with neurology and/or neurosurgery is recommended.
A probe-patent foramen ovale is present on 30 % of postmortem subjects but rarely has clinical significance as normal left atrial pressures exceed right atrial pressures. This situation can be reversed in mechanically ventilated patients due to positive pressure ventilation and coughing secondary to airway stimulation, increasing the risk of paradoxical emboli. Neurosurgical patients in the seated operative position are theoretically at increased risk of paradoxical embolism, but the evidence is conflicting whether this occurs any more frequently in practice.
Endocarditis
Manifestations of infective endocarditis (IE) are protean. The diagnosis is based upon evidence of cardiac vegetations and the presence of positive blood cultures [87]. Stroke risk correlates with the size of the vegetation and occurs in 30 % of patients with mitral valve endocarditis and in 10 % of patients with aortic valve endocarditis [88–94]. The risk of cerebral embolism diminishes rapidly after initiation of intravenous antibiotic therapy. Neurologic sequelae are not infrequent after cerebral embolism, with meningitis occurring in 10.7 %, followed by intracranial hemorrhage (9.2 %) and intracranial abscess (7.7 %). Infective aneurysms may occur as a result of microemboli. Reported sites of aneurysms include the aorta, mesentery, and distal MCA. The cerebral aneurysms are usually silent and can, occasionally, regress; delayed rupture after 6 months is very rare. Rupture of an infective cerebral aneurysm may be seen in 0.6–4 %.
The cornerstone of IE therapy is appropriately targeted antimicrobial therapy continued for 4–6 weeks. Early surgery [95–97] should be considered if the patient develops heart failure or if there is severe or rapid degeneration of the infected valve, recurrent emboli, or development of a perivalvular abscess. Cardiac surgery may need to be deferred in patients with large-volume ischemic strokes or those with intracranial hemorrhage. Overall, the mortality is lower after high-risk valve surgery than with conservative management alone when a restrictive surgical selection is employed.
Cardiac Arrest
Anoxic–ischemic encephalopathy (AIE) is the most devastating consequence of cardiac arrest, with historically few survivors returning to their previous levels of neurologic functioning. The use of therapeutic moderate hypothermia has been associated with significantly improved outcome following out-of-hospital ventricular fibrillation arrest [98, 99], and a recent prospective series indicates that a little more than one quarter of cardiac arrest survivors may gain functional independence in the long term [100, 101]. Currently, it appears reasonable to extrapolate these findings to comatose survivors of cardiac arrest in the inpatient setting. With greater implementation of hypothermia, existing prognostic models for post-cardiac arrest AIE [102] (anoxic–ischemic encephalopathy) have been seriously challenged [103, 104]. Hypothermia has multiple side effects including cardiac dysrhythmias, decreased cardiac output, pneumonia, atelectasis, decreased clearance of sedatives, shivering if paralytic agents are not used, hyperglycemia and decreased insulin secretion, and cold diuresis and hypovolemia.
Hypertensive Crisis
In a hypertensive emergency, the end organs most acutely affected are brain, heart, kidney, and large arteries. The most common complications are intracranial hemorrhage and pulmonary edema. Neurologic manifestations of hypertensive emergency are headache, nausea and vomiting, visual disturbances, confusion, and loss of consciousness. Hypertensive encephalopathy without hemorrhage may develop due to vasogenic edema and may be appreciable on neuroimaging as a specific subtype of PRES.
Altered Cerebral Autoregulation in Chronic Hypertension
Autoregulation of the cerebral vasculature allows vascular tone to correct for fluctuations in arterial blood pressure. Cerebral autoregulation is most protective of hypertensive fluctuations, but will also allow maintenance of adequate cerebral blood flow (CBF) at low blood pressures, provided mean arterial pressures (MAP) exists between 50 and 150 mmHg. In chronic hypertension, the CBF–MAP relationship is shifted to accommodate for the elevated blood pressures, hence increasing the risk of cerebral ischemia at the arterial blood pressure which would be tolerated in normal subjects. It is therefore reasonable to target higher blood pressures in patients with chronic hypertension.
Respiratory Failure
Hypoxia and Hypercapnia
Neurologic manifestations of acute hypoxemia include agitation and delirium, progressing to seizures, myoclonus, obtundation, and coma when hypoxemia is of sufficient magnitude or duration. After sudden prolonged or severe hypoxia, a global encephalopathy analogous to AIE may be seen. Incremental decreases in PaO2 may be better tolerated, as seen in high-altitude mountaineers. Myoclonus is a nonspecific finding, but is common in hypoxic encephalopathy. Myoclonic status (persistent myoclonus for the majority of the day post-event) portends poor outcome in comatose patients [105]. Coma survivors with intermittent myoclonus may occasionally develop Lance-Adams intention myoclonus on awakening, which itself may be a debilitating chronic condition [100, 106].
Neurologic manifestations of acute hypercapnia include somnolence, lethargy, and coma. Patients with chronic lung disease will have metabolically compensated hypercapnia with minimal neurologic expression. The cognitive impairment observed in patients with advanced COPD is likely reflective of both hypoxia and hypercapnia. Cognitive impairment with hypercapnia appears to correlate most with the change in PaCO2 from baseline, rather than the absolute value. In patients with baseline PaCO2 of 40 mmHg, significant neurological impairment occurs with a PaCO2 in the range of 60–80 mmHg; in COPD patients, the noticeable effect will occur at a similar delta from their baseline. The effect of PaCO2 of 90–100 mmHg in a baseline normocapnic patient may be equivalent to 1 MAC of anesthesia (roughly a normalized unit of full general anesthesia due to any agent).
ALI/ARDS
Nearly half of acute lung injury (ALI) survivors will have demonstrable long-term functional impairments [53, 67, 101, 107–110]. Survivors of ALI are at risk for significant long-term neurocognitive impairments [53, 107]. Cognitive sequelae in this population can derive from prolonged hypoxemia, hypotension, sepsis, and inflammation; however, the exact underlying pathophysiology is not certain. Delirium may be an epiphenomenon indicating neurologically significant critical illness, as delirium correlates with long-term cognitive sequelae in the critically ill [8] and with mortality at 6 months [1].
Impaired neurocognitive performance following ALI has been documented in several studies by Hopkins and colleagues. Global cognitive impairment was noted in 30 % of patients who were assessed 1 year after acute respiratory distress syndrome (ARDS), and significant impairment was noted in at least one domain of cognition in 55 % of study subjects [107]. In a follow-up study, significant residual impairment in at least one domain of testing was observed in 47 % of patients at 2 years [53]. At 6 year follow-up, the incidence of neurocognitive defect may be decreased to 25 % of patients [110].
Studies using head CT have shown that ALI survivors may be at increased risk for brain atrophy when compared to age-matched controls [111]. Although CTs in many ALI patients were normal, there was a significant increase in averaged brain atrophy as measured by volumetric ventricle-to-brain ratio compared to controls. In the study, there was no control for the timing of the imaging, and many CTs were performed early in the course of illness (within the first 2 weeks); the study failed to show correlation between degree of hypoxia and atrophy, although the pattern was similar to atrophy seen following AIE.
Sickness Behavior Syndrome
The sickness behavior syndrome (SBS) is an adaptive and evolutionarily advantageous physiologic and behavioral response to a systemic inflammatory state. Presenting signs include anorexia, fatigue, somnolence, social withdrawal, aching joints, fever, and chills. SBS is prominent in autoimmune disease and in sepsis, but may also play a role in malaise of other chronic conditions including heart failure, obesity, Alzheimer’s, stroke, and depression [112].
There may be several signaling pathways involved in SBS, both humoral and neural. IL-1, IL-6, and TNF-alpha play a prominent role. Pro-inflammatory cytokines activate vagal afferents to the brainstem, with input to the hypothalamus and limbic system. By interacting with the hypothalamic–pituitary axis, IL-1 can cause fever via induction of prostaglandin E2 and cortisol release. Autoimmune diseases activate a pathway involving anti-self T lymphocytes, in which the T-cells bind to CD40+ on B-cells, dendritic cells, and macrophages to produce pro-inflammatory cytokines that are independent of the pathway for SBS induced by bacterial lipopolysaccharide [113]. Although SBS is adaptive in mammals to learn to avoid poisons in the wild and to recuperate during illness, in the ICU the effect is maladaptive and may delay resolution of illness and recovery.
Sepsis-Associated Encephalopathy
Sepsis-associated encephalopathy (SAE) [114–122] is a disturbance of brain function arising in the setting of sepsis arising from a non-CNS source. It is characterized by alteration in mental status, diffuse slowing on EEG, typically a normal head CT, and normal CSF indicating absence of meningitis, all in the presence of systemic sepsis. In patients with bacteremia, 87 % had abnormal EEG and 70 % had neurologic symptoms ranging from lethargy to coma [114]. SAE is commonly viewed as a reversible condition; however, patients may develop long-lasting deficits. Often, patients emerging from SAE are noted to have ICU-AW which may take longer to resolve. ICU-AW is noted in up to 70 % of patients with SAE [65].
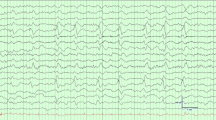
Clinical presentation may be subtle. CNS dysfunction may be one of the earliest signs of infection and may allow for timely diagnostic evaluation and therapy. Early presenting signs of SAE include inattention and fluctuating mental status consistent with acute delirium [114]. More severe SAE presents as coma. Motor manifestations may include velocity-dependent resistance to passive movement that diminishes as the limb is moved slowly (gegenhalten or paratonic rigidity) [114] but also asterixis, tremor, or myoclonus. The electroencephalogram (EEG) is very sensitive for sepsis-associated encephalopathy, even before clinically evident neurologic findings appear. Routine use of EEG in septic patients has been suggested as a means of categorizing patients with SAE [115]. The EEG change follows a progression with severity of encephalopathy and correlates with mortality. Early changes include slowing of the dominant rhythm: the thetas (19 % mortality), deltas (36 % mortality), the appearance of generalized triphasic waves (50 % mortality), and burst suppression (67 % mortality) [115]. While CT findings are generally unremarkable, MRI may reveal ischemic stroke or a pattern of leukoencephalopathy, which may represent breakdown of the blood–brain barrier [116]; these findings on MRI correlate with poor outcome [116]. CSF analysis is negative for infection, but elevated protein may be present. Serum biomarker S100B may be elevated in some patients but does not correlate with severity of illness [117].
The pathophysiology of SAE is not well understood. Elevated cytokine levels inhibit endothelial nitric oxide synthase (eNOS) resulting in vasoconstriction and impaired microcirculatory flow. Cerebral autoregulation is impaired in septic shock patients; thus, hypotension during septic shock may result in significant decreases in CBF [120]. The endothelial dysfunction in sepsis also produces a procoagulant state, potentially contributing to microvascular infarcts [116]. Endotoxemia may cause impairment of blood–brain barrier, leading to vasogenic edema and altering brain homeostasis. There may also be an alteration in the ratio of amino acids transported across the BBB, as aromatic amino acids are more readily transported than branch-chained amino acids. There is some indication that addition of branch-chained amino acids may be of benefit in the treatment of SAE [123].
Management of SAE is predicated on treatment of the underlying infection. Antibiotic therapy may not reverse the encephalopathy in all cases as more endotoxin may be initially released with antibiotic therapy, or a severe or irreversible injury may have already occurred. In addition to management of organ failure and metabolic disturbances, avoidance of neurotoxic drugs is recommended.
Liver Failure
The liver contributes importantly to normal CNS function. The brain is dependent on glucose homeostasis, which is maintained with the aid of the liver. In addition, the liver is involved in intermediate steps of metabolism for many substrates used in the brain. Most importantly, the liver is essential in eliminating toxic metabolites that would modulate CNS function.
Acute Liver Failure
Acute liver failure (ALF) is defined as the rapid development of encephalopathy and impaired synthetic function in a patient with previously normal liver function. The etiology is most commonly a toxic ingestion or viral hepatitis. Presenting symptoms are neurologic, often preceding any clinically evident jaundice. Mania, agitation, and delirium are common early findings, along with nausea, vomiting, and abdominal pain; the neurologic features evolve rapidly to coma. Generalized seizures are common. Cerebral edema [124, 125] is the principal consequence of ALF and has both cytotoxic and vasogenic components.
The cornerstone of management is to identify liver transplant candidates and preserve neurologic function. Transplantation may increase ALF survival from 15–20 % to 60–80 % [126, 127]. Admission to the ICU for aggressive management and rapid evaluation for transplantation are essential. ICP monitoring and ICP management with hypertonic saline or mannitol may allow treatment or prevention of brain herniation and irreversible neurologic injury. Medical management should include interventions to reduce serum ammonia, seizure prophylaxis, sedation, and induced hypothermia [124, 128].
Hepatic Encephalopathy
Chronic liver disease may produce encephalopathy with intermittent exacerbation. The encephalopathy is initiated by impaired clearance of metabolites from portosytemic shunting of blood in the setting of portal hypertension and, in late disease, loss of sustainable synthetic function due to cirrhosis; hepatic encephalopathy (HE) is a clinical diagnosis [129, 130]. The diagnosis can be supported by hyperammonemia, but severity does not always correlate well with blood ammonia levels. Clinical findings include short-term memory loss, hypersomnia, insomnia, lethargy, asterixis, slurred speech, erratic behavior, and coma (Table 21.8). Sensitive neuropsychiatric testing may be required to evaluate milder disease. Some patients may be functional in society at baseline.
Acute decompensation is associated with gastrointestinal bleeding events, due to the concomitant esophageal varices seen with portal hypertension. Infections commonly precipitate deterioration as well, notably subacute bacterial peritonitis, pneumonia, and urinary tract infection. Renal failure, malnutrition from cachexia, overaggressive diuresis and hypoperfusion, and exogenous narcotics or benzodiazepines may contribute a second cause of encephalopathy and make the disease clinically evident. There may be worsening of portosystemic shunt as after portal vein thrombosis or after TIPS procedure. Lastly, there may be an additional hepatic insult such as superimposed viral hepatitis, alcoholic hepatitis, or drug-induced liver injury.
EEG abnormalities in HE include – in order of worsening severity – theta rhythms, generalized triphasic waves, and predominant delta rhythm. The EEG may have characteristic high-amplitude low-frequency bursts. The most consistent laboratory finding is hypoalbuminemia and clotting factor deficiency. Other laboratory findings are inconsistent, but in general elevated serum ammonia levels are expected. Ammonia levels should be taken fasting and preferably from arterial samples. Elevated ammonia levels may exert its effect indirectly by inducing astrocyte dysfunction, thus explaining the occasional unlinking of hyperammonemia and neurologic effects [124]. HE is associated with loss of regulation of cerebral blood flow, impaired oxygen metabolism, and conversion of astrocytes to Alzheimer type 2 cells [131, 132]. A head CT should be performed to evaluate for brain edema and to rule out structural lesions. MRI is more sensitive and specific for cerebral edema and may demonstrate increased signal in bilateral basal ganglia on T1-weighted imaging.
The treatment of acute exacerbations should identify and remove precipitating factors. Lactulose or other cathartics may aid in the elimination of protein metabolites. Enteral neomycin, metronidazole, or rifaximin can be very helpful in reducing the bacterial flora of the gut. Long-term management requires limitation of enteral protein to 0.5 g/kg/day and titration of lactulose to adequate stool frequency and abatement of neurologic symptoms.
Nutritional and Malabsorptive Disorders in the Critically Ill
Nutritional disorders and malabsorptive disorders may occasionally produce neurologic symptoms in the ICU (Table 21.9). When present, deficiency of water-soluble vitamins such as thiamine, riboflavin, niacin, and pyridoxine is usually due to insufficient nutritional intake [133]. Deficiency of fat-soluble vitamins, such as vitamin A, D, E, and K, can occur in the ICU in the setting of postsurgical short gut syndrome, pancreatic insufficiency, hepatic disease with bile acid deficiency, colchicine, laxatives, Zollinger–Ellison syndrome, or poorly controlled celiac disease [134] or Crohn’s disease.
Wernicke’s Encephalopathy
Wernicke’s encephalopathy is a rare and preventable condition caused by thiamine deficiency. It is most commonly seen in patients with chronic alcoholism, cancer, and in late-stage AIDS. Clinical presentation includes ophthalmoplegia, nystagmus, gait ataxia, and altered mental status. MRI may reveal T2-hyperintense lesions surrounding the cerebral aqueduct and third ventricle and in the mamillary bodies and medial thalami. Treatment is with intravenous thiamine. The administration of glucose to susceptible patients without prior thiamine supplementation can precipitate Wernicke’s encephalopathy or worsen existing disease. In any patient who is potentially at risk for Wernicke’s encephalopathy, thiamine should be administered prior to giving any glucose containing solutions.
Encephalopathy of Renal Failure
Uremic Encephalopathy
Uremic encephalopathy can develop with both acute and chronic renal failure. Presenting symptoms include headache, tremor, myoclonus, obtundation, and coma. The cause of the neurologic dysfunction is thought to be due to accumulation of dialyzable toxins including urea, guanidino compounds, uric acid, hippuric acid, atypical amino acids, polyamines, phenols, acetone, glucuronic acid, carnitine, myoinositol, and phosphates. Uremic encephalopathy resolves with renal replacement therapy, yet a delay of 1–2 days is common before clinical improvement is seen.
Dialysis Disequilibrium Syndrome
An acute neurologic complication that may occur with hemodialysis is dialysis disequilibrium syndrome (DDS). Symptoms include headache, nausea, confusion, and ataxia. Severely affected patients may develop seizures, obtundation, and coma. This condition usually develops during or immediately after hemodialysis and is thought to be caused by rapid changes in serum osmolality leading to brain edema. DDS is self-limiting and symptoms generally resolve over several hours.
Encephalopathy Associated with Endocrine Disorders
Severe hypothyroidism and hyperthyroidism can cause an acute encephalopathy. Myxedema coma is the severest form of hypothyroidism and manifests with lethargy or coma in association with bradycardia, hypothermia, hyponatremia, and hypercapnic/hypoxemic respiratory failure. Thyrotoxicosis may present with a range of neurologic symptoms from psychosis and agitation to delirium, somnolence, and even coma. Acute adrenal insufficiency typically presents with circulatory shock and electrolyte abnormalities, yet it can also be associated with lethargy and coma. Hashimoto’s encephalopathy – also known as steroid-responsive encephalopathy with autoimmune thyroiditis – is a heterogenous syndrome of neurologic symptoms associated with anti-thyroid antibodies and/or autoimmune thyroid dysfunction. Its presentation can range from subacute, recurrent episodes of focal neurologic deficits to a rapidly progressive dementia or coma. Treatment is with corticosteroids.
Metabolic Encephalopathy
Common electrolyte disturbances that result in encephalopathy are hyponatremia, hypernatremia, hypoglycemia, and hyperglycemic crises. Acute hyponatremia may cause brain edema with clinical signs ranging from confusion to coma and death [135–140]. The severity of clinical presentation depends on the rate of decrease and the absolute serum sodium level. Correction of hyponatremia should be achieved in a controlled manner to avoid the development of an osmotic demyelination syndrome. Hypernatremia leads to neurologic dysfunction through a hyperosmolar state that effectively dehydrates the brain. The rate of correction of hypernatremia should also be cautious to prevent the development of cerebral edema.
Hypoglycemia can present with encephalopathy or occasionally as focal neurologic deficits, especially in patients with prior strokes or other brain lesions. Correction of hypoglycemia should be performed rapidly with intravenous dextrose to prevent permanent brain injury. Depending on the severity and duration of hypoglycemia, the clinical response to treatment may lag significantly behind the return to normoglycemia. Severe hyperglycemia resulting from decompensated diabetes mellitus is another important cause of encephalopathy. Brain dysfunction results from serum hyperosmolarity associated with acidosis and electrolyte depletion. Treatment priorities are intravascular volume resuscitation, intravenous insulin, and electrolyte repletion.
Summary
The neurologic expression of critical illness is prevalent and associated with adverse outcome. Both the central and the peripheral nervous system may be affected. Recognition may be delayed due to sedation and emphasis on systemic resuscitation. Intensive care providers must work systematically to identify and treat supervening neurologic disorders. Research is needed to discover biological mechanisms and implement preventive and therapeutic interventions.
References
Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62.
Ouimet S, Kavanaugh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73.
Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–7.
van den Boogaard M, Peters SAE, van der Hoeven JG, et al. The impact of delirium on the prediction of in-hospital mortality in intensive care patients. Crit Care. 2010;14:R146.
Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–900.
Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–62.
van den Boogaard M, Schoonhoven L, Evers AW, et al. Delirium in critically ill patients: impact on long-term health related quality of life and cognitive functioning. Crit Care Med. 2012;40:112–8.
Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–20.
Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29:1370–9.
Ely WE, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients – validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–10.
Luetz A, Heymann A, Radtke FM, et al. Different assessment tools for the intensive care unit delirium: which score to use? Crit Care Med. 2010;38:409–18.
CAM-ICU Worksheet 2010. http://www.mc.vanderbilt.edu/icudelirium/docs/CAM_ICU_worksheet.pdf. Accessed 16 May 2012.
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–64.
Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13(3):R77.
Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–304.
Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–34.
Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–6.
Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41.
Hipp DM, Ely EW. Pharmacological and nonpharmacological management of delirium in critically ill patients. Neurotherapeutics. 2012;9:158–75.
Girard TD, Panharipande PP, Carson SS. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38:428–37.
Lonergan E, Britton AM, Luxenberg J. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
Devlin JW, Skrobik Y, Riker RR. Impact of quetiapine on resolution of individual delirium symptoms in critically ill patients with delirium: a post-hoc analysis of a double-blind, randomized, placebo-controlled study. Crit Care. 2011;15(5):R215.
Devlin JW, Roberts RJ, Fong JJ. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–27.
Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009;35:781–95.
Saper CB. Brainstem modulation of sensation, movement, and consciousness. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of neural science. 4th ed. New York: McGraw-Hill; 2000. p. 897.
Wijdicks EFM. Coma and other states of altered awareness in the intensive care unit. In: Neurologic complications of critical illness. 3rd ed. New York: Oxford University Press; 2009.
Saper CB. Brainstem modulation of sensation, movement, and consciousness. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of neural science. 4th ed. New York: McGraw-Hill; 2000.
Wijdicks EFM, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58:585–93.
Wijdicks EFM, Sharbrough FW. New-onset seizures in critically ill patients. Neurology. 1993;43:1042–4.
Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–5.
Bartynski WS, Tan HP, Boadman JF, Shapiro R, Marsh JW. Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol. 2008;29:924–30.
Bartynksi WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR AM J Neuroradiol. 2008;29:1043–9.
Friese R. Sleep and recovery from critical illness and injury: a review of theory, current practice, and future directions. Crit Care Med. 2008;36:697–705.
Hardin KA. Sleep in the ICU potential mechanisms and clinical implications. Chest. 2009;136:284–94.
Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subject exposed to ICU noise. Sleep Med. 2005;6:423–8.
Bijwadia JS, Ejaz MS. Sleep and critical care. Curr Opin Crit Care. 2009;15:25–9.
Marzano C, Ferrara M, Curcio G, De Gennaro L. The effects of sleep deprivation in humans: topographical electroencephalogram changes in non-rapid eye movement (NREM) sleep versus REM sleep. J Sleep Res. 2010;19:260–8.
Weinhouse GL, Schwab RJ, Watson PL, Patil N, Vaccaro B, Pandharipande P. Review bench-to-bedside review: delirium in ICU patients – importance of sleep deprivation. Crit Care. 2009;13:234–42.
Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423–9.
Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048–54.
Watson P. Measuring sleep in critically ill patients: beware the pitfalls. Crit Care. 2007;11:159–60.
Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–63.
Nelson AB, Faraguna U, Tononi G, Cirelli C. Effects of anesthesia on the response to sleep deprivation. Sleep. 2010;33(112):1659–67, S1–S2.
Pal D, Lipinski WJ, Walker AJ, Turner AM, Mashour GA. State-specific effects of anesthesia on sleep homeostasis. Selective recovery of slow wave but not rapid eye movement sleep. Anesthesiology. 2011;114:302–10.
Mashour GA, Lipinski WJ, Matlen LB, Walker AJ, Turner AM, Schoen W, Lee U, Poe GR. Isoflurane anesthesia does not satisfy the homeostatic need for rapid eye movement sleep. Anesth Analg. 2010;110:1283–9.
Trompeo AC, Vidi Y, Locane MD, Braghiroli A, Mascia L, Bosma K, Ranieri VM. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol. 2011;77:1–2.
Tung A, Bergmann BM, Herrera S, Cao D, Mendelson WB. Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology. 2004;100:1419–26.
Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–78.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94.
Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med. 2010;38(7):1554–61.
Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–51.
Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–5.
Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–7.
Jackson JC, Gordon SM, Burger C, et al. Acute respiratory distress syndrome and long-term cognitive impairment: a case study [abstract]. Arch Clin Neuropsychol. 2003;18:688.
Rothenhäusler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23:90–6.
Jones C, Griffiths RD, Slater T, Benjamin KS, Wilson S. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med. 2006;32:923–6.
Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–41.
Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–44.
Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, Ali NA, Sharshar T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 suppl):S299–308.
Howard RS, Tan V, Z’Graggen WJ. Weakness on the intensive care unit. Pract Neurol. 2008;8:280–95.
Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: the electrophysiological components of a complex entity. Intensive Care Med. 2003;29:1505–14.
DeJonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphael JC, Outin H, Bastuji-Garin S. Paresis acquired in the intensive care unit a prospective multicenter study. JAMA. 2002;288(22):2859–67.
De Seze M, petit H, Wiart L, Cardinaud JP, Gaujard E, Joseph PA, Mazaux JM, Barat M. Critical illness polyneuropathy a 2-year follow-up study in 19 severe cases. Eur Neurol. 2000;43:61–9.
Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar A, Garnacho-Montero MC, Moyano-Del Estad MR. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27:1288–96.
Garnacho-Montero J, Amaya-Villar R, Garcia-Garmendia JL, Madrazo-Osuna J, Ortiz-Leyba C. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit Care Med. 2005;33(2):349–54.
Guarneri B, Bertolini G, Latronico N. Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J Neurol Neurosurg Psychiatry. 2008;79:838–41.
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93.
Latronico N, Peli E, Botteri M. Critical illness myopathy and neuropathy. Curr Opin Crit Care. 2005;11:126–32.
Leitjen FSS, Harinck-de Weerd JE, Poortvliet DCJ, de Weerd AW. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274:1221–5.
Maramattom BV, Wijdicks EFM. Acute neuromuscular weakness in the intensive care unit. Crit Care Med. 2006;34(11):2835–41.
Maramattom B, Wijdicks EFM, Sundt TM, Cassivi SD. Flaccid quadriplegia due to necrotizing myopathy following lung transplantation. Transplant Proc. 2004;36:2830–3.
Niskanen M, Karl A, Halonen P. Five-year survival after intensive care- comparison of 12,180 patients with the general population. Crit Care Med. 1996;24(2):1962–7.
Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131:1541–9.
Schweickert WD, Pohlman MC, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomized controlled trial. Lancet. 2009;373:1874–82.
Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33:1876–91.
Watling SM, Dasta JF. Prolonged paralysis in intensive care unit patients after the use of neuromuscular blocking agents: a review of the literature. Crit Care Med. 1994;22:884–93.
Riker RR, Shehabi Y, Bokesch PM, et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–99.
Pandharipande PP, Pun BT, Herr D, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–53.
Braakman HMH, Lodder J, Postma AA, Span LFR, Mess WH. Vasospasm is a significant factor in cyclosporine-induced neurotoxicity: case report. BMC Neurol. 2010;10:30.
Jain A, Kashyp R, Dodson F, Kramer D, Hamad I, Khan A, Eghestad B, Starzl TE, Fung JJ. A prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone, and mycophenolate mofetil in primary adult liver transplantation: a single center report. Transplantation. 2001;72:1091–7.
McDiarmid SV, Busuttil RW, Ascher NL, Burdick J, D’alessandro AM, Esquivel C, Kalayoglu M, Klein AS, Marsh JW, Miller CW, Schwartz ME, Shaw BW, SO SK. FK506 (tacrolimus) compared with cyclosporine for primary immunosuppression after pediatric liver transplantation. Transplantation. 1995;59:530–6.
Mueller AR, Platz KP, Bechstein WO, Schattenfroh N, Stoltenburg-Didinger G, Blumhardt G, Christe W, Neuhaus P. Neurotoxicity after orthotopic liver transplantation a comparison between cyclosporine and FK506. Transplantation. 1994;58:155–69.
Pfitzmann R, Klupp J, Langrehr JM, Uhl M, Neuhaus R, Settmacher U, Steinmueller T, Neuhaus P. Mycophenolatemofetil for immunosuppression after liver transplantation: a follow-up study of 191 patients. Transplantation. 2003;76:130–6.
Saner FH, Gensicke J, Olde Damink SWM, Pavlakovic G, Treckmann J, Dammann M, Kaiser GM, Sotiropoulos GC, Radtke A, Koeppen S, Beckebaum S, Cicinnati V, Nadalin S, Malago M, Paul A, Broelsch CE. Neurologic complications in adult living donor liver transplant patients: an underestimated factor? J Neurol. 2010;257:253–8.
Selzner N, Durand F, Bernuau J, Heneghan MA, Tuttle-Newhal JE, Belghiti J, Clavien PA. Conversion from cyclosporine to FK506 in adult liver transplant recipients: a combined north American and European experience. Transplantation. 2001;76:1061–5.
Umeda Y, Matsuda H, Sadamori H, Shinoura S, Yoshida R, Sato D, Utsumi M, Yagi T, Fujiwara T. Leukoencephalopathy syndrome after living-donor liver transplantation. Exp Clin Transplant. 2011;9:139–44.
Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infectious endocarditis. Clin Infect Dis. 2000;30:633–8.
Cabell CH, Pond KK, Peterson GE, et al. The risk of stroke and death in patients with aortic and mitral valve endocarditis. Am Heart J. 2001;142:75–80.
Derex L, Bonnefoy E, Delahaye F. Impact of stroke on therapeutic decision making in infective endocarditis. J Neurol. 2010;257:315–21.
Ruttmann E, Willeit J, Ulmer H, et al. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke. 2006;37:2094–9.
Rostagno C, Rosso G, Puggelli F, et al. Active infective endocarditis: clinical characteristics and factors related to hospital mortality. Cardiol J. 2010;16:566–73.
Amodeo MR, Clulow T, Lainchbury J, et al. Outpatient intravenous treatment for infective endocarditis: safety, effectiveness, and one-year outcomes. J Infect. 2009;59:387–93.
Bishara J, Leibovici L, Gartman-Israel D, et al. Long-term outcome of infective endocarditis: the impact of early surgical intervention. Clin Infect Dis. 2001;33:1636–43.
Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med. 2009;169(5):463–73.
Denk K, Vahl CF. Endokarditis: entscheidungshilfen fur den optimalen zeitpunkt zur operativen sanierung. Herz. 2009;34:198–205.
Wang A, Pappas P, Anstrom KJ, et al. The use and effect of surgical therapy for prosthetic valve infective endocarditis: a propensity analysis of a multicenter international cohort. Am Heart J. 2005;150:1086–91.
Perrotta S, Aljassim O, Jeppsson A, Bech-Hanssen O, Svensson G. Survival and quality of life after aortic root replacement with homografts in acute endocarditis. Ann Thorac Surg. 2010:90:1862–8.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63.
Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56.
Lance JW, Adams RD. The syndrome of intention or action myoclonus as a sequel to hypoxic encephalopathy. Brain. 1963;86:111–36.
Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304.
Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S, Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–10.
Bouwes A, Binnekade JM, Kuiper MA, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol. 2012;71:206–12.
Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–7.
Wijdicks EFM, Parisi JE, Sharbrough FW. Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol. 1994;35:239–43.
English WA, Griffin NJ, Nolan JP. Myoclonus after cardiac arrest: pitfalls in diagnosis and prognosis. Anaesthesia. 2009;64:908–11.
Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–6.
Hopkins RO, Weaver LK, Chan KJ, Orme JF. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc. 2004;10:1005–17.
Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31:1226–34.
Rothenhausler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23:90–6.
Hopkins RO, Gale SD, Weaver LK. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 2006;20(3):263–71.
Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–60.
Taraborrelli C, Palchykova S, Tobler I, Gast H, Birchler T, Fontana A. TNFR1 is essential for CD40, but not for lipopolysaccharide-induced sickness behavior and clock gene dysregulation. Brain Behav Immun. 2011;25:434–42.
Wilson JX, Young GB. Sepsis-associated encephalopathy: evolving concepts. Can J Neurol Sci. 2003;30:98–105.
Young GB, Bolton CF, Archibald YM, Austin TW, Wells GA. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol. 1992;9:145–52.
Sharshar T, Carlier R, Bernard F, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806.
Piazza O, Russo E, Cotena S, Esposito G, Tufano R. Elevated S100B levels do not correlate with severity of encephalopathy during sepsis. Br J Anaesth. 2007;99:518–21.
Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T. Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med. 2009;37(10 Supp.):S331–6.
Siami S, Annane D, Sharshar T. The encephalopathy in sepsis. Crit Care Clin. 2008;24:67–82.
Taccone FS, Castanares-Zapatero D, Peres-Bota D, Vincent JL, Berre J, Melot C. Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care. 2010;12:35–42.
Jackson AC, Gilbert JJ, Young B, Bolton CF. The encephalopathy of sepsis. Can J Neurol Sci. 1985;12:303–7.
Nauwynck M, Huyghens L. Neurological complications in critically ill patients; septic encephalopathy, critical illness polyneuropathy. Acta Clin Belg. 1998;53:92–7.
Freund HR, Ryan JA, Fischer JE. Amino acid derangements in patients with sepsis: treatment with branched chain amino acid rich infusions. Ann Surg. 1978;188:423–30.
Raghavan M, Marik PE. Therapy of intracranial hypertension in patients with fulminant hepatic failure. Neurocrit Care. 2006;4:179–89.
Gottlieb A, DeBoer KR. Brain preservation during orthotopic liver transplantation in a patient with acute liver failure and severe elevation in intracranial pressure. J Gastrointest Surg. 2005;9(7):888–90.
Lockwood AH. Hepatic encephalopathy. In: Aminoff MJ, editor. Neurology and general medicine. 3rd ed. Philadelphia: Churchill Livingstone; 2001.
Gotthardt D, Riediger C, Heinz Weiss K, Encke J, Schemmer P, Schmidt J, Sauer P. Fulminant hepatic failure: etiology and indications for liver transplantation. Nephrol Dial Transplant. 2007;22 suppl 8:viii5–8.
Jalan R, Olde Damink SWM, Deutz NEP, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology. 2004;127:1338–46.
Blei A, Cordoba J, et al. Hepatic encephalopathy. Am J Gastroenterol. 2001;96:1968–76.
Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy-definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th world congress of gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21.
Jalan R. Pathophysiological basis of therapy of raised intracranial pressure in acute liver failure. Neurochem Int. 2005;47:78–83.
Norenberg MD. A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy: progression and reversal of the disorder. Lab Invest. 1977;36:618.
Mancall EL. Nutritional disorders of the nervous system. In: Aminoff MJ, editor. Neurology and general medicine. 3rd ed. New York: Churchill Livingstone; 2001.
Chin RL, Latov N, Green PHR, et al. Neurologic complications of celiac disease. J Clin Neuromusc Dis. 2004;5:129–37.
Androgue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–9.
Androgue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–9.
Elhassan EA, Schrier RW. Hyponatremia: diagnosis, complications, and management including V2 receptor antagonists. Curr Opin Nephrol Hypertens. 2011;20:161–8.
Halawa I, Andersson T, Tomson T. Hyponatremia and risk of seizures: a retrospective cross-sectional study. Epilepsia. 2011;52(2):410–3.
Samuels MA, Seifter JL. Encephalopathies caused by electrolyte disorders. Semin Neurol. 2011;31:135–8.
Sterns RH. Severe symptomatic hyponatremia: treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107:656–64.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
LacKamp, A.N., Stevens, R.D. (2013). Neurologic Implications of Critical Illness and Organ Dysfunction. In: Layon, A., Gabrielli, A., Friedman, W. (eds) Textbook of Neurointensive Care. Springer, London. https://doi.org/10.1007/978-1-4471-5226-2_21
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5226-2_21
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5225-5
Online ISBN: 978-1-4471-5226-2
eBook Packages: MedicineMedicine (R0)