Abstract
Carbohydrates are involved in crucial physiological and pathological events. One can take advantage of carbohydrate-based interaction for drug discovery, diagnosis, antibiotics, vaccine, etc. This chapter deals with biosensors and microarrays that take advantage of carbohydrates-based interactions with a special interest in devices that are designed for medical applications. A large overview of glycochemistry, followed by the biological role of carbohydrates, is given. Carbohydrate-based biosensors are then described with special emphasis on surface chemistry and signal transduction. Finally, medically relevant applications illustrate the use of carbohydrates as recognition receptors in biosensing.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Glycosylation of proteins is a common post-translational modification, and eukaryotic cells are coated with complex carbohydrates associated with lipids or proteins forming the so-called glycocalix. Carbohydrates are the most abundant type of biomolecules on earth in term of biomass. Their biological role spans from the stabilisation of protein structure to their involvement in crucial biological events such as the development, growth or survival of a cell/organism (Varki 1993; Sharon and Lis 2004; Varki et al. 1999; Dwek 1996; Bertozzi and Kiessling 2001; Bishop et al. 2007). For instance, protein–carbohydrate interactions are involved in intracellular trafficking of proteins, cell adhesion, cell recognition, cell differentiation and the development of the neuronal network. They are also involved in pathological events such as inflammation, metastasis (Galonic and Gin, 2007) and host–pathogen interactions (Imberty et al. 2004; Seeberger and Werz 2007). For example, influenza virus takes advantage of the carbohydrate motifs present on the surface of the respiratory track endothelial cells for adhering to these cells (Skehel and Wiley 2000). The switch from avian influenza to human influenza sub-type is related to the subtle change of its ability to recognise the glycosylated motif of human respiratory track endothelial cell instead of the avian ones. The elucidation of these interactions is a critical issue not only in terms of fundamental research but also in terms of applied research for the development of diagnosis or new therapeutics.
Biophysical approaches such as calorimetry (Dam and Brewer 2002), nuclear magnetic resonance (Sandström et al. 2004) and crystallography (Berhe et al. 1999) have been successfully employed and have brought many valuable pieces of information. However, these techniques are not straightforward as well as are time- and material-consuming.
When dealing with carbohydrates, several limitations arise because of their chemical nature. Firstly, carbohydrate structures are extremely diverse. It has been calculated that a hexamer can lead to more than 1012 structures (Laine 1994). Secondly, carbohydrate synthesis is one of the most difficult and laborious synthetic work, and carbohydrates cannot be amplified enzymatically like DNA (by polymerase chain reaction) or cloned such as proteins. The consequence is that carbohydrate material is difficult to obtain in large amounts at affordable prices. Finally, carbohydrate interactions, especially with proteins, tend generally to be weak unless a multivalent interaction is involved, taking advantage of the so-called cluster effect (Lee and Lee 1995). Consequently, analysis of carbohydrate-mediated interactions requires analytical techniques with very low detection limit.
In this context, biosensors and microarrays have several advantages. They require very little amounts of biological material. In the case of microarrays, high-throughput analysis can be performed and they usually have very low detection limits (Feizi et al. 2003; Wang 2003; Jelinek and Kolusheva 2004). Almost all biosensors are based on a biological binding/recognition element (ligand) that performs a specific binding or biochemical reaction with a target. This interaction is then converted into a signal through a transducer.
The requirements for modern biosensors are sensitivity, selectivity and reproducibility. These parameters depend on a combination of many factors among which surface chemistry, ligand immobilisation, target physico-chemical properties and signal transduction are key factors.
This chapter concerns carbohydrate based biosensor in a broad sense, that is devices comprising bound bioactive carbohydrate ligands responsible for a specific interaction at the surface of a material. Here, the ligand considered will be a saccharide or an array of saccharides and more precisely mono or oligosaccharides.
At this stage, it is worth giving a few definitions. In the following, a probe is a molecule (a biomolecule) that is immobilised on the surface of a material in order to interact with a target. The target is the biological molecule to be detected in a biological fluid.
In order to avoid confusion, a biosensor is a device that can perform directly the detection of a biochemical interaction. This means that the biological interaction is measured in real time (Rasooly 2005). Surface plasmon resonance (SPR) or electrochemical detection belong to this class of biosensors. Another class of biosensors relies on indirect detection such as fluorescence detection (Rasooly 2005).
A biochip or microarray is a special class of biosensor as it allows for the simultaneous detection of multiple interactions. Common to both cases, the ligands are immobilised on the surface of a material (plane or particles) that can be active as a transducer (electrochemically active or optically active for SPR) or inert (glass in the case of fluorescence).
In this chapter, we are concerned with carbohydrate-based biosensing. Therefore, we will first give an overview on the physico-chemical properties of carbohydrates, as these are critical for obtaining and designing the probes. We will then focus on their biological role. We will then give an overview of carbohydrate-based biosensing from their immobilisation, as this is critical for obtaining reliable devices and to the signal transducer. Finally, we will give some applications described in the literature.
2 General Aspects of Glycochemistry
2.1 Introduction
Carbohydrates were named after their composition as hydrates of carbon, but they can be also called saccharides from greek σα´κχαρον meaning sugar. They are polyfunctional biomolecules with the general formula C n (H2O) n (e.g. n = 6 for glucose). The biological aspects of carbohydrates have long been underestimated because of their complexity in comparison to the three other classes of biomolecules. Polypeptides (proteins) and polynucleotides (e.g. DNA or RNA) are linear polymers, whereas carbohydrates are either linear or branched polymers, thereby increasing drastically the number of possible combinations (Laine 1994; Werz et al. 2007). Recent advances in glycobiology have demonstrated the numerous roles of carbohydrates in a series of biological processes such as storage and transport of energy, signal transduction, fertilisation, immune responses, pathogenesis, blood clotting, virus infection and cell-to-cell communication (Bertozzi and Kiessling 2001; Sharon and Lis 2004; Dwek 1996; Varki 1993; Varki et al. 1999). These fundamental discoveries found several applications in the design of carbohydrate-based drugs and vaccines against various diseases (Werz and Seeberger 2005b) such as malaria (Hölemann and Seeberger, 2006; Seeberger and Werz 2007), cancer (Galonic and Gin, 2007; Livingston and Ragupathi 2006) and AIDS (McReynolds and Gervay-Hague, 2007; Scanlan et al. 2007). The ever-increasing number of chemists, biochemists and biologists who are involved in research with carbohydrates is testimony to the fact that there is still a lot more work to be achieved in glycochemistry and glycobiology.
2.2 Structural Aspects and Chemistry of Carbohydrates
Monosaccharides are classified according to the number of carbon atoms, which ranges from 4 (tetroses), 5 (pentoses), 6 (hexoses) and up to 10 atoms. Aldoses (Fig. 7.1) and ketoses (Fig. 7.2) are polyhydroxylated aldehydes or ketones, respectively, which can cyclise intramolecularly into hemiacetals. The nucleophilic attack of a hydroxyl group on the carbonyl will provide two diastereoisomeric hemiacetals called α- and β-anomers. Cyclisation can occur between O-4 and O-5 to form a five-membered ring designated as furanose or a six-membered pyranose ring, respectively (Fig. 7.3). d-Aldoses are derived from d-glyceraldehyde and their enantiomers (l-aldoses) from l-glyceraldehyde. Atom numbering starts at the most oxidised end of the carbon chain (e.g. carbonyl group for aldoses) and increases along the carbon chain.
The cyclic structures of monosaccharides can be represented according to several projections (Fig. 7.4). The Fischer projection was the first one to be applied to acyclic forms of sugars, where the carbon chain is drawn vertically with the most oxidised carbon atom (carbonyl) at the top. Later, Haworth designed a projection for a more realistic description of cyclic forms of carbohydrates. The chair conformation provides the most accurate description of the geometry of the carbohydrate ring and should be preferred to other projections. Mills and zig-zag projections are useful when considering the stereochemistry of each carbon atom along the chain.
2.2.1 Mutarotation
Carbohydrates are single stereoisomers in their crystalline form. Nevertheless, optical rotation of a single anomer in aqueous solution slowly changes with time to reach a constant value. Mutarotation (Pigman and Isbell 1968; Isbell and Pigman 1969) refers to this equilibration process where an α-anomer would equilibrate to a mixture of α- and β-anomers through the ring-opened aldehyde form and re-cyclisation into its opposite anomer (Fig. 7.5). This equilibrium is catalysed by acids or bases. Carbohydrates are therefore present as a mixture of acyclic and five- or six-membered cyclic forms with two different anomers in equilibrium in aqueous solutions with ratios that can be determined by NMR spectroscopy (Table 7.1).
2.2.2 The Anomeric Effect and the Exo-anomeric Effect
The anomeric effect (Edward and Puskas 1962) was studied by Lemieux (Praly and Lemieux 1987) as the tendency for an electronegative substituent at the anomeric centre to prefer the axial orientation instead of the less hindered equatorial position (Edward and Puskas 1962; Praly and Lemieux 1987). Two explanations can be invoked based on dipolar moments or hyperconjugation (Fig. 7.6). Equatorial configuration of an electronegative anomeric substituent results in the formation of two similar dipoles repelling each other and destabilizing the molecule. On the contrary, axially orientated substituents create roughly opposed dipoles accounting for a lower energy state. The second explanation to the anomeric effect is the presence of a stabilizing interaction through hyperconjugation between an unshared electron pair of the endocyclic oxygen atom and the σ* orbital of the anomeric bond (e.g. C–Br).
The orientation of the substituent at the anomeric centre (aglycon) can be described by the exo-anomeric effect (Fig. 7.7) (Praly and Lemieux 1987; Perez and Marchessault 1978; Thoegersen and Lemieux 1982) In this case, the exocyclic anomeric oxygen atom’s lone pair is interacting with the anti-bonding orbital of the endocyclic C–O bond. The exo-anomeric effect can be observed for both α- and β-anomers.
2.3 Glycosylation Methods
The formation of a glycosidic bond occurs through the nucleophilic displacement of a leaving group at the anomeric centre of a glycosyl donor by an alcohol of a glycosyl acceptor (e.g. ROH) to provide the corresponding O-glycoside (Fig. 7.8) (Fügedi 2006; Schmidt 1986; Veeneman 1998; Whitfield and Douglas 1996). This reaction is usually promoted by a third partner (promoter) increasing the reactivity of the glycosyl donor.
The substitution at the 2-position plays an important role in the stereochemical outcome of the glycosylation process (Fig. 7.9). When a non-participating group is present at the 2-position, the major glycoside obtained is the α-anomer because of the stabilization through the anomeric effect. However, a participating group at the 2-position creates a cationic bis-acetal intermediate hindering the α-face of the pyranose ring and therefore favours the nucleophilic attack of the glycosyl acceptor from the β-face to give rise to the 1,2-trans glycoside. During this process, the alcohol could also form an orthoester which can be rearranged into the desired 1,2-trans glycoside under Lewis acid catalysis (Kochetkov et al., 1967, 1971).
Each glycosylation method can be distinguished through the glycosyl donor/promoter system involved (Table 7.2). Acetalation of a reducing sugar with an alcohol in the presence of a protic acid promoter was used as one of the first glycosylation method by Fischer (Fischer 1893; Fischer 1895). It is probably the most appropriate method for the synthesis of simple glycosides (e.g. methyl, benzyl, allyl). Glycosylation was then performed from glycosyl halides in the presence of heavy metal salts by Koenigs and Knorr (Igarashi 1977; Koenigs and Knorr 1901). Glycosyl fluorides can also be used as glycosyl donors and are much more stable than their chlorinated or brominated analogues (Mukaiyama et al. 1981). They can be activated under specific conditions compatible with most protecting groups. Peracetylated carbohydrates are particularly convenient for large-scale synthesis and can be also involved in a glycosylation as glycosyl donors but their activation sometimes requires drastic conditions (Helferich and Schmitz-Hillebrecht 1933). Glycosyl trichloroacetimidates were then introduced by Schmidt and are activated under acidic conditions with either Brønsted or Lewis acids usually in the presence of molecular sieves and at low temperature (Schmidt and Michel 1980; Pougny and Sinay 1976). Arylthioglycosides are highly stable even when stored for long periods on shelves and can also accommodate a large number of chemical reactions on the pyranose ring. They can be activated by thiophilic reagents under mild conditions, and formation of the oxonium intermediate proceeds through the addition of the electrophilic promoter to the sulphur atom (Garegg 1997; Ferrier et al. 1973; Fügedi et al. 1987). Activation can also take place under single electron transfer, electrochemical or even high pressure conditions. n-Pentenyl glycosides were introduced by Fraser-Reid as glycosyl donors and are activated through halogenation of the terminal double bond followed by intramolecular cyclisation to provide the oxonium intermediate and a halomethyl-2-furane (Fraser-Reid et al. 1988; Fraser-Reid et al. 1992). Glycosyl phosphites (Kondo et al. 1992; Martin and Schmidt 1992; Zhang and Wong 2000) react with alcohols at low temperature in the presence of Lewis acids affording the corresponding glycosides, and glycosyl phosphates (Hashimoto et al. 1989; Vankayalapati et al. 2002) can be activated by protic acids. Activation of selenoglycosides (Mehta and Pinto 1991; Mehta and Pinto 1993) can be achieved under similar conditions as for thioglycosides, by photo-induced or single electron transfer or iodine. They can be selectively activated in the presence of thioglycosides.
All these glycosylation methods have found numerous applications for the synthesis of natural products or for the selective formation of the desired anomer. One should also consider the influence of protecting groups on the pyranose ring, which sometimes affect the glycosylation outcome.
2.4 Chemo-enzymatic Glycosylation Methods
Among the powerful methods of glycosylation listed above, only Fischer glycosylation can accommodate a non-protected carbohydrate where the hydroxyl functions are not masked by protecting groups. Glycosyltransferases and glycosidases are natural enzymes capable of creating or hydrolyzing glycosidic bonds in an aqueous medium, using native sugars and with high stereo- and regioselectivities. Their use as “glycosylating agents” or promoters has attracted a lot of interest in the scientific community and a series of applications are now available for the synthesis of complex oligosaccharidic structures (Qian et al. 2001). This technology requires both biochemical and chemical skills since enzymes sometimes need to be mutated for obtaining the expected result. Sialylation is one of the most difficult glycosylation because of the poor stability of activated sialic acid derivatives. The biochemical engineering of sialyltransferases can readily provide a large series of sialylated derivatives ranging from the glycosylation of simple alcohols to mono- or disaccharides (Drouillard et al. 2006; Blixt et al. 2005; Huang et al. 2006b; Dumon et al. 2006; Muthana et al. 2007; Yu et al. 2006).
2.5 Glycoconjugates
Carbohydrates play an important role in a large series of biological processes and are usually present in nature either in their native form or as glycoconjugates where a sugar residue is associated to a lipid (glycolipids), a protein (glycoprotein) or a peptide (glycopeptides), along with several other natural products containing sugars. The chemical conjugation of carbohydrate moieties to proteins or polyvalent species will create neoglycoproteins or neoglycoconjugates, respectively, which can find numerous applications in biology and biochemistry. After introducing a functionalised linker at the anomeric position of a carbohydrate through standard glycosylation methods, the other end of this arm will be used for its conjugation to the desired multivalent scaffold (Fig. 7.10).
The conjugation of carbohydrate derivatives to multivalent species has been achieved through a wide range of chemical ligation techniques. When considering the formation of neoglycoproteins, the chemist will take advantage of the reactive amino group from the lysine residues present at the surface of proteins and therefore use a series of reactions involving an amine function (Fig. 7.11). Similarly, neoglycoproteins can be prepared by reacting the thiol groups of cystein residues of proteins with thiol or maleimide functions (Fig. 7.12).
The above-mentioned techniques can be also applied to multivalent non-natural scaffolds for the preparation of neoglycoconjugates. In these cases, the nature of functionalities accessible is much broader. 1,3-Dipolar cycloaddition between an alkyne and an azido derivative to form 1,2,3-triazoles, developed by Huisgen (Huisgen et al., 1965, 1969) in the late 1960s, was recently introduced into the realm of techniques for conjugation and rapidly found a large number of applications in glycobiology (Fig. 7.13). This methodology is now accessible due to the improved Cu(I)-catalysed processes developed more recently (Kolb et al. 2001; Rostovtsev et al. 2002; Bock et al. 2006; Tornoe et al. 2002; Gil et al. 2007; Bouillon et al. 2006; Morvan et al. 2007). This chemical ligation technique is highly attractive since the reactive species involved are pretty much unreactive to the chemical functions present in biological media, the reaction does not create any by-product and they can be performed in water, at low temperature and on reasonable time scales.
2.6 Examples of Naturally Occurring Carbohydrates
2.6.1 Structures of Some Mono- and Disaccharides
A large number of monosaccharides incorporating a single carbohydrate residue are present along with several disaccharides composed of two sugar moieties (Fig. 7.14). Lactose is a disaccharide present in milk and fructose in fruits, while saccharose is the most common food sweetener. Ribose is a major component of RNA-forming nucleotide building blocks. Xylose is also called the wood sugar as it is one of the main components of trees and plants along with the disaccharide cellulose. Fucose is a 6-deoxy sugar found in mammalian, insect and plant cell surface.
2.6.2 Nucleosides
RNA and DNA can be differentiated through the nature of the carbohydrate involved in their structural subunits (nucleosides). Ribose is the sugar involved in RNA, while DNA is based on 2-deoxy-D-ribose lacking one hydroxyl group at the 2-position. The four constituents of RNA are based on a ribose residue linked to a pyrimidine: uracil (U) or cytosine (C), or to a purine: adenine (A) or guanine (G). In the DNA series, thymine (T) replaces uracil while the three other nucleobases are similar (Fig. 7.15).
2.6.3 Oligosaccharides
Carbohydrates can be associated through glycosidic linkages to form oligosaccharides possessing a wide range of biological aspects. The Lewis blood group antigens are oligosaccharides present on the surface of red blood cells and distinguish the human blood groups. These oligomers differ by the substitution (R) at the 3-position of the non-reducing galactosyl residue (Fig. 7.16).
Sialylated oligosaccharides are involved in a series of viral infection events. The substitution of lactose by sialic acid either at the 3′ or 6′ position differentiates between avian and human flu, respectively (Fig. 7.17) (Shinya et al. 2006).
Oligosaccharides can also adopt cyclic forms conferring a very different set of chemical and physical properties. Cyclodextrins are natural cyclic oligomers composed of six or more glucose units linked through an α-1,4-glycosidic bond (Fig. 7.18) (Nepogodiev and Stoddart 1998). Their inside cavity is highly hydrophobic and can form host–guest complexes with a series of molecules of various interests in the pharmaceutical industry (drug release) or for environmental applications (interactions with toxic compounds).
2.6.4 Polysaccharides
Polymeric carbohydrates are also widely present in nature. Cellulose is a β(1→4)-glucose polymer with a linear shape present in plants, while amylose is the corresponding α(1→4)-polyglucoside and is organized in a helical structure (Fig. 7.19). Polysaccharides are also employed for energy storage, like glycogen which is a branched polysaccharide based on the same α(1→4)-glucose polymeric chain as in amylose. Chitin is a polymeric β(1→4)-N-acetyl glucosamine and is the main component of the exoskeletons of crustaceans and insects.
2.6.5 Glycosaminoglycans
Heparin and heparan sulphate are the most complex members of the glycosaminoglycans (GAGs) family, which also includes chondroitin sulphate, keratin sulphate and dermatan sulphate (Fig. 7.20) (Bishop et al. 2007; Capila and Linhardt 2002). These highly negatively charged polysaccharides are displayed on the extracellular matrix or on the membrane of the cell. Heparin is an anticoagulant and is used for the treatment of heart diseases. It is composed of a tetrasaccharidic repeating unit with a major sequence highly conserved throughout the polymer chain and a variable sequence in which the sulphation/acetylation sites are not systematically functionalised. Heparan sulphate is a less sulphated version of heparin where the major sequence of heparin is not sulphated and the amino group acetylated.
2.6.6 Carbohydrate-Based Drugs from Natural and/or Synthetic Sources
A series of natural products containing carbohydrates have been isolated and their structure elucidated. Some of them are now used as drugs for the treatment of various diseases. Nojirimycin is produced by Streptomyces strains (Inouye et al. 1966). It is used as an antibiotic and inhibits α-glucosidases, thereby preventing the normal glycosylation of proteins (Fig. 7.21). Calicheamycin γ I1 is isolated from Micromonospora echinospora and is used as an antibiotic (Nicolaou and Smith 1992). It binds to the minor groove of DNA showing a high degree of base pair sequence specificity which can be attributed to the oligosaccharide residues. Kanamycins are antibiotics affecting the 30S ribosomal subunit and preventing the correct translation of RNA (Maeda et al. 1957). Indeed, the proteins are biosynthesized but fold incorrectly, thus destroying the bacterium. Daunomycin is an anti-cancer chemotherapy agent of the anthracycline family and isolated from Streptomyces peucetius (Arcamone et al. 1964). Acarbose is an inhibitor of α-glucosidase used for the treatment of type 2 diabetes mellitus (Junge et al. 1984). Streptomycin is an antibiotic drug obtained from the actinobacterium Streptomyces griseus and inhibits bacterial growth by damaging cell membranes and blocking protein synthesis (Majumdar and Kutzner 1962). Anthrose is found on the surface of Bacillus anthracis. This antigen is a very promising target for the development of an anti-anthrax vaccine (Tamborrini et al. 2006).
A series of nucleoside analogues have been approved by the Food and Drug Administration (FDA) for the treatment of HIV infection (AIDS) (Fig. 7.22). Zidovudine (AZT) was one of the first nucleoside approved by FDA and acts as a chain terminator for HIV reverse transcriptase (HIV-RT). Although resistance to AZT frequently develops, the association of Lamivudine (3TC) and AZT can cause AZT-resistant HIV strains to revert to AZT-sensitive strains. Abacavir is a carbocyclic nucleoside analogue significantly less toxic than AZT and appears to have a stronger anti-HIV effect than any of the approved nucleoside analogues. The association of AZT, 3TC and Abacavir in a tri-therapy strategy improves considerably the life expectancy of AIDS (or HIV-infected) patients.
Fondaparinux has been recently marketed by Sanofi-Aventis as an heparan sulphate analog for the treatment of heart diseases (Fig. 7.22) (Petitou and van Boeckel 2004).
3 Biological Role
The carbohydrates are probably best known for their role in energy metabolism. But like proteins, some carbohydrates perform structural functions, providing scaffolding for bacterial and plant cell walls (cellulose, pectin), connective tissues such as cartilage in animals, and exoskeleton shells (chitin) in arthropods. Carbohydrates are also covalently bound with proteins (glycoproteins) or lipids (glycolipids) on cell surfaces. The glycoproteins and glycolipids play important roles in cell–cell recognition processes and in signal transduction
3.1 The Glycocalix and Extracellular Matrix Polysaccharides
The surface of most types of cells is covered by a carbohydrate-rich coat, the glycocalyx, which plays an important functional role in cell–cell and cell–matrix interactions. These specific contacts are realized by using attractive forces between cell-surface oligosaccharides and proteins (lectins) (Hakomori 1991; Hakomori 2002; Rojo et al. 2002). It is now accepted that these interactions strongly depend on divalent cations and show high specificity and low affinity. However, its mechanism is not yet clarified because of the difficulty in analysing weak affinity interactions. The main challenge to understand and control interactions between carbohydrates and lectins is the low affinity of the interactions. Nature overcomes this problem by a polyvalent presentation of ligands and receptors at the cell surface (Mammen et al. 1998).
Studies of the terminal carbohydrate residues of the glycocalyx of immune cells have shown that cell–cell and cell–matrix interactions, which play an important functional role in various processes of the immune response, are mediated at least in part by these carbohydrate residues (Hounsell 2000). The use of lectin histochemistry in lymphatic organs of chickens demonstrated that carbohydrate residues are differentially expressed in the cells of the immune system of chickens (Jorns et al. 2003). For example, in the thymus, mannose as well as N-acetyl glucosamine (GlcNAc)-specific lectins labelled macrophages, epithelial reticulum cells and lymphocytes within the cortex. In the bursa of Fabricius, the brush border of the lining epithelium, the macrophages and the endothelium were labelled by mannose-specific lectins.
Because glycoconjugates, including proteoglycans, glycoproteins and glycolipids, are mainly localized in the plasma membrane and in the extracellular matrix of cells, they are considered as useful cell-surface biomarkers and play crucial roles in signal transduction and cell adhesion. To illustrate this, the vascular endothelium is presented in the following.
The endothelial glycocalyx is considered to be connected to the endothelium through several “backbone” molecules, mainly proteoglycans and also glycoproteins. Proteoglycans consist of a core protein to which one or more glycosaminoglycan chains are linked. There are five types of glycosaminoglycan chains: heparan sulfate, chondroitin sulfate, dermatan sulfate, keratan sulfate and hyaluronan (or hyaluronic acid). They are linear polymers of disaccharides with variable lengths that are modified by sulfation and/or (de)acetylation to variable extents. The disaccharides are each composed of a uronic acid and a hexosamine. In vasculature, heparan sulfate proteoglycans represent 50–90% of the total amount of proteoglycans present in the glycocalyx (Pries et al. 2000). Hyaluronan, another important glycosaminoglycan, differs from others because it is not linked to a core protein. Its exact link to the cell membrane is not known, but it can be bound to the receptor CD44 (Nandi et al. 2000).
Besides the proteoglycans, well-defined glycoproteins bearing small (2–15 sugar residues) and branched carbohydrate side chains connect the glycocalyx to the endothelial cell membrane. These endothelial cell adhesion molecules play a major role in cell recruitment from the bloodstream and in cell signalling. The three families of cell adhesion molecules present in the endothelial glycocalyx are the selectin family, the integrin family and the immunoglobulin superfamily. Selectins are carbohydrate-binding glycoproteins. These glycoproteins were designated as E-, P- and L-selectin, according to the cell type on which the selectin was found (i.e. endothelium, platelets, and lymphocytes, respectively). E-selectin and P-selectin are both involved in leukocyte–endothelial cell interactions (Sperandio 2006). The carbohydrate ligands that are recognised by these selectins have been identified. E-selectin recognizes sialyl Lewis X (sLeX) on the surface of neutrophils. P-selectin also binds sLeX on neutrophils or leukocytes with a lower affinity. L-selectin weakly recognises sLeX on endothelial cells, but the affinity is higher with a sulphate group on the 6-position of Gal or, perhaps more likely, on the 6-position of the GlcNAc residue. Integrins are heterodimeric molecules composed of non-covalently bound α (18 different α-subunits) and β (8 β-subunits) subunits. Integrins are found on many cell types, including endothelial cells, leukocytes, and platelets. These integrins, such as α2β1, α5β1 and α6β1, bind to multiple extracellular matrix ligands and are responsible for interactions with laminin, fibronectin, and collagen. Many studies have focused and still focus on the interactions between these integrins and the sub-endothelial matrix during angiogenesis (Ruegg and Mariotti 2003). The best known immunoglobulin superfamily of glycoproteins includes intercellular adhesion molecule 1 and 2 (ICAM-1 and -2), vascular cell adhesion molecule 1 (VCAM-1) and platelet/endothelial cell adhesion molecule 1 (PECAM-1). They act as ligand for integrins on leukocytes and platelets and are crucial mediators of leukocyte homing to the endothelium and subsequent diapedesis. In addition, glycoproteins of the endothelial glycocalyx display functionality in coagulation, fibrinolysis and homeostasis.
Leukocyte adhesion to endothelial cells is a complex process involving capture of free-flowing leukocytes from the bloodstream, rolling on the endothelial surface, deceleration and, eventually, leukocyte immobilization (firm adhesion). Studies have shown that disruption of heparan sulphate proteoglycans of the endothelial glycocalyx stimulates firm adhesion of leukocytes (Constantinescu et al. 2003). Indeed, heparan sulfate proteoglycans are abundant on the endothelial cell surface, are highly negatively charged due to the presence of numerous sulfate groups, and bind numerous plasma proteins, contributing to the glycocalyx charge and thickness (Esko 1999; Yanagishita and Hascall 1992). The integrity of vascular endothelium depends on the number of negatively charged molecules at the endothelial cell surface. In myocardial tissue oedema, the decrease of microvascular fluid is associated with a reduction of negatively charged molecules (Gotloib et al. 1992).
Glycoconjugate antigens are also utilized as unique markers in the developing brain and central nervous system (CNS) (Yu and Yanagisawa 2006; Yanagisawa and Yu 2007; Zhang 2001). Indeed, basic fibroblast growth factor (bFGF) is important for sustaining multipotency and enhancing proliferation of neural stem cells (NSCs)/neural precursor cells (NPCs). The signal of bFGF is mediated by receptors localized in the cell surface, but additional co-factors are also required. One of these co-factors is heparan sulfate proteoglycan (HSPG; Fig. 7.1). In addition to HSPGs, chondroitin sulfate proteoglycans (CSPGs) are also expressed in NSCs/NPCs. Nerve/glial antigen 2 (NG2) is a CSPG expressed in heterogeneous cell populations, including glial precursor cells and/or NSCs (Belachew et al. 2003). Furthermore, NG2 displays high affinity for bFGF and platelet-derived growth factor (PDGF)-AA, which are mitogens of NSCs/NPCs and oligodendrocyte progenitor cells (Goretzki et al. 1999).
Another class of carbohydrate-bearing molecules are glycosphingolipids (GSLs) which are composed of a core lipid moiety (ceramide) linked to a carbohydrate chain (Yu et al. 2004). GSLs having one or more sialic acid residues are named gangliosides. GSLs are most abundant in nervous tissues and are localized on the plasma membrane of cells. Studies have shown that certain GSLs are expressed in NSCs/NPCs like GD2 in human and mouse NSCs (Klassen et al. 2001) and GD3, GT1b and GQ1b in mouse NPCs(Yanagisawa et al. 2005).
Because of the biological significance of NSCs in neurogenesis, glycoconjugates are expected to play a significant role in developing clinical uses of NSCs for the treatment of a variety of neurodegenerative disorders. First, glycoconjugates can be used as biomarkers to define and isolate NSCs and their progenies. They also may be used for enriching NSCs. In addition, glycoconjugates may be useful for inducing differentiation of NSCs into specific lineages.
3.2 Carbohydrates in Host–Pathogen Interactions and Metastasis
Carbohydrates can serve as key elements in various molecular recognition processes, including bacterial and viral infections, cell adhesion in inflammation and metastasis.
The glycosylated phosphatidyl inositols (GPIs) are ubiquitous eukaryotic glycolipids covalently linked to the C-terminus of membrane-associated proteins. They provide an alternative mechanism for the attachment of proteins to the plasma membrane. However, studies have shown that glycoinositol phospholipids (GIPLs) not linked to either protein or polysaccharide are major cell-surface glycolipids in trypanosomatids, and are closely related to the structure of the GPI–protein anchor (Ferguson 1999). The GIPL of the parasite Trypanosoma cruzi, the causative agent of Chagas’ disease, contains a unique glycan chain expressing slight variations among distinct T. cruzi isolates, and linked through a non-N-acetylated glucosamine residue to an inositol phosphorylceramide, mainly composed of an N-lignoceroyl-dihydrosphingosine (Carreira et al. 1996). The β-D-Galf-(1→3)-α-D-Manp substructure is involved in its antigenicity (Mendonça-Previato et al. 1983). Biological effects of T. cruzi GIPL and its isolated glycan and lipid moieties were investigated on cells of the immune system. Dos Reis et al. (2002) found that GIPLs are bioactive against every cell type that has been tested.
Leishmania spp. are protozoan parasites that cause a spectrum of diseases in humans, ranging from self-limiting cutaneous infections to visceral leishmaniasis. Control of this disease, which affects more than 20 million people worldwide, has been hampered by the absence of a vaccine, limitation with frontline drugs and the increased transmission as a result of co-infections with HIV (Croft et al. 2006). The promastigote stages of Leishmania are coated by a thick glycocalyx composed of glycosyl phosphatidyl inositol (GPI)-anchored proteins, the GPI-anchored phosphoglycan, lipophosphoglycan (LPG) and GIPLs (Naderer et al. 2004). Analysis of Leishmania mutants lacking individual or multiple surface components indicates that only LPG plays a critical role in macrophage infection, while loss of other surface components has relatively little affect on promastigote virulence in the mammalian host (McConville et al., 2007). The plasma membrane of Leishmania amastigotes is unusual in several other respects. It contains relatively high levels of externally exposed phosphatidyl serine, which may provide a mechanism for entering host cells via apoptotic cell receptors without activating microbicidal processes or proinflammatory responses (Wanderley et al. 2006).
The study of N-linked glycosylation has become an area of intense interest because of its importance in viral virulence and immune evasion for several prominent human pathogens such as Hendra (Carter et al. 2005), severe acute respiratory syndrome coronavirus (SARS-CoV) (Oostra et al. 2006), influenza, hepatitis C (Goffard et al. 2005) and West Nile (Hanna et al. 2005). HIV and influenza, two clear threats to human health, have been shown to rely on expression of specific oligosaccharides to evade detection by the host immune system.
Influenza viruses express two envelope proteins that are involved in virulence, neuraminidase (NA) and hemagglutinin (HA). Influenza hemagglutinin binds to sialic acids attached to membrane glycosphingolipids, proteoglycans and glycoproteins. It was shown that the linkage between sialic acid and the second sugar (usually galactose) could determine whether an influenza virus binds to avian or human cells (Carroll et al. 1981; Rogers and Paulson 1983), and that a single mutation in the hemagglutinin (HA) was sufficient to change binding from α-2–3′ to α-2–6′ sialyl-lactose and host cells from avian to human, or vice versa (Rogers et al. 1983; Rogers et al. 1985). Now it is generally accepted that human influenza viruses bind to α-2–6′ sialic acids and avian viruses bind to α-2–3′ sialic acids (Baum and Paulson 1990; Matrosovich et al. 1997), while swine influenza viruses are reported to bind mainly to α-2–6′ sialic acids and also to α-2–3′ sialic acid receptors (Ito et al. 1998; Rogers and D’Souza 1989).
Infection by dengue virus (DV), a mosquito-borne flavivirus leading to haemorrhagic fever in humans, has undergone a global resurgence in the past 40 years such that almost half the world’s population are currently living at risk in dengue-endemic areas. The four serotypes of DV (DV1–DV4) bind to a number of opsonic and non-opsonic receptors on cells of the mononuclear phagocyte lineage including DC-SIGN (Navarro-Sanchez et al. 2003; Tassaneetrithep et al. 2003) and glycosaminoglycans (Chen et al. 1997). Recently, Miller et al. (Miller et al. 2008) have shown that the mannose receptor (MR) is a functional receptor for DV infection of human macrophage. MR and DC-SIGN both contain lectin domains, but differ distinctly in terms of ligand specificity. MR binds to terminal mannose, fucose and N-acetyl glucosamine, whereas DC-SIGN binds to mannose within high-mannose oligosaccharides and fucosylated glycans (Taylor et al. 2005; Feinberg et al. 2001).
Carbohydrate antigen sialyl Lewis a (CA19-9) is the most frequently applied serum tumour marker for diagnosis of cancers in the digestive organs. Moreover, malignant transformation is associated with changes in the glycosylation of cell-surface proteins and lipids. In tumour cells, alterations in cellular glycosylation may play a key role in their metastatic behaviour. Indeed, GD2 ganglioside is a major constituent of neuroectodermal tumors (Livingston 1995). Studies suggest that peptides mimicking GD2 ganglioside inhibit tumour growth through antibody and/or CD4+ T-cell-mediated mechanisms.
Oligosaccharide expression is highly relevant to many cancers. Many studies show that alterations in N-linked oligosaccharides of tumour cells are associated with carcinogenesis, invasion and metastasis (Varki 1993; Dwek 1996). Numerous studies have demonstrated a correlation between hyaluronan expression and the malignant properties of various kinds of cancer. Furthermore, hyaluronan oligosaccharides have been shown to inhibit several tumour cell types via disruption of receptor–hyaluronan interaction. So hyaluronan oligosaccharides could have potent antitumour effects due in part to the abrogation of hyaluronan-rich cell-associated matrices (Hosono et al. 2007).
Recent work has highlighted the importance of altered cell–matrix interactions in the acquisition of malignant characteristics of glioblastoma (Yamada and Araki 2001). Several studies have provided evidence that hyaluronan–cell interactions may play a role in glioma invasiveness. For example, addition of exogenous hyaluronan enhances glioma cell migration and invasion in vitro, and this effect is blocked by antibodies or anti-sense oligonucleotides to CD44, a cell-surface hyaluronan receptor (Merzak et al. 1994; Koochekpour et al. 1995; Okada et al. 1996; Tsatas et al. 2002).
4 Carbohydrate-Based Biosensors
Saccharide probes have been immobilised on a variety of substrates ranking from plane substrates such as polymer (Fukui et al. 2002), glass (Blixt et al. 2004), diamond (Chevolot et al. 1999) or gold (Seo et al. 2007; Miura et al. 2002; Smith et al. 2003) to nanoparticles or quantum dots (Larsen et al. 2006; Robinson et al. 2005; Huang et al. 2005). Both plane and spherical substrates are important to consider, as both can be used for biosensing.
4.1 Obtaining Saccharide Probes
Custom-synthesised peptides or nucleic acids are commercially available, and powerful synthetic tools such as automatic synthesisers are on the market. Furthermore, they can be amplified by PCR or cloning. On the other hand, oligosaccharides are tedious to synthesise and cannot be amplified. They are of synthetic or natural origin. Consequently, they are often available in limited quantities and expensive in the market.
Also, as mentioned in the introduction, since they have a very high structural diversity because of the fact that they can be linear or branched and the anomeric carbon can be α or β, they can be in the chair or boat conformation and the carbon involved in the glycoside linkage can vary.
Furthermore, all the functional groups are hydroxyls with special reactivity for the one carried by the anomeric carbon atom and for the primary alcohol. This means that the synthesis of oligosaccharides requires selective protections and deprotections of the desired hydroxyl function for coupling of monosaccharides through a glycosyl bond. Some authors have developed automated solid-phase synthesis, while some others have used a “one-pot” approach (Feizi et al. 2003; Werz and Seeberger 2005a; Seeberger 2008; Plante et al. 2001). Still others take advantage of enzymes (glycosidases or glycosyltranferases) to perform the synthesis of oligosaccharides. Glycosidases are enzymes that under usual conditions hydrolyse the glycosidic bond. However, as this reaction is reversible, under defined conditions, the enzyme can catalyse the formation of the linkage. Glycosyltransferases are enzymes that catalyse the glycosidic linkage formation between two saccharides. They are more efficient than glycosidases, giving better yields. However, the number of different enzymes is limited and expensive. The natural substrate of the enzyme is usually expensive as well. These reasons have motivated some authors to engineer whole bacteria with genes coding for the different enzymes required for oligosaccharides synthesis (Fort et al. 2005).
Nevertheless, synthesis of a considerable number of complex carbohydrates has been achieved but often in limited amounts and at high cost.
In the following section, the different methods employed for surface immobilisation of carbohydrates are described, followed by a paragraph on signal transduction and finally some applications.
4.2 Surface Physicochemistry: Non-specific Adsorption and Immobilisation
One can picture the saccharide–protein interaction as a key–lock interaction. In consequence, like protein arrays (Gordus and Mac Beath 2006), carbohydrate arrays require the preservation of the 3-D conformations of the saccharide ligand in order to perform specific biomolecular recognition with their receptor. In addition, their accessibility and orientation are major issues especially in the case of immobilised probes where the degrees of freedom are fewer than in solution. Therefore, the surface chemistry employed for immobilisation (the chemical reaction for immobilisation, but also the structure of the spacer arm) of the saccharide and the physico-chemical properties of the surface (and ligand) are major issues in maintaining the biological activity, as they will play key roles not only in the preservation of the 3-D conformation but also in the probe availability. For example, weak interactions between the surface and the probe can influence the orientation of the probe. Surface organisation of the probe (dense packing which depends among other things on the spacer arm structure) can also influence the probe availability. Organisation of the water molecules at the surface of the material may also play a role in the thermodynamics of biological recognition at the surface of the material.
The immobilisation should
-
proceed with good and reproducible yield for a given probe;
-
proceed with the same yield independently of the probe (especially in the case of glycoarray);
-
be oriented in order to preserve the “key–lock” interaction between the probe and the target and to obtain a reproducible device;
-
allow the accessibility of the probe by the target although the degree of freedom is reduced; and
-
allow quality control of immobilised probes.
The performances (lower detection limit, reliability, robustness, etc.) of the microdevices will greatly depend upon this step, as the target capture by the probe will depend on the probe availability (not taking into account diffusion and microfluidic-related phenomena (Raynal et al. 2004)).
The detection technique will determine the choice of the substrate to be used and, therefore, the subsequent coupling reactions. For example, gold is the substrate of choice for SPR detection, whereas glass is often preferred with fluorescent detection. In one case, mercapto-based coupling agents have been used (Schreiber 2000), whereas silane-based coupling agents are preferred with glass.
Furthermore, the background signal depends on non-specific adsorption of the target. It should be reduced to avoid high background.
In the following, we will discuss the various strategies employed for reducing non-specific adsorption, as well as the various strategies for immobilising carbohydrates, on surfaces of materials, more specifically mono and oligosaccharides.
Finally, the collective behaviour of the molecules such as surface organisation of probe molecules can influence the affinity of a target for a probe as discussed in section “Surface Cluster Effect or Not.”
4.2.1 Non-specific Adsorption
A protein in solution adsorbs on a surface if the total Gibbs free energy is negative. In a simple medium where only one protein is present, the entropic factor (Norde 1985; Norde et al. 1986) seems to be the driving force for protein adsorption. Entropy is affected by the dehydration of both the surface and the protein, rearrangement of the protein structure and rearrangement of charged groups. The dehydration effect on hydrophobic surfaces can be viewed as the disruption of an ordered ice-like layer of water at the surface of the materials by the incoming protein. This disruption is due to surface–protein hydrophobic interactions that are stronger than the surface–water and protein–water interactions. This leads to an increase in entropy. On hydrophilic surfaces, the driving force is the change in protein structure (Norde et al. 1986).
In case of complex media, such as biological fluids, where different proteins coexist, the adsorption phenomenon can be regarded as a competitive adsorption (Vroman effect). The composition of the adsorbed layer may be very different from that of the bulk solution (Vroman 1962; Bamford et al., 1992).
From the above considerations, the following conclusions can be made:
-
Protein adsorption on a surface depends on a combination of factors such as the solution physico-chemical characteristics (pH, ionic strength, temperature, composition, etc.), on the physico-chemical properties of the protein(s) and on the physico-chemical properties of the material surface (surface energy, roughness, surface chemistry, etc.).
-
Protein adsorption can be reduced by two major means:
-
By pre-adding a protein that will readily adsorb on the surface and displace the protein that one wants to analyse: BSA (bovine serum albumin), HSA (human serum albumin), casein, etc. The choice of one blocking protein over another will depend on the protein to be analysed.
-
By developing a surface on which protein adsorption is thermodynamically not favoured. Neutral hydrophilic polymers such as polyethylene glycol (PEG) (Desai and Hubbell 1991; Jeon et al. 1991; Lee et al. 1997), polyhydroxyethyl methacrylate, polyvinyl alcohol, poly-N-vinyl 2-pyrrolidone, etc. seem to be ideal in this case. Some authors have described the use of polymers mimicking the lipid double layer (Ishihara et al. 1998; Ruiz et al. 1999).
In the case of carbohydrate-based biosensors, Table 7.3 summarises the blocking strategies commonly described in the literature. It has to be ensured that the blocking protein does not have glycosylation motifs that can compete with the saccharide ligands and may interfere with the target molecule. Some authors have demonstrated that BSA can hide the probe immobilised at the surface from the target (Lesaicherre et al. 2002; Mac Beath and Schreiber, 2000).
4.2.2 Immobilisation of Saccharide Ligands
The immobilisation of the saccharides at the surfaces of the biosensor has been achieved through three main strategies:
-
Physisorption
-
Covalent immobilisation
-
Biochemical-based interaction
4.2.2.1 Physisorption
Physisorption (Fukui et al. 2002; Wang et al. 2002; Satoh et al. 1999; Stoll et al. 2000; Palma et al. 2006) of carbohydrate ligands relies on the weak interactions between the molecules to be immobilised and the surface. This is a convenient technique, as it does not require surface functionalisation. Nitrocellulose or polystyrene are mainly used as the substrates.
In the case of low molecular weight species such as oligosaccharides, their interactions with the surface are too weak and require the modification of the saccharide with an anchoring tail for increasing the interaction, e.g. a lipid, a fluorescent tag (Jaipuri et al. 2008), a protein or a polymer such as polyacrylamide. Such a tail can be introduced by the condensation of the azido functional group with sp2- and sp3-bearing molecules (Fazio et al. 2002), by aminoreduction (Fukui et al. 2002) or by the condensation of amine with isocyanate (Fazio et al. 2004). The group of Wang at Stanford University and the group of Feizi at Imperial College, London, have developed neoglycoconjugates bearing such tails for their immobilisation on nitrocellulose membranes or nitrocellulose-coated glass slides. Oligosaccharides (2–20-mers) are linked to amino phospholipid 1,2-dihexadecyl-sn-glycero-3-phosphoethanolamine or its anthracene derivative by reductive amination. The anthracene derivative allows quality control of the relative surface density of the probes (Palma et al. 2006). The group of Wong has derivatised oligosaccharides with a C14 alkyl chain through Cu(I)-catalysed Huisgen 1,3-dipolar cycloaddition. A similar strategy was used by Huang et al. (2005). These two groups have demonstrated in various publications that this strategy is very efficient, allowing the immobilisation of femtomoles of compounds and with a very good signal-to-noise ratio.
4.2.2.2 Covalent Immobilisation
The covalent immobilisation of the saccharide has been achieved by reacting with functionalised surfaces with unmodified saccharides or functionalised glycosides (saccharide bearing a functionalised aglycon).
4.2.2.2.1 a Immobilisation of Underivatised Saccharides (Fig. 7.23)
Activation of the hydroxyl functions can be achieved with a compound leading to good leaving groups under nucleophilic attack. Activating agents (CNBr, CNCl, divinylsulphone, isocyanate, etc.) have been reported for immobilisation of oligo- and polysaccharides. However, divinylsulphone may lead to intra-reactions between two adjacent hydroxyl groups.
Immobilisation of carbohydrate through their reductive end: a base Schiff or reductive amination, b oxime, c glycosyl amide bound formation and d thiazolidine. Immobilisation of underivatised saccharides usually take advantage of the reducing end of the saccharides as anchoring point, though immobilisation by the O-3, O-6 and N-2 have been described (Larsen et al. 2006; Seo et al. 2007)
Jouan et al. (1996) have described the immobilisation of saccharides by divinylsulfone cross-linking of amino groups and oligosaccharide hydroxyl groups. The main drawback is that the hydroxyl groups react randomly with the amino groups.
Reductive amination takes advantage of the reaction of the reducing end of the carbohydrate (or aldehydes obtained after oxidation under mild conditions agent such as nitrous acid) with an amine or an hydrazine followed by the reduction of the Schiff base by hydrides. Many examples have been reported. However, it requires long reaction times and the yields are low. To circumvent these drawbacks, other authors have immobilised saccharides through hydrazide (Lee and Shin 2005; Satoh and Matsumoto 1999) or oxime (Zhou and Zhou 2006) linkages. Thiazolidine and glycosyl amide bonds have also been described (Larsen et al. 2006). The hydrazide linkage is poorly stable at low pH, while oximes are stable over a wider range of pH (Larsen et al. 2006). Nevertheless, the immobilisation of unmodified saccharides is advantageous with complex carbohydrates from natural sources. However, the main drawbacks are that the immobilisation reactions are limited to reactions involving the reducing end of the saccharides (and consequently cannot be used with non-reducing sugars) and they often lead to the opening of the reducing end ring while it may be crucial to maintain the structure of the molecule (Larsen et al. 2006).
4.2.2.2.2 b Immobilisation of Saccharide Derivatised with a Functional Aglycon
It can be advantageous to derivatise the saccharide with a functionalised aglycon allowing for the subsequent immobilisation. Two main strategies can be envisioned. One is the use of an aglycon bearing a function that can react directly with the surface, such as thiolated or disulfide aglycons, which permits the direct immobilisation of the glycoside onto the metal surfaces (gold in particular, Fig. 7.24). The second is chemically modifying the surface with a hetero cross-linker bearing a functional group for reaction with the surface and a second functional group for reaction with the aglycon (Fig. 7.24).
Immobilisation reactions of glycosides: (1) reaction of thiol with gold, (2) reaction of disulfide with gold, (3) photochemistry using aryl-diazirine, (4) and (5) addition of thiol to maleimide group, (6) disulfide bond formation by oxidation of thiol functions, (7) amide formation by reaction of activated carboxylic group with amine, (8) amide/epoxy substitution, (9) Diels–Alder cycloaddition, (10) copper I-accelerated regiospecific 1, 3-dipolar cycloaddition, (11) Staudinger ligation and (12) reaction between a cyanuric acid chloride and an aminophenyl group
Direct reaction. Thiol functions have strong affinity for noble metals such as gold, silver, indium, etc. (see the corresponding chapter 2). Voltametric studies (Wink et al. 1997) have shown that upon adsorption thiols are deprotonated, leading to a covalent bond between the thiolate and the gold metal. Disulfide can also strongly bind to gold; however, the resulting films are less compact and less ordered. Many authors have taken advantage of these interactions for the immobilisation of thiol- (or disulfide-) containing biomolecules. In the case of carbohydrates, a mercapto aglycon should be introduced into the molecule.
Miura et al. (2002) have reported the immobilisation of Gb3 mimics (a saccharide ligand of the shigatoxins 1 and 2) bearing a disulfide aglycon on a gold substrate. The surface density of the immobilised Gb3 mimics was evaluated with quartz micro-balance measurements. The area per molecules was estimated to be 54 Å2, leading to an estimated surface density of approximately 2 × 1014 molecules per cm2. Ratner et al. have studied the affinity of the protein cyanovirin-N with mannosyl oligosaccharides. Immobilisation on gold substrates for SPR-binding affinity measurements was achieved with thiol derivatives of mannosyl oligosaccharides (Ratner et al. 2004).
Gold nanoparticles are often functionalised with saccharide ligands by mean of thiol reaction with gold (Chen et al. 2005). Robinson et al. (2005) have reported the synthesis of N-acetyl glucosamine encapsulated quantum dots (QDs). CdSe/ZnS core shell QDs were synthesized and subsequently encapsulated with pyridine. They were then allowed to react with disulfide-functionalised GlcNAc in the presence of NaBH4, leading to GlcNAc-modified QDs. They have demonstrated by NMR an IR spectroscopy the reaction between the sulphur atom and the QD and the presence of the GlcNAc.
The synthesis of mannose-containing CdSe/ZnS QD has also been reported using mannose-containing phosphine oxide (Larsen et al. 2006). CdS QD modified with maltose or Lewis X was achieved using thiol or disulfide glycosides in the presence of sodium sulfide and cadmium nitrate (Larsen et al. 2006).
Other authors (Chevolot et al. 2001; Chevolot et al. 1999) have derivatised mono- and di-saccharides with photoactivatable aryl-diazirine. Immobilisation was performed by irradiation at 350 nm, leading to a reactive carbene, which reacted with the substrate. The advantage of this chemistry is the versatility of the reaction with respect to the substrate and the possibility of using photolithography for obtaining specific patterns of carbohydrate domains on the surface. In such a way, the immobilisation of galactose was performed on silicon, silicon nitride, diamond and polystyrene. Time-of-flight secondary ion mass spectroscopy (ToF-SIMS) analysis (Leonard et al. 1998; Léonard et al. 1998; Léonard et al. 2001) demonstrated that the hydroxyl groups present on diamond surfaces were involved in the immobilisation reaction. The resulting surface density was estimated to be in the range of 1012–1013 molecules per cm2. Square features of 25 µm were obtained using photolithography.
Surface immobilisation of saccharides can be achieved by the surface copolymerisation of saccharide-containing monomers with monomers (without saccharides). Dubois et al. (2005) have developed an electrochemical approach based on a polypyrrole-coated electrode displaying pendant carbohydrates.
Reaction with functionalised surfaces. Several covalent immobilisations of oligosaccharides through reaction between an aglycon and a functionalised surface are described in the literature. The introduction of the aglycon can be performed by various means (refer to the corresponding paragraph on glycoside formation 7.2.3).
Surface functionalisation with the desired function such as carboxylic acid, amine, thiol and maleimide is achieved with a hetero-bifunctional cross-linker or coupling reagent. These molecules possess two functions: one that reacts with the surface and the other with the function of the aglycon. If necessary, the latter function can be protected during the reaction with the substrate. The coupling reaction may require the activation of one function, such as NHS activation of a carboxylic function, for reaction with an amine (see the corresponding chapter 2).
The usual yields are between a few percent and 10–20%, leading to a surface density between 1012 and 1013 molecules per cm2. Therefore, high biomolecule concentrations are used for the immobilisation (mM range). To increase the yield, the saccharide solution is allowed to dry. This can lead to spot non-homogeneity, such as donut-like spots. This can be circumvented by adding moistening agents and surfactants (Dugas et al. 2005) and/or with good control of the evaporating conditions (relative humidity, temperature).
After performing the coupling reaction, the unreacted surface function should be disabled to perform further reaction. This is done by reacting the surface with a molecule bearing the same function as the aglycon but at a higher concentration. Usually this molecule is commercially available and cheap. This operation is sometime called “capping” of the surface. For example, NHS-activated surfaces after reaction with an amine-bearing carbohydrate are allowed to react with ethanolamine to deactivate the unreacted NHS esters (Fig. 7.25).
Immobilisation of carbohydrate have been achieved by taking advantage of
-
thiol reactivity towards double bonds such as maleimido groups (Park et al. 2004; Brun et al. 2006; Ratner et al. 2004; Houseman et al. 2003) or towards thiol groups under oxidative condition, leading to the formation of a disulphide bond (Shin 2007);
-
amine reactivity (or hydrazine) towards carboxylic (Blixt et al. 2004) (Consortium for functional glycomic (CFG)), towards aldehydes (Biskup et al. 2005) or towards epoxy groups (Lee and Shin 2005);
-
cycloaddition reaction such as Diels–Alder (Houseman and Mrksich 2002) by copper I-accelerated regiospecific 1,3-dipolar cycloaddition (Huang et al. 2006a; Bryan et al. 2004; Seibel et al. 2006);
-
Staudinger ligation (Kohn et al. 2003);
-
the reaction between a cyanuric acid chloride and an aminophenyl group(Schwarz et al. 2003); or
-
functional macromolecules such as glycoproteins, neoglycoproteins or polysaccharides.
Biskup et al. (2005) have reported the synthesis of amino, aldehyde and carboxylic acid glycoside for their immobilisation on amino- or formyl-functionalised glass slides.
A tremendous effort from the CFG led to a library of 200 (now 400) amino glycosides for subsequent immobilisation on NHS ester-activated glass slides. These slides have been used for a variety of applications such as antibody to viral protein affinity profiling.
The group of Seeberger in Zurich has reported the immobilisation of amine-derivatised heparin oligosaccharides onto NHS ester surfaces (de Paz et al. 2006), thiol oligomannosyl onto maleimide-functionalised surface (Brun et al. 2006) or aminoglycosides with disuccinimydyl carbonate or disuccinimidyl tetrapolyethyleneglycol cross-linker (Disney and Seeberger 2004a).
For 1,3-cycloaddition as well as Staudinger ligation, one takes advantage of azido-functionalised carbohydrates. In the latter case, Schwartz et al. have generated amine-functionalised surfaces using fourth-generation PMAM, a fourth-generation dendrimer, for increasing the number of reaction sites (amines). The surfaces were further treated to form the phosphane group, allowing subsequent Staudinger ligation (Kohn et al. 2003).
Macromolecules have been used as spacers for the immobilisation of carbohydrates taking advantage of functional groups such as amines, carboxylic acids, thiols or diazirines.
For example, BSA glycoconjugates and glycoproteins were immobilised on epoxy-derivatised glass slides (Manimala et al. 2006; Ratner et al. 2004; Adams et al. 2003). The saccharides were covalently link to BSA by reaction of thiol-modified carbohydrates with maleimide-derivatised BSA (Adams et al. 2003; Ratner et al. 2004) or by reaction of NHS ester-modified carbohydrate with BSA (Manimala et al. 2006; Manimala et al. 2005). The neoglycoprotein was then immobilised on NHS ester-activated surfaces (Ratner et al. 2004; Adams et al. 2003) or epoxy-derivatised glass slides (Manimala et al. 2005). Seeberger’s group has used these strategies for immobilising carbohydrates on plane substrates as well as on microspheres (Adams et al. 2003).
Angeloni et al. (2005) described the immobilisation of oligosaccharides after surface functionalisation with Optodex. The latter is a dextran molecule bearing aryl-diazirine, allowing surface immobilisation by converting the diazirine into a reactive carbene under light activation.
As one can see, many different reactions have been employed for achieving the immobilisation of saccharides. However, it is difficult to draw a general conclusion, as the surface densities of the probes are rarely given and the washing protocols are often not the same. Dugas et al. (2004) have demonstrated that the final surface density of aminolinker-derivatised oligonucleotides immobilised on the NHS-activated ester surface is greatly dependent on the washing step. A quick rinsing with water led to 1014 molecules per cm2, whereas rinsing with hot water led to 1013 molecules per cm2. A further rinsing with SDS led to 3 × 1011 molecules per cm2. This means that a significant amount of the probes can be adsorbed rather than covalently linked depending on the washing steps. In consequence, surface-oriented immobilisation can also be effected.
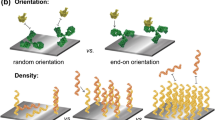
Linker effect. Affinities of targets for surface-immobilised probes depend on the saccharide structures and also on the aglycon structure. Miura et al. (2002) have demonstrated that shigatoxins 1 and 2 affinities for immobilised Gb3 mimics (a saccharide ligand of the shigatoxins 1 and 2) were affected by the aglycon alkyl chain length. Gb3 mimics were derivatised with a disulfide bearing 2 or 10 carbon alkyl chains as a spacer. The resulting molecules were self-assembled onto gold surfaces and the affinity of Shigatoxins 1 and 2 were studied. They found that Shigatoxin 1 binds to 2-carbon-derivatised Gb3 five times more strongly than 10-carbon-derivatised Gb3, while Shigatoxin 2 binds more strongly to 10-carbon-derivatised Gb3. Some authors (Sato et al. 1998) have demonstrated that the availability of the carbohydrate probe can be spoiled by steric hindrance due to compact packing. It may that the observed difference is related to the surface organisation of the probe. Indeed, it was demonstrated that the C10 long spacer forms compact layers compared to the C3 long spacer (Grubor et al. 2004).
Huang et al. (2006a) compared the affinity of antibodies for Globo H analogues. Globo H is a glycosphingolipid which is highly expressed on a wide range of tumoral cell lines. They synthesised four analogues of increasing complexity of the saccharide moiety of Globo H with two different aglycons bearing either an amine function or an azido function. The amino derivatives were immobilised on NHS ester-functionalised surfaces, while the azido derivatives were immobilised on alkyne-bearing surfaces through 1,3 dipolar cycloaddition. They found that the affinity of the mouse antibodies (MBr1 or VK-9) for the derivatives linked through an amide function was higher than those linked through a 1,2,3-triazol ring. They interpreted the observation as a difference of solubility between the two types of derivatives, a difference in the coupling yield or the better suitability of the shorter linker for binding.
The overall affinity of the target for the immobilised probe is a complex process, which should be viewed as the combination of the contribution of probe structure, probe surface density, probe availability (which is related to the probe orientation and to the physico-chemical properties of the spacer), the surface physico-chemical properties of the substrate and the target physico-chemical properties (Yeung and Leckband 1997).
4.2.2.3 Biochemical Interaction Based Immobilisation
Bochner et al. (2005) reported the use of streptavidin–biotin interaction for the immobilisation of sialic acids. One hundred and eighty biotinylated glycosides were immobilised on streptavidin-coated polystyrene titer plates and on streptavidin-coated gold surfaces for SPR measurements (CFG). Linhardt’s group immobilised biotinylated heparin for studying the affinity and the kinetic of factor P/heparin interaction (Muñoz et al. 2005). Heparin was conjugated with biotin by the reaction of sulfo-NHS-LC-biotin with the free amino groups of unsubstituted glucosamine residues in heparin.
Chevolot et al. reported the use of DNA hybridisation for surface immobilisation of glycomimetics (Bouillon et al. 2006; Chevolot et al. 2007). Glycomimetics were synthesised as follows: azido glycosides were attached to pendant propargyl residues on a phosphorylated scaffold by means of solid-supported 1,3-dipolar cycloaddition. The architecture (distance between two saccharides residues, hydrophilic–lipophilic balance) was designed by adjusting the linker between two pendant propargyl residues. Each glycomimetic was tagged with a DNA sequence bearing a cyanine 3 (Cy3) dye. The resulting molecules were immobilised on a DNA array (Dugas et al. 2004) bearing complementary sequence by means of DNA/DNA hybridisation. The fluorescent dye borne by the oligonucleotide permits a quality control of the immobilised molecules. Hybridisation was performed with a 1 µM solution of Cy3 –DNA glycomimetic, leading to a probe surface density of 1–4 ×1010 molecules per cm2 as estimated by Dugas et al. with radiolabelled oligonucleotides immobilised under the same conditions. The resulting analytical device had a lower detection limit in the nanomolar range (albeit the use of a 1 µM solution of glycomimetic), which is similar to what is described in the literature where typical immobilisation solution concentrations are in the millimolar range.
In the field of protein array, Niemeyer et al. (Wacker et al. 2004) have used a similar strategy called DNA directed immobilisation (DDI). They also found that immobilisation by means of hybridisation give lower detection limit of the device, which is improved, as opposed to direct covalent immobilisation. They hypothesised that the improvement was due to the rigid structure of the DNA duplex and to the reversibility of the hybridisation allowing the denser packing of the probes.
Another advantage of the DDI strategy is that biological recognition between the glycomimetics and the lectin can be first performed in solution before immobilisation of the complex on the solid support by hybridisation, circumventing the limitations due to the surface proximity.
4.2.2.4 Surface Cluster Effect or Not?
Individual protein–carbohydrate interactions have binding constants in the millimolar to low micromolar range, with apparent low specificity. However, under multivalent interactions in a Velcro-type mode, these interactions can be enhanced. This is the so-called cluster effect as defined by Lee in 1994(Lee and Lee, 1994, 1995): “binding affinity enhancement exhibited by a multivalent carbohydrate ligand over and beyond that expected from the concentration increase resulting from its multivalency.”
Two different mechanisms have been proposed to explain the cluster effect (Pohl and Kiessling 1999):
-
Due to multivalency, the local concentration of the ligand is high at the receptor-binding site allowing an increase of 5- to 10-fold in binding affinities.
-
Due to intra molecular binding, which is the chelate effect, a multivalent ligand binds with greater affinity for a multivalent receptor than the monovalent ligand, similar to the mechanism described in inorganic chemistry. It leads to an exponential increase of the affinity.
Several reviews have been published on this topic (Lundquist and Toone 2002; Mammen et al. 1998; Turnbull and Stoddart 2002).
In the case of biosensors, packing of carbohydrate at the surface of a material should permit taking advantage of the cluster as opposed to solution assays. For example, in the case of neoglycolipid-based glycol array (Jelinek and Kolusheva 2004; Stoll et al. 2000), lipid clustering and surface oligosaccharide organisation allowed higher affinity of proteins for the saccharides. On the contrary, Sato et al. observed a decreased WGA affinity by the high probe surface. They studied the affinity of WGA (Wheat Germ Agglutinin) for different glycolipids and found that the maximum affinity of WGA for GM 3 and GM4 was for a monolayer containing 20% GM (Sato et al. 1998). Similar results were found by Ebara (Ebara and Okahata 1994) with concanavalin A and glycolipids. This phenomenon was attributed to steric hindrance due to the compact packing of the carbohydrate heads in gangliosides monolayers (not mixed) limiting the access to WGA.
These apparent contradictory results suggest that the surface-based cluster effect is dependent on surface density of the probes and their surface organisation. This latter parameter is dependent on interactions between the probes through van der Waals interactions, polar, ionic forces and hydrogen bonds. Usually, when immobilising carbohydrates via a functional aglycon with a functionalised surface, the surface densities of the probes are below that of a densely packed monolayer. On the other hand, direct immobilisation of glycosides leads to denser layers.
4.3 Transduction
The specific biological interaction between the probe and the target can be studied by optical, gravimetric or electrochemical detection and more recently by mass spectroscopy (MS) or by the use of nanoparticles-based assays.
The detection of biomolecular interactions may require the pre-labelling of the target prior to detection or may be label free and exploit the change of physico-chemical properties induced by the biological recognition.
In the first case, strategy consists in labelling the molecule to be detected (directly or via sandwiches) or grafting different markers on probes (Cy3) and targets (Cy5) for discriminating the specific molecular interaction in a better way and requires appropriated techniques for detecting the signal associated with the label. The markers can be fluorescent, radioactive or electroactive species. Detection by fluorescence is a semi-quantitative and sensitive technique with high spatial resolution but the quenching and blenching phenomena must be monitored. Nowadays, read-out and analyses are made with standard commercial fluorescence scanners. Detection using radioactive labels such as 125I, 32P or 33P is a more quantitative method but the weak spatial resolution and both the inherent safety and waste disposal problems have directed studies towards alternative solutions. Spe-cificity of the recognition signal must be ensured by careful washing protocols to remove non-specifically labelled probes adsorbed out of the surface without breaking the specific binding. A drawback is that the detection is performed a posteriori, out of the liquid environment and in dry conditions, which hides the analysis of the kinetics of the interactions. Advantage of these methods is that they are particularly well adapted for the parallel and massive detection analysis of numerous spots, as for instance with the DNA biochips performed by Affymetrix containing more 400,000 spots per cm2.
In the second case, the detection strategy is based on the measure of the change of properties such as temperature, mass, electrical, optical and chemical (pH) parameter. The necessary development of new and reliable tools designed for detecting low parameter change has considerably increased on the two last decades. The release of commercial systems such as microcalorimeter, quartz crystal microbalances (QCM), field effect transistors (FETs), surface plasmon resonance (SPR) and, more recently, platforms based on piezoelectric acoustic sensors (principally bulk acoustic wave (BAW)) and thickness shear mode (TSM) sensors are leading a large number of studies determining binding specificities, affinities, kinetics and conformational changes associated with a molecular recognition event. The investigations concerned a large variety of interactions involving small molecular weight ligands, carbohydrates, proteins, nucleic acids, viruses, bacteria, cells as well as lipidic and polymeric interfaces. Beyond the direct and in situ interaction detection, the main advantage of this strategy is its ability to obtain kinetics informations on molecular interactions.
4.3.1 Optical Detection
4.3.1.1 Fluorescence
Fluorescence is one of the most commonly used detection techniques in the field of microarrays. It relies on fluorescent dyes (see Table 7.4). These molecules are capable, under illumination with a monochromatic light corresponding to their absorption maximum, of reaching an excited state. They return to the ground state by emitting a photon. The energy of the outcoming photon is less than that of the absorbed photon due to loss of energy by a non-radiative process. The difference between the maxima (in wavelength) in the excitation spectrum and the emission spectrum is called the Stokes shift. The ratio between absorbed photon and emitted photon is the fluorescence quantum yield. Table 7.4 gives some of the commonly used fluorescent dyes. Fluorescein and related molecules (Alexa, Oregon green, rhodamine, etc.) are four-ring structures with conjugated double bounds. FITC (fluorescein isothiocyanate) is affordable but has several drawbacks (Schena 2003). It is sensitive to self-quenching and photobleaching, and the fluorescent signal is influenced by pH (Schena 2003). The parent molecules of FITC (Alexa, Texas red, Oregon green, etc.) have additional polar, aliphatic and/or hetero cyclic groups that influence their excitation and emission spectra. These molecules are in general photostable. Furthermore, Alexa dyes are insensitive to pH change over a broad range, have high fluorescence quantum yield and a long half-life of the excited state (Schena 2003).
A second family of fluorescent dyes is of the indocarbocyanine series. The most used ones are Cy3 and Cy5. The cyanine dyes are bright, are stable to photobleaching, and are commercially available with chemical function allowing their conjugation with biomolecules. Their fluorescent signal is affected by ozone exposure as low as 5–10 ppb for Cy5 and above 100 ppb for Cy3 (Fare et al. 2003).
A third family of organic dyes used in microarrays applications is the BODIPY (boron-dipyrromethene) series. Next to these organic dyes, QDs are emerging as an alternative. Huang et al. (2005) have compared the lower detection limits for a FITC-labelled lectin plant and a QD-labelled lectin plant (Con A) interacting with immobilised D-glucopyrannoside. They found that the QD-label-based method was at least a 100 times more sensitive than the FITC approach in detecting ConA interaction.
A biosensing experiment relies on (a) the labelling of the target or (b) the detection of the target through a second interaction using a sandwich technique with antibodies, for example (Fig. 7.26).
Principle of fluorescent detection: (a) When target is previously labelled, then the recognition interaction is directly detected. (b) Sandwich technique consists in a first recognition step between probe and unlabelled target following by a secondary recognition step between the formed complex and a labelled interacting protein
Dyes have been modified for allowing their direct conjugation with molecules bearing amine, thiol, etc. In a fluorescent experiment, after incubation of the device with the protein of interest, the substrate is washed and dried and the remaining fluorescent signal is detected with a microarray scanner.
One of the main advantages of fluorescence is that the technique is very sensitive, allowing the detection of plant lectin with concentration as low as a few tens of picomolars (Manimala et al. 2006). However, generally no real-time experiment can be performed. Furthermore, the washing step is compulsory and critical in terms of background levels. It is tricky to find an optimum between removing non-specifically adsorbed protein and maintaining a protein that interacts weakly with the carbohydrates.
Instruments on the market (Zeptosens (http://www.zeptosens.com/en/; Disney and Seeberger 2004b)) take advantage of evanescent field for fluorescent dye excitation. By this means, real-time experiments can be performed without washing the surface. Indeed, evanescent field decreases exponentially with the distance to the surface. Consequently, only the fluorescent dyes next to the surface are excited.
Fluorescent-labelled plant lectins have been widely used as model protein for the validation of carbohydrate microarrays. The best lower detection limits, depending on the considered model, are in the nanomolar range.
Stevens et al. (2006) have used a double sandwich for probing the interaction of the influenza virus hemagglutinins of different sub-types for more than 200 saccharides displayed on a microarray. Due to the low affinity of the hemagglutinin–carbohydrate interaction, they developed an approach based on two antibodies. The hemagglutinins (HA) bear a His6 tag. First, hemagglutinins are allowed to interact with the microarray. Next, the slides are incubated with a labelled anti-His6 mouse antibody followed by the incubation with a second labelled antibody directed against mouse antibody. With this approach, the signal is amplified, allowing the probing of weak interactions.
Usually, fluorescence give semi-quantitative results. The group of Shin (Shin 2007) has developed a procedure for quantitative analysis by determining the IC50 based on inhibition experiments (Fig. 7.27). In brief, the microarray was incubated with increasing amounts of a soluble inhibitor. The concentration that produces 50% of inhibition is called the IC50.
An original glycoarray was developed using internally encoded microspheres (Adams et al. 2003). Each population of microspheres has a unique carbohydrate molecule covalently attached and a unique spectral signature due to an internal fluorescent dye. The microspheres are randomly spread over an imaging optical fibre. Due to the spectral signature, each microsphere is identified at the surface of the fibre with regard to the immobilised carbohydrate and position. Interactions with labelled Concanavalin A, a plant lectin, and Cyanovirin A, an HIV-inactivating protein issued from a cyanobacterium, are performed and analysed by fluorescent imaging.
4.3.1.2 Surface Plasmon Resonance and Surface Plasmon Resonance imaging
Surface plasmons are surface electromagnetic waves propagating at the metal/dielectric interface. Surface plasmons can be excited by an incident polarised light especially by means of an evanescent field. The total internal reflectance of the incident light will be affected as a function of the incident or reflection angle leading to a sharp minimum.
The position of this minimum is dependent on the refractive index and thickness of the surface layer. Change in the surface refractive index on the surface of the metal will thus be probed by either measuring the change in total internal reflectance or by the change in the minimum position.
Two configurations can be used: namely, the Otto set-up and the Kretschmann set-up. The Kretschmann set-up is the most widely used. In this set-up, a thin layer of gold, typically 50 nm, is evaporated on a glass substrate (with an intermediate adhesive layer such as chromium). An incident polarised light travels across the glass, and an evanescent field penetrates through the metal film and excites the surface plasmons of the outer side of the metal film.
Its use for biosensing began in the late 1980s (Lundquist and Toone 2002) and was further implemented in the early 2000s with the first commercially available surface plasmon resonance imaging (SPRi) apparatus.
SPR for detecting carbohydrate-based interactions has been reported with antibodies (Zou et al. 1999; Young et al. 1999), membrane proteins, viruses proteins (Ratner and Seeberger 2007), chemokine (de Paz et al. 2007), fibroblast growth factor (de Paz et al. 2007), plant lectin (Smith et al. 2003; Satoh and Matsumoto 1999), hSiglec7 (Karamanska et al. 2008) and enzyme (Park and Shin 2007).
SPR offers many advantages: firstly, it is a label-free detection technique; secondly, it provides rates (K on and K off) allowing the determination of affinity constants; and thirdly, it is a real-time measurement. The main drawback relates to its lower detection limit, which is not as good as that of fluorescence. Also, the on and off rates seem to be affected by the flow vector (mass transport) and by the orientation of the molecules at the surface of the sensor (Lundquist and Toone 2002). Recent developments have focused on coupling SPR plates with matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (Lundquist and Toone 2002).
4.3.1.3 Dual Polarization Interferometry
Yates (Yates et al. 2006) has pointed out another optical technique based on dual polarization interferometry (DPI), which measures real-time changes in the mass and the geometry of molecules bound to a surface. DPI measures in phase both TE (transverse electric) and TM (transverse magnetic) polarizations of light. Using Maxwell’s equations, one unique value for thickness and for refractive index of the layer of molecules is true for both phases of light. DPI gives absolute measurements and allows full characterisation in terms of mass, thickness and refractive index of the layer to be formed. It allows measurements in real time of probe coverage and probe orientation and then to follow in real time the biological interaction. The measurement by DPI immobilization of streptavidin on a biotinylated surface followed by the capture of the reducing end biotinylated heparin oligosaccharides is given as an example.
4.3.2 Gravimetric Detection: Quartz Crystal Microbalance
Among the analytical tools available to investigate the biological interactions, QCM holds a special place because this direct measurement does not require probe-labelling and allows monitoring the binding affinity. In their recent review, Cooper and Singleton (2007) register a total of 1,404 publications referencing QCM in the Web of Sciences database, of which 569 specifically involved molecular recognition studies in the period 2001–2005.
In 1959, QCM was developed by G. Sauerbrey (Sauerbrey 1959) for correlating changes in the oscillation frequency of a piezoelectric crystal with the mass deposited on it. He simultaneously developed a method for measuring the characteristic frequency (resonant frequency) and its changes by using the crystal as the frequency-determining component of an oscillator circuit. His method continues to be used as the primary tool in QCM experiments for the conversion of frequency to mass and is valid in nearly all applications under vacuum or gas.
The Sauerbrey’s equation is given below:
where f 0 is resonant frequency (Hz), Δf is the frequency change (Hz), Δm is the mass change (g), A is the piezoelectrically active crystal area (m2), ρ q is the density of quartz (ρ q = 2.648 g/cm3), ρ q the shear modulus of quartz for AT-cut crystal (μ q = 2.947 × 1011 g/cm s2) and ν q is the transverse wave velocity in quartz (3,340 m/s).
QCM measures the mass per unit area by measuring the change in frequency of a quartz crystal resonator. The resonance is disturbed by the addition or removal of a small mass on the surface of the acoustic resonator. When a thin film is attached to the sensor crystal, the frequency decreases. If the film is thin and rigid, the decrease in frequency is proportional to the mass of the film. In this way, the QCM operates as a very sensitive balance. The mass of the adhering layer is calculated by using the Sauerbrey’s relation. For instance, with a crystal of resonant frequency 6 MHz, and considering an active crystal surface of 0.2 cm2 and a frequency resolution of 1 Hz, we can detect a change of 2.46 ng. For a long time, the main application of QCM consisted in monitoring thin-film deposition in vacuum or in gas phase (King, 1964). When QCM was used in liquid environments (Bruckenstein and Shay 1985), the number of applications increased dramatically, in particular in the biology field. For instance, an adsorbed protein monolayer on a surface electrode of 1 cm2 adds a mass of a few hundred nanograms (Muratsugu et al. 1993). But, the Sauerbrey equation developed for oscillation in air must be applied only to rigid masses attached to the crystal. Besides the mass, other physico-chemical parameters (such as viscosity, roughness, temperature) can induce a change in the resonant frequency. Thus, in liquid environment, Kanazawa and Gordon (1985) showed that quartz crystal microbalance measurements can be performed, in which case a viscosity-related decrease in the resonant frequency will be observed:
where ρ l is the density of the liquid and η l is the viscosity of the liquid.
The advantage of QCM in biological measurements is connected with its ability to monitor the changes of small masses in real time, and such measurements can be performed by use of the native or synthetic molecules without any supplementary labelling.
Specifically, a QCM is constituted of a thin quartz crystal resonator disc attached with metal electrodes plated onto the upper and lower faces of the disc. Gold is usually used as the electrode material. When an alternating electric field is applied across the quartz crystal between the upper and lower electrodes, a mechanical oscillation of the characteristic frequency (f) is generated in the crystal. This resonant frequency shifts as a function of the additional bound material occurring at the upper surface. For adapting this system to molecular recognition, the metal surface is specifically biofunctionalised with a selective, passivating layer on which the analyte-specific receptor is immobilized. Appropriate flow channels with monitored microfluidics allow controlled introduction of the analyte. Nowadays, commercial QCM integrate user-friendly software for characterising the biomolecular interactions at the surface. A large variety of surface coatings have been proposed, allowing several optimized chemical treatments and biomolecule as the receptor grafting depending on the aimed interactions. Apart from the standard gold material, which is the most commonly used sensor surface because Au is chemically inert and easy to modify in different ways, we can find in product catalogues (Q Sense) electrode materials such as silicon dioxide whose main advantage is the ability to form lipid bilayers which act as a platform for further immobilisation of molecules; stainless steel surfaces used for studies of biofilm formation; titanium-coated surfaces mainly used for implant research; metals and oxides (aluminium trioxide, platinum, tantalum, tantalum nitride, tungsten, copper, chromium, iridium, iron, silicon carbide, iron carbide, silver and cobalt); and more recently commercially available polymers (polysterene, PMMA) deposited by spin coating.
QCM-D. In biochemical applications, as macromolecules (proteins, polymers, etc.) deposited on the surface do not constitute a thin rigid layer, their viscoelastic behaviour leads to a vibrational energy loss by dissipation. That is why, in addition to measuring the frequency, the dissipation is often measured to help with the analysis.
The dissipation (D) is defined as:
where E lost is the energy lost (dissipated) during one oscillation cycle and E stored is the total energy stored in the oscillator.
The QMC-D consists in measuring the dissipation in the crystal by recording the response of a freely oscillating crystal at its resonance frequency. This also gives the opportunity to jump between the fundamental frequency and overtones (e.g. 15, 25 and 35 MHz). By working at multiple frequencies and applying a viscoelastic model (the so-called Voight model), the adhering film can be characterized in detail; viscosity, elasticity and correct thickness may be extracted even for soft films when certain assumptions are made.
The QCM can also be combined with other surface-analytical instruments. The electrochemical QCM (EQCM) is particularly advanced (Schumacher 1990; Buttry and Ward 1992). Using the EQCM, one can determine the ratio of mass deposited at the electrode surface during an electrochemical reaction to the total charge passed through the electrode. This ratio is called the “current efficiency.” This methods allows one to compare the deposited mass value estimated from current flowing between the working electrode (which is also the resonant electrode of QCM) and the counter electrode with the value deduced from the frequency shift of the QCM. The shrewdness of the method concerns the double use of the electrode (as the resonant QCM electrode and as the electrochemical working electrode) and the possible electrical cutting of the two functions (electrochemical and piezoelectric transducer) thanks the two different ranges of frequency.
The binding between two molecules can be quantitatively described by two parameters: the association equilibrium constant and the association rate. QCM measurements give access to these parameters. But we must keep in mind that theses values are strong depending on the experimental conditions (temperature, solution ionic strength and pH value). They also reflect structural properties of the binding sites. For instance, conformation of proteins on the surface affect drastically their recognition activities, the spacer between the surface and receptor appears as crucial factor and, in the case of lectin–carbohydrate interaction, the number of sugar monomers in the oligosaccharide chain strongly influence the interaction. Thus, uncontrolled immobilisation of receptor on biosensor surface could lead to meaningless results. The large number of parameters influencing the experimental results make a direct comparison of values (sensitivity, assay time, detection limit, association rate)reported in the literature very difficult. In their review, Cooper and Singleton (2007) have collected several tables comparing QCM assays and results for DNA sequences, for virus and phage, for selected pathogenic bacteria and for small-molecule detection. This drawback is also noticed by Lebed et al. (2006). Therefore, they discuss carefully the lectin–carbohydrate affinity values obtained by QCM measurement and compare them with others’ previously published results.
Nevertheless, this technique used in the similar and controlled conditions allows comparison of the binding behaviour of a set of monosialogangliosides (GM1, GM2, GM3, GM4) towards wheat germ agglutinin as described by Sato et al. (1998) as far back as 1998. More recently, Pei et al. (2007) demonstrated the great interest in the use of QCM sensor for rapid identification of glycosyldisulfide lectin inhibitors from a dynamic combinatorial library. Gold-plated quartz crystals were drop-coated with polystyrene before being covered with mannan in phosphate-buffered saline (PBS), and complete blocking was achieved with BSA to obtain a stable baseline. The plant lectin concanavalin A was injected as target species alone or in presence of potential inhibitor library components. Between each screening of libraries, the Con A binding to the surface must be carefully released.
Yates et al. (2006) affirm that QCM-D provides a unique means to explore the change in mass and the molecular flexibility of oligosaccharides, GAG (glycoaminoglycans) chains and entire proteoglycans and their complexes with proteins thanks to the ability to form membranes mimetics on the sensor surface in an environment that approximates that of the cell surface.
Nagase et al. (2003) used a lectin coated on the silver face of a piezoelectric crystal biosensor for oligosaccharide analysis. They used it for the detection of dissolved sugars and identification of erythrocytes, and obtained a detection limit approaching 100 cells.
Miura et al. (2004) used the QCM for investigating the biological functions of their synthetic glycoconjugate polymers with respect to lectins and Shiga toxin-1 (Stx-1) produced by the pathogen Escherichia coli. QCM detector was covered by a Gb3C10 SAM. First they demonstrated that the addition of Stx-1 to aqueous solutions induced a frequency reduction depending on the concentration. The binding affinity of Stx-1 to Gb3C10 SAM was estimated to be 1.2 × 108 (M−1). Secondly, upon addition of their glycoconjugates, they exhibited a notable inhibitory activity for the adhesion of Stx-1 to the Gb3C10 SAM (degree of inhibition going from 15% up to 70% versus glycoconjugate).
4.3.3 Electrochemical Detection
The use of electrochemical methods involves the molecular recognition-induced redox reaction. This is particularly true for enzymatic systems. The most basic amperometric enzyme electrode concerns glucose, which was first oxidized within an immobilized layer of glucose oxidase and then determined at a conducting (platinum or carbon) electrode by measuring the current resulting either from the oxidation of hydrogen peroxide or reduction of diatomic oxygen consumed by the enzymatic reaction (Updike and Hicks 1967).
An electrochemical biosensor for determination of the tumour marker-bound sialic acid (b-SIA) was reported by Aubeck et al. (1993). This sensor consisted of a copolymer-immobilized bilayer containing the enzyme sialidase, placed in contact with a H+ selective indicator membrane. The release of sialic acid following enzymatic cleavage resulted in a local pH change monitored by the proton-sensitive indicator electrode.
Potentiometric detection can be used if the binding of biomolecules induces large non-equilibrium potentiometric responses. One type of device named ISFET (ion sensitive field effect transistor) sensors uses this event and is derived from MOSFET (metal oxide semiconductor field effect transistor), the most important component in integrated circuits. In 1970, Bergveld et al. (Van Kerhof et al. 1993) showed the sensitivity towards ions H+ of a MOSFET without metallic grid when the blocking silicon layer is directly in contact with the solution.
ISFET is made of a p-type silicon substrate with two areas of n-doping (called source and drain) on which metallic electrodes are applied. The channel between the source and the drain is covered of a thin blocking SiO2 layer. Metallisation of this layer forms the gate, which is the electrode monitoring the conductivity in the channel. In the case of ISFET, an ion-sensitive membrane is put in place of the metallic layer. This structure is intrinsically blocked, so no current flows between source and drain because whatever the potential applied at this two areas, one of the p-n junction is in reverse polarisation. When a positive voltage V G is applied between the gate and the source, the majority of holes tend to be repelled while the minority electrons of the p-substrate move to the channel. For V G > V T (V T is the threshold voltage), the electron density becomes larger than the hole density and the formation of n-type channel ensures electrical continuity between the source and the drain; the current flows from source to the drain. I D is a function of V G and V D voltages. If V D < V G – V T, the FET is in the linear range; for V D > V G – V T , FET is in the saturation range.
V T = V T0 + y 0, where V T0 is relative to the ISFET characteristics and y 0 is the potential between the sensitive membrane and the electrolytic solution.
Taking into account the equilibrium between the ion analyte in solution and its chemical conformation inside the sensitive membrane, y 0 is dependent of the activity of the ions to be detected. The following figure gives the shape of I D variation for ISFET pH with an alumina sensitive membrane.
The high concentration of negative charges on heparin makes the detection of the variations in charge density in porous membranes mounted on ISFET easy. Thus, heparin biosensors based on the ISFET concept exhibit very high sensitivity thresholds of between 0.1 and 1 units per millilitres (Van Kerhof et al. 1993).
A similar transducer can be used for detecting enzymatic reactions. In fact, catalysed enzymatic reactions of hydrolase or oxidase type, respectively, consume or produce protons. The presence of the resulting products from the enzymatic reactions can be detected through the pH variation induced at the ISFET surface on which specific enzymes have been grafted. In this case, ISFET is called ENFET (enzymatic field effect transistor). Numerous molecular species can be detected by ENFET: for example, urease, glucose, organophosphorous pesticides. This type of transducer was also adapted for the detection of DNA hybridization. In this case, the SiO2 thin film was coated with an APTS (3-aminopropyltriethoxysilane) layer on which single-strand DNA was immobilised. This FET put in contact with a solution containing complementary DNA strands shows a strong drain current resulting from the field effect induced by the arrival of the negative charge borne by DNA strands under hybridisation (Souteyrand et al. 1997). This device was called GENFET. To exploit this field effect on a microarray, Souteyrand et al. (2000) described a technique based on the optoelectrochemical impedance measurement in which electrical excitation is replaced by modulated light for scanning the surface of a electrolyte/DNA modified dielectric layer/semiconductor and for detecting directly the hybridisation on spots.
4.3.4 Mass Spectrometry
Mass Spectroscopy used as a detector has the advantages of being label free and of allowing the determination of the target structure after interaction in a complex medium. Bryan et al. (2004) reported the use of cleavable linker (disulfide) for subsequent electron spray ionisation MS. The use of porous silicon as a matrix for MALDI-TOF analysis was also proposed. Chen et al. (2005) used carbohydrate encapsulated gold nanoparticles for capturing proteins. After purification of reacted material from the unreacted one by centrifugation, the proteins were subjected to protease digestion. The remaining peptides were analysed by MALDI-TOF for the determination of the amino acid involved in the binding. Enzymatic reaction by enzymes can also be monitored by MALDI-TOF MS (Park and Shin 2007; Su and Mrksich 2002; Laurent et al. 2008).
4.3.5 Nanoparticles
The use of saccharide-modified nanoparticles (polymeric, metallic or semiconductor) for biosensing purpose is attractive, as it allows purification from a complex mixture of reacted products (by centrifugation, or magnetic field) and the multivalent display of carbohydrate. They can be used advantageously for in vivo bioimaging in transmission electron microscopy or confocal microscopy, or for glycoprofiling by mass spectroscopy (after purification and release of reacted molecules) (Larsen et al. 2006).
Carbohydrate-modified core magnetic nanoparticles could be advantageously employed for in vivo imaging by magnetic resonance imaging (MRI). Indeed, in the literature (Harris et al. 2006; Perez et al. 2002; Perez et al. 2003; Perez et al. 2004), the detection of biomolecules using super paramagnetic iron nanoparticles (and MRI) is reported. The assay is based on modified nanoparticles that self-assemble in the presence of the targeted biomolecule. The spin–spin relaxation time of surrounding water molecules is then enhanced and can be imaged by MRI, allowing the detection of the target even in complex media.
The multivalent nature of carbohydrate interactions should be suitable for this type of biosensors. However, to our knowledge, this has not been reported for probing carbohydrate–protein interaction. This would be useful for in vivo imaging in diagnosis where abnormal carbohydrate–protein interactions take place.
5 Biosensors: Applications
5.1 Antibody/Antigen
Carbohydrate biosensing can be applied to the design of sugar-based vaccine or for antibody profiling for diagnosis (IgMs profiling for tumor detection, natural auto-antibodies profiling, etc.).
The cell sphingolipid Globo H possesses a hexasaccharide moiety. It is an antigenic carbohydrate such as T antigen, which is highly expressed on a variety of tumoral cell lines. Huang et al. (2006a) reported the design of a glycoarray based on Globo H and truncated Globo H ligands for understanding Globo H interaction with antibodies MBr1 and VK-9. MBr1 and VK-9 are mouse monoclonal antibodies targeting the Globo H epitope. The epitopes (including Globo H) were synthesised using “one-pot” programmable synthesis and modified with an aglycon bearing an amino or an azido function. The glycosides were covalently immobilised on a glass support using ester-activated or alkyne-modified glass slides. Affinities for MBr1 and VK-9 were probed with FITC-labelled secondary antibodies. It was shown that the monoclonal antibodies bind the terminal tetrasaccharides as well as the natural hexasaccharides and that the fucose residue is compulsory for binding. However, serum from patients with breast cancer binds both the fucosylated and defucosylated pentasaccharide, probably due to the polyclonal nature of the serum. According to the authors, their carbohydrate platform was more effective and more sensitive than ELISA for probing the affinity of antibodies for Globo H (Fig. 7.28) with the use of very minute amounts of the material.
5.2 Enzyme/Carbohydrates
Another application of carbohydrate microarray is to understand the structural features that govern substrate specificity of enzymes processing carbohydrates. The group of Mathieu (Chevolot et al. 2001) demonstrated that α-2,6-sialyltransferase was active on lactose-modified polystyrene. Detection was performed using radiolabelled CMP-Neu5Ac. The group of Shin (Shin 2007) showed, using N-GlcNAc and O-Fuc-α-modified glass slide, that LacNac could be successfully synthesised on chip using β-1,4-galactosyltransferase. They further illustrated that complex oligosaccharides could be synthesised using sequential β-1,4-galactosyltransferase followed by α-2,3′-sialyltransferase. Each step of the synthesis was characterised using FITC-labelled specific lectin.
Aminoglycosides are a broad-spectrum class of antibiotics due to their interaction with bacterial 30S ribosome. Their efficiency has decreased as a result of the emergence of resistant strains. The resistance mechanism is usually due to enzymatic modification of the molecules such as aminoglycoside acetyl transferases. Disney et al. have used aminoglycoside microarrays for probing the affinity of three acetyl transferases (from Salmonella enterica and from Mycobacterium tuberculosis) towards aminoglycoside antibiotics (Disney and Seeberger 2004a; Disney et al. 2004). They also studied new aminoglycosides as inhibitors of these enzymes. Based on their microarray experiment, they identified new aminoglycosides with low affinity towards the acetyl transferases, meaning they are less susceptible to enzymatic transformation by the bacteria, as well as being a potent inhibitor.
5.3 Carbohydrates/Lectins
Plant lectins are widely used usually as a model to assess biosensor/microarray in terms of specificity. Here, we will focus on three examples where carbohydrate biosensing was used advantageously with the perspectives of medical applications for
-
analysing ligand affinity of Dectin-1, a C-type leukocytes lectin involved in phagocytosis and inflammation (Palma et al. 2006);
-
analysing the specificity of hemagglutinins from modern and pandemic influenza virus (Stevens et al. 2006); and
-
determining the affinity profile of GP120-binding protein. GP120 is a hyper-mannosylated protein of the HIV envelope. These mannose residues are involved in the binding of the virus to the host cells (Adams et al. 2004).
Plama et al. studied the binding of Dectin-1, a C-type leukocytes lectin involved in phagocytosis and inflammation that binds both β-1,3 and β-1,3/β-1,6 rich β-glucans (Palma et al. 2006). They developed a bioassay based on neoglycolipids oligosaccharides immobilised through physisorption onto nitrocellulose membranes. Oligosaccharides comprise mammalian oligosaccharides and oligosaccharides issued from the depolymerisation of natural polysaccharides. Fluorescence was used for detection. They showed that Dectin-1 binds exclusively β-1,3-linked glucose oligosaccharides. Ten to eleven glucose residues per oligosaccharides were compulsory for good binding.
GP120 is a hyper-mannosylated protein of the HIV envelope. The mannose residues are involved in the entry of the virus into the host cells by interacting with CD4, a chemokine family receptor present at the surface of T-lymphocytes, macrophages, dendritic cells, etc. Furthermore, the mannose residues are involved in protecting the virus from antibodies directed against GP120 by hiding the targeted epitope. This mannose oligosaccharide has been considered a potential target for the design of vaccine (it interacts with the 2G12 antibody (Calarese et al. 2005)) or as potential target for prophylactic measures. In the latter case, proteins interacting with the hyper-mannosylated moiety of GP120 would prevent the entry of the virus into the host cell. Adams et al. designed a glyco array bearing different mannose and studied the affinities of 2G12, CD4, Scytovirin (a potential anti-HIV protein), Cyanovirin N (CVN, a cyano bacteria protein with potential anti-HIV properties) and the dendritic cell lectin DC SIGN involved the internalisation of the virus. Detection was performed with FITC labelling (Adams et al. 2004). It was shown that each protein has specific requirements concerning the structure of the mannosyl oligosaccharide. For example, an α-1–2 linkage alone is necessary for the interaction of 2G12. The binding profile of the Scytovirin showed that the terminal α-1–2 mannose linkage at the terminal non-reducing end is required.
Stevens et al. took advantage of the CFG platform for studying the affinities of hemagglutinin of different strands of the influenza virus (Stevens et al. 2006). Influenza virus is an enveloped virus with a segmented negative RNA genome. Its envelope bears two proteins: hemagglutinin (HA, around 5,000 copies) and neuraminidase (3–500 copies). HAs interact with the sialic acids present at the surface of the host cell, permitting adhesion of the virus. It is the specificity of the HA towards the sialic acid linkage that determines the host specificity of the influenza virus. Human influenza viruses preferentially bind the α-2–6 linkage present at the surface of the epithelial cells of the upper respiratory track and lungs, while the avian ones recognise the α-2–3 linkage present in epithelial cells of the intestine of birds. Therefore, the subtle switch from avian to human subtype is related to the HA affinity to α-2–6.
Stevens et al. used a glycoarray bearing 200 oligosaccharides for studying the specificity of HAs from modern and pandemic influenza virus for α-2,6′/α-2′,3 linkage, and also the influence of sulfation, fucosylation and sialylation at position 2. They showed that the species barrier of the New York 1918 variant (human pandemic variant) could be reverted to an avian virus by a single mutation of the Asp190Glu. The species barrier from avian to human pandemic, as defined by the specificity of 1918 viruses (New York and South Carolina), can be jumped by the likely avian progenitor by only changing two amino acids of their HA.
5.4 Whole Cells
Using whole cells, the biosensor takes advantage of the natural multivalency of carbohydrate binding on the cell surface. Indeed, in nature, multivalency is often achieved by the self-association of lectine at the cellular surface upon interaction with carbohydrates (Nimrichter et al. 2004). Several authors have reported the use of whole-cell detection by means of carbohydrate/whole cell interactions.
Disney and Seeberger (2004b) have reported the use of microarrays as a means for probing bacteria (E. coli) affinities for immobilised oligomannose. E. coli strains were stained with a fluorescent cell-permeable nucleic acid dye and incubated on the microarray. They demonstrated that the platform allows discriminating strains according to their affinities for mannose residues. This issue is critical since the ability of screening cells’ affinity for different carbohydrate affinities is related to their pathogenicity. The ability of the microarray to sense multiple carbohydrate-based interactions in one single experiment allows profiling pathogen–carbohydrate affinities. This should permit establishing a “finger print” like identification of the pathogen present in a complex medium. They also showed that they could identify E. coli from complex media such as a serum containing erythrocytes. Anti-adhesive molecule activities were evaluated using such arrays. They compared the IC90s of mannose, p-nitro mannose and mannose-containing polymers.
Chevolot et al. (2001)tested the effect of galactose and a lactose-derivatised surface on primary rat hepatocytes. They found that their viability and xenobiotic metabolism were not affected, while neutral red uptake and MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) activity increased with galactose surface density. Neutral red uptake is related to the lysosomal activity. They hypothesised that this may related to the natural lysosomal activity of hepatocytes in correlation with the asialoglycoprotein receptor.
Adhesion of primary chicken hepatocytes and CD4+ human T cell onto glycoarrays (bearing 200 and 45 structures, respectively) were studied by Nimrichter et al. (2004) using fluorescent staining of the cells. Primary chicken hepatocytes express a C-type lectin that binds to N-acetyl glucosamine (GlcNac) present at the non-reducing terminal position of oligosaccharides. They found that the cells did bind as expected onto spots bearing GlcNAc at the terminal non-reducing end independently of the linkage and orientation. The hepatocytes did not adhere onto spots bearing galactose residues at the terminal non-reducing end. CD4+ human T cell from healthy individual express affinity towards Lewis X structure mediated by an L-selectin. On a 45-structure-bearing chip, they showed that the presence of the fucosyl residues was compulsory for obtaining good adhesion. They also showed that the adhesion was influenced by the staining protocol. According to the authors, the main limitations are the control of detachment forces, the low compatibility of cells with the robotics and high-throughput liquid and long-term storage. Adhesion of pathogens such as E. coli (Nimrichter et al. 2004) or viruses (Amonsen et al. 2007) was also reported.
6 Concluding Remarks
Carbohydrate biosensors have demonstrated to be powerful tools for studying glycobiolgy. They should find applications in fundamental research, diagnosis or drug discovery. However, availability of carbohydrate ligands in terms of synthesis, biosynthesis or extraction from natural sources remains a key issue. Furthermore, fundamental understanding should be addressed. For example, the influence of surface chemistry on the subsequent protein–carbohydrate interaction has not been widely studied, even though it may influence the overall results.
Most reported biosensors have so far focused on carbohydrate–protein interactions. However, other interactions may be of crucial interest such as carbohydrate–carbohydrate interactions, DNA–carbohydrate interactions, etc. Finally, to our knowledge, very few integrated (i.e., with integrated microfludic) systems have been reported. Such systems could in theory improve applications by reducing the amount of needed material, decreasing the experimental time, etc.
Abbreviations
- Ab:
-
Antibody
- Ac:
-
Acetyl
- AIDS:
-
Acquired immune deficiency syndrome or acquired immunodeficiency syndrome
- AZT:
-
Azidothymidine or Zidovudine
- BAW:
-
Bulk Acoustic Wave
- bFGF:
-
Basic fibroblast growth factor
- BSA:
-
Bovine Serum Albumin
- Bz:
-
Benzoyl
- CD4:
-
Cluster of differentiation 4
- CD44:
-
Cluster of differentiation 44
- CFG:
-
Consortium for functional glycomics
- CMP-Neu5Ac:
-
Cytidine monophosphate-N-acetylneuraminic-acid
- CNS:
-
Central nervous system
- ConA:
-
Concanavalin A
- CSPG:
-
Chondroitin-sulfate proteoglycan
- Cy3:
-
Indodicarbocyanine 3
- Cy5:
-
Indodicarbocyanine 3
- DC-SIGN:
-
Dendritic cell-specific intercellular adhesion molecule- 3-Grabbing Non-integrin
- DDI:
-
DNA directed immobilization
- DNA:
-
Deoxyribonucleic acid
- DPI:
-
Dual polarisation interferometry
- DV:
-
Dengue virus
- EC:
-
Erythrina crista-galli lectin
- ENFET:
-
Enzymatic field effect transistor
- EQCM:
-
Electrochemical quartz crystal microbalance
- FDA:
-
Food and drug administration
- FET:
-
Field effect transistor
- FITC:
-
Fluorescein isothiocyanate
- GAG:
-
Glycosaminoglycans
- GIPL:
-
Glycoinositol phospholipid
- GlcNAc:
-
N-Acetylglucosamine
- GPI:
-
Glycosylphosphatidylinositol
- GSLs:
-
Glycosphingolipids
- HA:
-
Hemagglutinin
- HIV:
-
Human immunodeficiency virus
- HIV-RT:
-
Human immunodeficiency virus reverse transcriptase
- HSA:
-
Human serum albumin
- HSPG:
-
Heparan-sulfate proteoglycan
- ICAM:
-
Inter-cellular adhesion molecule
- ISFET:
-
Ion sensitive field effect transistor
- MALDI-ToF:
-
Matrix-assisted laser desorption/ionization time of flight
- MOSFET:
-
Metal oxide semiconductor field effect transistor
- MR:
-
Mannose receptor
- MRI:
-
Magnetic resonance imaging
- MS:
-
Mass spectroscopy
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NA:
-
Neuramidase
- NG2:
-
Nerve/Glial antigen 2
- NHS:
-
N-Hydroxysuccinimide
- NIS:
-
N-iodosuccinimide
- NMR:
-
Nuclear magnetic resonance
- NPC:
-
Neuronal precursor cells
- NSC:
-
Neuronal stem cells
- PCR:
-
Polymerase chain reaction
- PECAM:
-
Platelet Endothelial cell adhesion molecule
- PEG:
-
Poly(ethylene glycol)
- QCM:
-
quartz crystal microbalance
- QCM-D:
-
quartz crystal microbalance with dissipation monitoring
- QD:
-
Quantum dot
- RNA:
-
Ribonucleic acid
- SAM:
-
Self assembled monolayer
- SARS:
-
Severe acute respiratory syndrome
- SDS:
-
Sodium dodecyl sulfate
- SLe:
-
Sialyl lewis
- SPR:
-
Surface plasmon resonance
- Tf:
-
Trifluoromethanesulphonate
- TMS:
-
Trimethylsilyl
- TSM:
-
Thickness shear mode
- VCAM:
-
Vascular cell adhesion molecule
- WGA:
-
Wheat-germ agglutinin
References
Adams EW, Ratner DM, Bokesch HR, Mc Mahon JB, O’ Keefe BR, Seeberger PH (2004) Oligosaccharide and glycoprotein microarrays as tools in HIV glycobiology; glycan-dependent gp120/protein interactions. Chem Biol 11:875–881
Adams EW, Ueberfeld JM, Ratner DM, O’ Keefe BR, Walt DR, Seeberger PH (2003) Encoded fiber-optic microsphere arrays for probing protein–carbohydrate interactions. Ang Chem Inter Ed Engl 42:5317–5320
Amonsen M, Smith DF, Cummings RD, Air GM (2007) Human parainfluenza viruses hPIV1 and hPIV3 bind oligosaccharides with α2-3-linked sialic acids that are distinct from those bound by H5 avian influenza virus hemagglutinin. J Virol 81:8341–8345
Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, Guinchard S, Kochhar S, Sigrist H, Sprenger N (2005) Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 15:31–41
Angyal SJ (1984) The composition of reducing sugars in solution. Adv Carbohydr Chem Biochem 42:15–68
Arcamone F, Franceschi G, Orezzi P, Cassinelli G, Barbieri W, Mondelli R (1964) Daunomycin I. The structure of daunomycinone. J Am Chem Soc 86:5334–5335
Aubeck R, Eppelsheim C, Bräuchle C, Hampp N (1993) Potentiometric thick-film sensor for the determination of the tumour marker bound sialic acid. Analyst 118:1389–1392
Bamford CH, Cooper SL, Tsurutta T (eds) (1992) The Vroman effect: festschrift in honour of the 75th birthday of Dr. Leo Vroman. VSP, Utrecht
Baum LG, Paulson JC (1990) Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histol Supp 40:35–38
Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V (2003) Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 161:169–186
Berhe S, Schofield L, Schwarz RT, Gerold P (1999) Conservation of structure among glycosylphosphatidylinositol toxins from different geographic isolates of Plasmodium falciparum. Mol Biochem Parasitol 103:273–278
Bertozzi CR, Kiessling LL (2001) Chemical glycobiology. Science 291:2357–2364
Bishop JR, Schuksz M, Esko JD (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446:1030–1037
Biskup MB, Müller JU, Weingart R, Schmidt RR (2005) New methods for the generation of carbohydrate arrays on glass slides and their evaluation. ChemBioChem 6:1007–1015
Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, Van Die I, Burton DR, Wilson IA, Cummings R, Bovin NV, Wong C-H, Paulson JC (2004) Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA 10:17033–17038
Blixt O, Vasiliu D, Allin K, Jacobsen N, Warnock D, Razi N, Paulson JC, Bernatchez S, Gilbert M, Wakarchuk W (2005) Chemoenzymatic synthesis of 2-azidoethyl-ganglio-oligosaccharides GD3, GT3, GM2, GD2, GT2, GM1, and GD1a. Carbohydr Res 340:1963–1972
Blumenschein TM, Friedrich N, Childs RA, Saouros S, Carpenter EP, Campanero-Rhodes MA, Simpson P, Chai W, Koutroukides T, Blackman MJ, Feizi T, Soldati-Favre D, Matthews S (2007) Atomic resolution insight into host cell recognition by Toxoplasma gondii. EMBO J 26:2808–2820
Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL (2005) Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem 280:4307–4312
Bock VD, Hiemstra H, Van Maarseveen JH (2006) CuI-catalyzed alkyne-azide “Click” cycloadditions from a mechanistic and synthetic perspective. Eur J Org Chem 2006:51–68
Bouillon C, Meyer A, Vidal S, Jochum A, Chevolot Y, Cloarec JP, Praly JP, Vasseur JJ, Morvan F (2006) Microwave assisted “click” chemistry for the synthesis of multiple labeled-carbohydrate oligonucleotides on solid support. J Org Chem 71:4700–4702
Bruckenstein S, Shay M (1985) An in situ weighing study of the mechanism for the formation of the adsorbed oxygen monolayer at a gold electrode. J Electroanal Chem Interface Electrochem 188:131–136
Brun MA, Disney MD, Seeberger PH (2006) Miniaturization of microwave-assisted carbohydrate functionalization to create oligosaccharide microarrays. ChemBioChem 7:421–424
Bryan MC, Fazio F, Lee H-K, Huang C-Y, Chang A, Best MD, Calarese DA, Blixt O, Paulson JC, Burton D, Wilson IA, Wong C-H (2004) Covalent display of oligosaccharide arrays in microtiter plates. J Am Chem Soc 126:8640–8641
Buttry DA, Ward MD (1992) Measurement of interfacial processes at electrode surfaces with the electrochemical quartz crystal microbalance. Chem Rev 92:1355–1379
Calarese DA, Lee H-K, Huang C-Y, Best MD, Astronomo RD, Stanfield RL, Katinger H, Burton DR, Wong C-H, Wilson IA (2005) Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci USA 102:13372–13377
Capila I, Linhardt RJ (2002) Heparin-protein interactions. Ang Chem Inter Ed Engl 41:390–412
Carreira JC, Jones C, Wait R, Previato JO, Mendonça-Previato L (1996) Structural variation in the glycoinositolphospholipids of different strains of Trypanosoma cruzi. Glycoconjugate J 13:955–966
Carroll SM, Higa HH, Paulson JC (1981) Different cell-surface receptor determinants of antigenically similar influenza virus hemagglutinins. J Biol Chem 256:8357–8363
Carter JR, Pager CT, Fowler SD, Dutch RE (2005) Role of N-linked glycosylation of the Hendra virus fusion protein. J Virol 79:7922–7925
Chen Y-J, Chen S-H, Chien Y-Y, Chang Y-W, Liao H-K, Chang C-Y, Jan M-D, Wang K-T, Chun-Cheng Lin C-C (2005) Carbohydrate-encapsulated gold nanoparticles for rapid target-protein identification and binding-epitope mapping. ChemBioChem 6:1169–1173
Chen Y, Maguire T, Hileman RE, Jonathan R, Fromm JR, Esko JD, Linhardt RJ, Marks RM (1997) Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med 3:866–871
Chevolot Y, Bouillon C, Vidal S, Morvan F, Meyer A, Cloarec J-P, Jochum A, Praly J-P, Vasseur J-J, Souteyrand E (2007) DNA-based carbohydrate biochips: a platform for surface glyco-engineering. Ang Chem Inter Ed Engl 46:2398–2402
Chevolot Y, Bucher O, Léonard D, Mathieu H-J, Sigrist H (1999) Synthesis and characterization of a photoactivatable glycoaryldiazirine for surface glycoengineering. Bioconjugate Chem 10:169–175
Chevolot Y, Martins J, Milosevic N, Léonard D, Zeng S, Malissard M, Berger EG, Maier P, Mathieu H-J, Crout DHG, Sigrist H (2001) Immobilisation on polystyrene of diazirine derivatives of mono and disaccharides: biological activities of modified surfaces. Bioorg Med Chem 9:2943–2953
Constantinescu AA, Vink H, Spaan JAE (2003) Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscl Throm Vas 23:1541–1547
Cooper MA, Singleton VT (2007) A survey of the 2001 to 2005 quatrz microbalance biosensor literature: applications of acoustic physics to the analysis of biomolecular interactions. J Mol Recognit 20:154–184
Croft SL, Sundar S, Fairlamb AH (2006) Drug resistance in leishmaniasis. Clin Microbiol Rev 19:111–126
Dam TK, Brewer CF (2002) Thermodynamic studies of lectin-carbohydrate interactions by isothermal titration calorimetry. Chem Rev 102:387–429
De Paz JL, Moseman EA, Noti C, Polito L, Von Andrian UH, Seeberger PH (2007) Profiling heparin-chemokine interactions using synthetic tools. ACS Chem Biol 2:735–744
De Paz JL, Noti C, Seeberger PH (2006) Microarrays of synthetic heparin oligosaccharides. J Am Chem Soc 128:2766–2767
Desai NP, Hubbell JA (1991) Solution technique to incorporate polyethylene oxide and other water-soluble polymers into surfaces of polymeric biomaterials. Biomaterials 12:144–153
Disney MD, Seeberger PH (2004a) Aminoglycoside microarrays to explore interactions of antibiotics with RNAs and proteins. Chem Eur J 100:3308–3314
Disney MD, Seeberger PH (2004b) The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem Biol 11:1701–1707
Disney MD, Magnet S, Blanchard JS, Seeberger PH (2004) Aminoglycoside microarrays to study antibiotic resistance. Ang Chem Inter Ed Engl 43:1591–1594
Dosreis GA, Peçanha LMT, Bellio M, Previato JO, Mendonça-Previato L (2002) Glycoinositol phospholipids from Trypanosoma cruzi transmit signals to the cells of the host immune system through both ceramide and glycan chains. Microbes Infect 4:1007–1013
Drouillard S, Driguez H, Samain E (2006) Large-scale synthesis of H-antigen oligosaccharides by expressing Helicobacter pylori α-1, 2-fucosyltransferase in metabolically engineered Escherichia coli Cells. Ang Chem Inter Ed Engl 45:1778–1780
Dubois M-P, Gondran C, Renaudet O, Dumy P, Driguez H, Fort S, Cosnier S (2005) Electrochemical detection of Arachis hypogaea (peanut) agglutinin binding to monovalent and clustered lactosyl motifs immobilized on a polypyrrole film. Chem Commun:4318–4320
Dugas V, Broutin J, Souteyrand E (2005) Droplet evaporation study applied to DNA chip manufacturing. Langmuir 21:9130–9136
Dugas V, Depret G, Chevalier B, Nesme X, Souteyrand E (2004) Immobilization of single-stranded DNA fragments to solid surfaces and their repeatable specific hybridization: covalent binding or adsorption? Sens Actuators B Chem 101:112–121
Dumon C, Bosso C, Utille J-P, Heyraud A, Samain E (2006) Production of Lewis x tetrasaccharides by metabolically engineered Escherichia coli. ChemBioChem 7:359–365
Dwek RA (1996) Glycobiology: toward understanding the function of sugars. Chem Rev 96:683–720
Dyukova VI, Dementieva EI, Zubtsov DA, Galanina OE, Bovin NV, Rubina AY (2005) Hydrogel glycan microarrays. Anal Biochem 347:94–105
Ebara Y, Okahata Y (1994) A kinetic study of Concanavalin A binding to glycolipid monolayers by using a quartz-crystal microbalance. J Am Chem Soc 116:11209–11212
Edward JT, Puskas I (1962) Stereochemical studies: IV. Acid-catalyzed equilibration of α- and β-forms of 3-alkoxy-4-oxa-5α-cholestanes. The anomeric effect. Can J Chem 40:711–717
Esko J (1999) Proteoglycans and glycosaminoglycans. In: Varki A, Cummings R, Esko J, Freeze H, Hart GW, Marth J (eds) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, New York, pp 145–161
Fare TL, Coffey EM, Dai H, He YD, Kessler DA, Kilian KA, Koch JE, Leproust E, Marton MJ, Meyer MR, Stoughton RB, Tokiwa GY, Wang Y (2003) Effects of atmospheric ozone on microarray data quality. Anal Chem 75:4672–4675
Fazio F, Bryan MC, Lee HK, Chang A, Wong CH (2004) Assembly of sugars on polystyrene plates: a new facile microarray fabrication technique. Tetrahedron Lett 45:2689–2692
Fazio F, Bryan MC, Blixt O, Paulson JC, Wong C-H (2002) Synthesis of sugar arrays in microtiter plate. J Am Chem Soc 124:14397–14402
Feinberg H, Mitchell DA, Drickamer K, Weis WI (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163–2166
Feizi T, Fazio F, Chai W, Wong C-H (2003) Carbohydrate microarrays—a new set of technologies at the frontiers of glycomics. Curr Opin Struct Biol 13:637–645
Ferguson MA (1999) The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci 112:2799–2809
Ferrier RJ, Hay RW, Vethaviyasar N (1973) A potentially versatile synthesis of glycosides. Carbohydr Res 27:55–61
Fischer E (1893) Ueber die glucoside der alkohole. Ber Deut Chem Gesell 26:2400–2412
Fischer E (1895) Ueber die verbindungen der zucker mit den alkoholen und ketonen. Ber Deut Chem Gesell 28:1145–1167
Fort S, Birikaki L, Dubois MP, Antoine T, Samain E, Driguez H (2005) Biosynthesis of conjugatable saccharidic moieties of GM(2) and GM(3) gangliosides by engineered E-coli. Chem Commun 2558–2560
Fraser-Reid B, Konradsson P, Mootoo DR, Udodong U (1988) Direct elaboration of pent-4-enyl glycosides into disaccharides. J Chem Soc Chem Commun 823–825
Fraser-Reid B, Udodong U, Wu Z, Ottosson H, Merritt JR, Rao CS, Roberts C, Madsen R (1992) n-Pentenyl glycosides in organic chemistry: a contemporary example of serendipity. Synlett 927–942
Fügedi P (2006) Glycosylation methods. In: Levy DE, Fügedi P (eds) The organic chemistry of sugars. Taylor & Francis, New York, pp 89–180
Fügedi P, Garegg PJ, Lönn H, Norberg T (1987) Thioglycosides as glycosylating agents in oligosaccharide synthesis. Glycoconj J 4:97–108
Fukui S, Feizi T, Galustian C, Lawson AM, Chai W (2002) Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat Biotechnol 20:1011–1017
Galonic DP, Gin DY (2007) Chemical glycosylation in the synthesis of glycoconjugate antitumour vaccines. Nature 446:1000–1007
Garegg PJ (1997) Thioglycosides as glycosyl donors in oligosaccharide synthesis. Adv Carbohydr Chem Biochem 52:179–205
Gil MV, Arévalo MJ, López O (2007) Click chemistry - What’s in a name? Triazole synthesis and beyond. Synthesis 1589–1620
Goffard A, Callens N, Bartosch B, Wychowski C, Cosset F-L, Montpellier C, Dubuisson J (2005) Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J Virol 79:8400–8409
Gordus A, Mac Beath G (2006) Circumventing the problems caused by protein diversity in microarrays: implications for protein interaction networks. J Am Chem Soc 128:13668–13669
Goretzki L, Brug MA, Grako KA, Stallcup WB (1999) High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem 274:16831–16837
Gotloib L, Shostak A, Galdi P, Jaichenko J, Fudin R (1992) Loss of microvascular negative charges accompanied by interstitial edema in septic rats heart. Circ Shock 36:45–56
Grubor NM, Shinar R, Jankowiak R, Porter MD, Small GJ (2004) Novel biosensor chip for simultaneous detection of DNA-carcinogen adducts with low temperature fluorescence. Biosens Bioelectron 19:547–556
Hakomori S-I (1991) Carbohydrate-carbohydrate interaction as an initial step in cell recognition. Pure Appl Chem 63:473–482
Hakomori S-I (2002) The glycosynapse. Proc Natl Acad Sci USA 99:225–232
Hanna SL, Pierson TC, Sanchez MD, Ahmed AA, Murtadha MM, Doms RW (2005) N-Linked glycosylation of west nile virus envelope proteins influences particle assembly and infectivity. J Virol 79:13262–13274
Harris TJ, Von Maltzahn G, Derfus AM, Ruoslahti E, Bhatia SN (2006) Proteolytic actuation of nanoparticle self-assembly. Ang Chem Inter Ed Engl 45:3161–3165
Hashimoto S-I, Honda T, Ikegami S (1989) A rapid and efficient synthesis of 1,2-trans-β-linked glycosides via benzyl- or benzoyl-protected glycopyranosyl phosphates. J Chem Soc Chem Commun 685–687
Helferich B, Schmitz-Hillebrecht E (1933) Eine neue methode zur synthese von glykosiden der phenole. Ber Deut Chem Gesell (A and B Series) 66:378–383
Hölemann A, Seeberger PH (2006) Synthetic carbohydrate-based antimalarial vaccines and glycobiology. In: Bewley CA (ed) Protein-carbohydrate interactions in infectious diseases. Royal society of Chemistry, Cambridge, UK, pp 151–174
Hosono K, Nishida Y, Knudson W, Knudson CB, Naruse T, Suzuki Y, Ishiguro N (2007) Hyaluronan oligosaccharides inhibit tumorigenicity of osteosarcoma cell lines MG-63 and LM-8 in vitro and in vivo via perturbation of hyaluronan-rich pericellular matrix of the cells. Am J Pathol 171:274–286
Hounsell EF (2000) Glycobiology of the immune system. In: Ernst B, Hart GW, Sinay P (eds) Carbohydrate in chemistry and biology. Wiley-VCH, New-York
Houseman BT, Mrksich M (2002) Carbohydrate arrays for the evaluation of protein binding and enzymatic modification. Chem Biol 9:443–454
Houseman BT, Gawalt ES, Mrksich M (2003) Maleimide-functionalized self-assembled monolayers for the preparation of peptide and carbohydrate biochips. Langmuir 19:1522–1531
Huang CY, Thayer DA, Chang AY, Best MD, Hoffmann J, Head S, Wong CH (2006a) Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc Natl Acad Sci USA 103:15–20
Huang G-H, Liu T-C, Liu M-X, Mei X-Y (2005) The application of quantum dots as fluorescent label to glycoarray. Anal Biochem 340:52–56
Huang K-T, Wu B-C, Lin C-C, Luo S-C, Chen C, Wong C-H, Lin C-C (2006b) Multi-enzyme one-pot strategy for the synthesis of sialyl Lewis X-containing PSGL-1 glycopeptide. Carbohydr Res 341:2151–2155
Huisgen R, Fraunberg K, Sturm HJ (1969) Cycloadditionen von pyridyl- und pyrimidyl-aziden. Tetrahedron Lett 10:2589–2594
Huisgen R, Knorr R, Möbius L, Szeimies G (1965) 1.3-Dipolare cycloadditionen, XXIII. einige beobachtungen zur addition organischer azide an CC-dreifachbindungen. Chem Ber 98:4014–4021
Igarashi K (1977) The Koenigs-Knorr reaction. Adv Carbohydr Chem Biochem 34:243–283
Imberty A, Wimmerová M, Mitchell EP, Gilboa-Garber N (2004) Structures of the lectins from Pseudomonas aeruginosa: insights into the molecular basis for host glycan recognition. Microbes Infect 6:221–228
Inouye S, Tsuruoka T, Niida T (1966) The structure of nojirimycin, a piperidinose sugar antibiotic. J Antibiotics Ser A 19:288–292
Isbell HS, Pigman W (1969) Mutarotation of sugars in solution: part II. Adv Carbohydr Chem Biochem 24:14–65
Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N (1998) Why do phospholipid polymers reduce protein adsorption? J Biomed Mater Res 39:323–330
Ito T, Nelson J, Couceiro SS, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Websterr G, Kawaoka Y (1998) Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 72:7367–7373
Jaipuri FA, Collet BYM, Pohl NL (2008) Synthesis and quantitative evaluation of Glycero-d-manno-heptose binding to concanavalin A by fluorous-tag assistance. Ang Chem Inter Ed Engl 47:1707–1710
Jelinek R, Kolusheva S (2004) Carbohydrate biosensors. Chem Rev 104:5987–6015
Jeon SI, Lee JH, Andrade JD, De Gennes PG (1991) Protein–surface interactions in the presence of poly-ethylene oxideI. Simplified theory. J Colloid Interface Sci 142:149–158
Jorns J, Mangold U, Neumann U, Van Damme EJM, Peumans WJ, Pfuller U, Schumacher U (2003) Lectin histochemistry of the lymphoid organs of the chicken. Anat Embryol 207:85–94
Jouan A, Hatakeyama T, Murakami K, Miyamoto Y, Yamasaki N (1996) An assay for lectin activity using microtiter plate with chemically immobilized carbohydrates. Anal Biochem 237:188–192
Junge B, Heiker F-R, Kurz J, Muller L, Schmidt DD, Wunsche C (1984) Untersuchungen zur struktur des α-glucosidaseinhibitors acarbose. Carbohydr Res 128:235–268
Kanazawa KK, Gordon IJG (1985) The oscillation frequency of a quartz resonator in contact with liquid. Anal Chim Acta 175:99–105
Karamanska R, Clarke J, Blixt O, Macrae JI, Zhang JQ, Crocker PR, Laurent N, Wright A, Flitsch SL, Russell DA, Field RA (2008) Surface plasmon resonance imaging for real-time, label-free analysis of protein interactions with carbohydrate microarrays. Glycoconj J 25:69–74
King WH Jr (1964) Piezoelectric sorption detector. Anal Chem 36:1735–1739
Klassen H, Schwartz MR, Bailey AH, Young MJ (2001) Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci Lett 312:180–182
Kochetkov NK, Bochkov AF, Sokolavskaya TA, Snyatkova VJ (1971) Modifications of the orthoester method of glycosylation. Carbohydr Res 16:17–27
Kochetkov NK, Khorlin AJ, Bochkov AF (1967) A new method of glycosylation. Tetrahedron 23:693–707
Koenigs W, Knorr E (1901) Ueber einige derivate des traubenzuckers und der galactose. Ber Deut Chem Gesell 34:957–981
Kohn M, Wacker R, Peters C, Schröder H, Soulère L, Breinbauer R, Niemeyer CM, Waldmann H (2003) Staudinger ligation: a new immobilization strategy for the preparation of small-molecule arrays. Ang Chem Inter Ed Engl 42:5830–5834
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Ang Chem Inter Ed Engl 40:2004–2021
Kondo H, Ichikawa Y, Wong C-H (1992) β-Sialyl phosphite and phosphoramidite: synthesis and application to the chemoenzymic synthesis of CMP-sialic acid and sialyl oligosaccharides. J Am Chem Soc 114:8748–8750
Koochekpour S, Pilkington GJ, Merzak A (1995) Hyaluronic acid/CD44H interaction induces cell detachment and stimulates migration and invasion of human glioma cells in vitro. Int J Cancer 63:450–454
Laine RA (1994) A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 10(12) structures for a reducing hexasaccharide: the Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 4:759–767
Larsen K, Thygesen MB, Guillaumie F, Willats WGT, Jensen KJ (2006) Solid-phase chemical tools for glycobiology. Carbohydr Res 341:1209–1234
Laurent N, Voglmeir J, Wright A, Blackburn J, Pham NT, Wong SCC, Gaskell SJ, Flitsch SL (2008) Enzymatic glycosylation of peptide arrays on gold surfaces. ChemBioChem 9:1–5
Lebed K, Kulik AJ, Forró L, Lekka M (2006) Lectin–carbohydrate affinity measured using a quartz crystal microbalance. J Colloid Interface Sci 299:41–48
Lee JH, Jeong BJ, Lee HB (1997) Plasma protein adsorption and platelet adhesion onto comb-like PEO gradient surfaces. J Biomed Mater Res 34:105–114
Lee MR, Shin I (2005) Fabrication of chemical microarrays by efficient immobilization of hydrazide-linked substances on epoxide-coated glass surfaces. Angew Chem Int Ed Engl 44:2881–2884
Lee RT, Lee YC (1994) Neoglycoconjugates: Preparation and Applications. In: Lee RT, Lee YC (eds) Enhanced biochemical affinities of multivalent neoglycoconjugates. Academic Press, San Diego, pp 23–50
Lee YC, Lee RT (1995) Carbohydrate-protein interactions: basis of glycobiology. Acc Chem Res 28:321–327
Leonard D, Chevolot Y, Bucher O, Haenni W, Sigrist H, Mathieu HJ (1998) ToF-SIMS and XPS study of photoactivatable reagents designed for surface glycoengineering - part 2. N-[m-(3-(trifluoromethyl)diazirine-3-yl)phenyl]-4-(-3-thio(-1-D-galactopyrannosyl)-maleimidyl)butyramide (MAD-Gal) on diamond. Surf Interface Anal 26:793–799
Léonard D, Chevolot Y, Bucher O, Sigrist H, Mathieu H-J (1998) ToF-SIMS and XPS study of photoactivatable reagents designed for surface glycoengineering. Part I. N-(m-(3-(trifluoromethyl)diazirine-3-yl)phenyl)-4-maleimido-butyramide (mad) on silicon, silicon nitride and diamond. Surf Interface Anal 26:783–792
Léonard D, Chevolot Y, Heger F, Martins J, Crout DHG, Sigrist H, Mathieu HJ (2001) ToF-SIMS and XPS study of photoactivatable reagents designed for surface glycoengineering: part III: 5-carboxamidopentyl N-[m-[3-(trifluoromethyl) diazirin-3-yl] phenyl b-d-galactopyranosyl]-(1->4)-1-thio-b-d-glucopyranoside (lactose aryl diazirine) on diamond. Surf Interface Anal 31:457–464
Lesaicherre M-L, Uttamchandani M, Chena GYJ, Yao SQ (2002) Developing site-specific immobilization strategies of peptides in a microarray. Bioorg Med Chem Lett 12:2079–2083
Livingston PO (1995) Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside-KLH conjugate vaccines. Immunol Rev 145:147–166
Livingston PO, Ragupathi G (2006) Cancer vaccines targeting carbohydrate antigens. Hum Vaccin 2:137–143
Lundquist JJ, Toone EJ (2002) The cluster glycoside effect. Chem Rev 102:555–576
Mac Beath G, Schreiber SL (2000) Printing proteins as microarrays for high-throughput function determination. Science 289:1760–1763
Maeda K, Ueda M, Yagishita K, Kawaji S, Kondo S, Murase M, Takeuchi T, Okami Y, Umezawa H (1957) Studies of kanamycin. J Antibiotics Ser A 10:228–231
Majumdar SK, Kutzner HJ (1962) Studies on the biosynthesis of streptomycin. Appl Environ Microbiol 10:157–168
Mammen M, Choi S-K, Whitesides GM (1998) Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Ang Chem Inter Ed Engl 37:2754–2794
Manimala JC, Roach TA, Li Z, Gildersleeve JC (2006) High-throughput carbohydrate microarray analysis of 24 lectins. Ang Chem Inter Ed Engl 45:3607–3610
Manimala JC, Li Z, Jain A, Vedbrat S, Gildersleeve JC (2005) Carbohydrate array analysis of anti-Tn antibodies and lectins reveals unexpected specificities: implications for diagnostic and vaccine development. ChemBioChem 6:2229–2241
Martin TJ, Schmidt RR (1992) Efficient sialylation with phosphite as leaving group. Tetrahedron Lett 33:6123–6126
Matrosovich MN, Gambaryan AS, Teneberg S, Piskarev VE, Yamnikova SS, Lvov DK, Robertson JS, Karlsson K-A (1997) Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology 233:224–234
McConville MJ, De Souza D, Saunders E, Likic VA, Naderer A (2007) Living in a phagolysosome; metabolism of leishmania amastigotes. Trends Parasitol 23:368–375
McReynolds KD, Gervay-Hague J (2007) Chemotherapeutic interventions targeting HIV interactions with host-associated carbohydrates. Chem Rev 107:1533–1552
Mehta S, Pinto MB (1991) Phenylselenoglycosides as novel, versatile glycosyl donors. Selective activation over thioglycosides. Tetrahedron Lett 32:4435–4438
Mehta S, Pinto MB (1993) Novel glycosidation methodology. The use of phenyl selenoglycosides as glycosyl donors and acceptors in oligosaccharide synthesis. J Org Chem 58:3269–3276
Mendonça-Previato L, Gorin PAJ, Braga AF, Scharfstein J, Previato JO (1983) Chemical structure and antigenic aspects of complexes obtained from epimastigotes of Trypanosoma cruzi. Biochemistry 22:4980–4987
Merzak A, Koocheckpour S, Pilkington GJ (1994) CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res 54:3988–3992
Miller JL, Dewet BJM, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S (2008) The mannose receptor mediates dengue virus infection of macrophages. PLOS Pathog 4:e17
Miura Y, Sasao Y, Dohi H, Nishida Y, Kobayashi K (2002) Self-assembled monolayers of globotriaosylceramide (Gb3) mimics: surface-specific affinity with shiga toxins. Anal Biochem 310:27–35
Miura Y, Wada N, Nishida Y, Mori H, Kobayashi K (2004) Chemoenzymatic synthesis of glycoconjugate polymers starting from nonreducing disaccharides. J Polym Sci Part A Polym Chem 42:4598–4606
Morvan F, Meyer A, Jochum A, Sabin C, Chevolot Y, Imberty A, Praly J-P, Vasseur J-J, Souteyrand E, Vidal S (2007) Fucosylated pentaerythrityl phosphodiester oligomers (PePOs): automated synthesis of DNA-based glycoclusters and binding to Pseudomonas aeruginosa lectin (PA-IIL). Bioconjugate Chem 18:1637
Mukaiyama T, Murai Y, Shoda S-I (1981) An efficient method for glucosylation of hydroxy compounds using glycopyranosyl fluoride. Chem Lett 10:431–432
Muñoz EM, Yu H, Hallock J, Edens RE, Linhardt RJ (2005) Poly(ethylene glycol)-based biosensor chip to study heparin–protein interactions. Anal Biochem 343:176–178
Muratsugu M, Ohta F, Miya Y, Hosokawa T, Kurosawa S, Kamo N, Ikeda H (1993) Quartz crystal microbalance for the detection of microgram quantities of human serum albumin: relationship between the frequency change and the mass of protein adsorbed. Anal Chem 65:2933–2937
Muthana S, Yu H, Huang S, Chen X (2007) Chemoenzymatic synthesis of size-defined polysaccharides by sialyltransferase-catalyzed block transfer of oligosaccharides. J Am Chem Soc 129:11918–11919
Naderer T, Vince JE, Mcconville MJ (2004) Surface determinants of leishmania parasites and their role in infectivity in the mammalian host. Curr Mol Med 4:649–665
Nagase T, Nakata E, Shinkai S, Hamachi I (2003) Construction of artificial signal transducers on a lectin surface by post-photoaffinity-labeling modification for fluorescent saccharide biosensors. Chem Eur J 9:3660–3669
Nandi A, Estess P, Siegelman MH (2000) Hyaluronan anchoring and regulation on the surface of vascular endothelial cells is mediated through the functionally active form of CD44. J Biol Chem 275:14939–14948
Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier J-L, Arenzana-Seisdedos F, Desprès P (2003) Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep 4:723–728
Nepogodiev S, Stoddart JF (1998) Chemistry of cyclic oligosaccharides. In: Boons G-J (ed) Carbohydrate chemistry. Blackie Academic & Professional, New York, pp 322–383
Nicolaou KC, Smith AL (1992) Molecular design, chemical synthesis, and biological action of enediynes. Acc Chem Res 25:497–503
Nimrichter L, Gargir A, Gortler M, Alstock RT, Shtevi A, Weisshauss O, Fire E, Dotan N, Schnaar RL (2004) Intact cell adhesion to glycan microarrays. Glycobiology 14:197–203
Norde W (1985) Probing protein adsorption via microcalorimetry. In: Andrade JD (ed) Surface and interfacial aspects of biomedical polymers, vol. 2. Proteins adsorption. Plenum publishing, New York, pp 263–294
Norde W, Macritchie F, Nowicka G, Lyklema J (1986) Protein adsorption at solid-liquid interfaces: reversibility and conformation aspects. J Colloid Interface Sci 112:447–456
Okada H, Yoshida J, Sokabe M, Wakabayashi T, Hagiwara M (1996) Suppression of CD44 expression decreases migration and invasion of human glioma cells. Int J Cancer 66:255–260
Oostra M, De Haan CAM, De Groot RJ, Rottier PJM (2006) Glycosylation of the severe acute respiratory syndrome coronavirus triple-spanning membrane proteins 3a and M. J Virol 80:2326–2336
Palma AS, Feizi T, Zhang Y, Stoll MS, Lawson AM, Diaz-Rodrıguez E, Campanero-Rhodes MA, Costa J, Gordon S, Brown GD, Chai W (2006) Ligands for the β-Glucan Receptor, Dectin-1, Assigned Using “Designer” Microarrays of Oligosaccharide Probe (Neoglycolipids) Generated from Glucan Polysaccharides. J Biol Chem 281:5771–5779
Park S, Lee MR, Pyo SJ, Shin I (2004) Carbohydrate chips for studying high-throughput carbohydrate-protein interactions. J Am Chem Soc 126:4812–4819
Park S, Shin I (2007) Carbohydrate microarrays for assaying galactosyltransferase activity. Org Lett 9:1675–1678
Pei Z, Yu H, Theurer M, Walden A, Nilsson P, Yan M, Ramstrom O (2007) Photogenerated carbohydrate microarrays. ChemBioChem 8:166–168
Perez JM, Josephson L, O’ Loughlin T, Hogemann D, Weissleder R (2002) Magnetic relaxation switches capable of sensing molecular interactions. Nat Biotechnol 20:816–820
Perez JM, Simeone FJ, Saeki Y, Josephson L, Weissleder R (2003) Viral-induced self-assembly of magnetic nanoparticles allows the detection of viral particles in biological media. J Am Chem Soc 125:10192–10193
Perez JM, Simeone FJ, Tsourkas A, Josephson L, Weissleder R (2004) Peroxidase substrate nanosensors for MR imaging. Nano Lett 4:119–122
Perez S, Marchessault RH (1978) The exo-anomeric effect: experimental evidence from crystal structures. Carbohydr Res 65:114–120
Petitou M, Van Boeckel CAA (2004) A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Ang Chem Inter Ed Engl 43:3118–3133
Pigman W, Isbell HS (1968) Mutarotation of sugars in solution: part I. Adv Carbohydr Chem Biochem 23:11–57
Plante OJ, Palmacci ER, Seeberger PH (2001) Automated solid-phase synthesis of oligosaccharides. Science 291:1523–1527
Pohl NL, Kiessling LL (1999) Scope of multivalent ligand function: lactose-bearing neoglycopolymers by ring-opening metathesis polymerization. Synthesis 1515–1519
Pougny J-R, Sinay P (1976) Reaction d’imidates de glucopyranosyle avec l’acetonitrile. Applications synthetiques. Tetrahedron Lett 17:4073–4076
Praly J-P, Lemieux RU (1987) Influence of solvent on the magnitude of the anomeric effect. Can J Chem 65:213–223
Pries AR, Secomb TW, Gaehtgens P (2000) The endothelial surface layer. Pflug Archiv Eur J Physiol 440:653–666
Qian X, Sujino K, Ratcliffe RM, Palcic MM (2001) Glycosyltransferases in oligosaccharide synthesis. In: Wang PG, Bertozzi CR (eds) Glycochemistry: principles, synthesis and applications. Marcel Dekker, New York, pp 535–566
Rasooly A (2005) Biosensor technologies. Methods 37:1–3
Ratner DM, Adams EW, Su J, O’keefe BR, Mrksich M, Seeberger PH (2004) Probing protein-carbohydrate interactions with microarrays of synthetic oligosaccharides. ChemBioChem 5:379–383
Ratner DM, Seeberger PH (2007) Carbohydrate microarrays as tools in HIV glycobiology. Curr Pharm Des 11:173–183
Raynal F, Plaza F, Beuf A, Carrière P, Souteyrand E, Martin J-R, Cloarec J-P, Cabrera M (2004) Study of a chaotic mixing system for DNA chip hybridization chambers. Phys Fluids 16:63–66
Robinson A, Fang J-M, Chou P-T, Liao K-W, Chu R-M, Lee S-J (2005) Probing lectin and sperm with carbohydrate-modified quantum dots. ChemBioChem 6:1899–1905
Rogers GN, Paulson JC, Daniels RS, Skehel JJ, Wilson IA, Wiley DC (1983) Single amino acid substitutions in Influenza haemagglutinin change receptor binding specificity. Nature 304:76–78
Rogers GN, D’souza BL (1989) Receptor binding properties of human and animal H1 influenza virus isolates. Virology 173:317–322
Rogers GN, Daniels RS, Skehel JJ, Wiley DC, Wang XF, Higa HH, Paulson JC (1985) Host-mediated selection of influenza virus receptor variants. Sialic acid-alpha 2, 6Gal-specific clones of A/duck/Ukraine/1/63 revert to sialic acid-alpha 2, 3Gal-specific wild type in ovo. J Biol Chem 260:7362–7367
Rogers GN, Paulson JC (1983) Receptor determinants of human and animal Influenza virus isolates: differences in receptor specificity of H3 hemagglutinin based on species of origin. Virology 127:361–373
Rojo J, Morales JC, Penades S (2002) Carbohydrate-carbohydrate interactions in biological and model systems. Host-guest chemistry. Springer-Verlag, Berlin, pp 45–92
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Ang Chem Inter Ed Engl 41:2596–2599
Ruegg C, Mariotti A (2003) Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cell Mol Life Sci 60:1135–1157
Ruiz L, Fine E, Vörös J, Makohliso SA, Léonard D, Johnston DS, Textor M, Mathieu HJ (1999) Phosphorylcholine-containing polyurethanes for the control of protein adsorption and cell attachment via photoimmobilized laminin oligopeptides. J Biomater Sci Pol Ed 10:931–955
Sandström C, Berteau O, Gemma E, Oscarson S, Kenne L, Gronenborn AM (2004) Atomic mapping of the interactions between the antiviral agent cyanovirin-N and oligomannosides by saturation-transfer difference NMR. Biochemistry 43:13926–13931
Sato T, Serizawa T, Ohtake F, Nakamura M, Terabayashi T, Kawanishi Y, Okahata Y (1998) Quantitative measurements of the interaction between monosialoganglioside monolayers and wheat germ agglutinin (WGA) by a quartz-crystal microbalance. Biochim Biophys Acta 1380:82–92
Satoh A, Fukui E, Yoshino S, Shinoda M, Kojima K, Matsumoto I (1999) Comparison of methods of immobilization to enzyme-linked immunosorbent assay plates for the detection of sugar chains. Anal Biochem 275:231–235
Satoh A, Matsumoto I (1999) Analysis of interaction between lectin and carbohydrate by surface plasmon resonance. Anal Biochem 275:268–270
Sauerbrey GZ (1959) Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z Phys 155:206–222
Scanlan CN, Offer J, Zitzmann N, Dwek RA (2007) Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature 446:1038–1045
Schena M (2003) Microarray analysis. Wiley, Hoboken
Schmidt RR (1986) New methods for the synthesis of glycosides and oligosaccharides - Are there alternatives to the Koenigs-Knorr method? [New Synthetic Methods (56)]. Ang Chem Inter Ed Engl 25:212–235
Schmidt RR, Michel J (1980) Facile synthesis of α- and β-O-glycosyl imidates; preparation of glycosides and disaccharides. Ang Chem Inter Ed Engl 19:731–732
Schreiber F (2000) Structure and growth of self-assembling monolayers. Prog Surf Sci 65:151–256
Schumacher R (1990) The quartz microbalance: a novel approach to the in-situ investigation of interfacial phenomena at the solid/liquid junction. Ang Chem Inter Ed Engl 29:329–343
Schwarz M, Spector L, Gargir A, Shtevi A, Gortler M, Altstock RT, Dukler AA, Dotan N (2003) A new kind of carbohydrate array, its use for profiling antiglycan antibodies, and the discovery of a novel human cellulose-binding antibody. Glycobiology 13:749–754
Seeberger PH, Werz DB (2007) Synthesis and medical applications of oligosaccharides. Nature 446:1046–1051
Seeberger PH (2008) Automated oligosaccharide synthesis. Chem Soc Rev 37:19–28
Seibel J, Hellmuth H, Hofer B, Kicinska A-M, Schmalbruch B (2006) Identification of new acceptor specificities of glycosyltransferase with the aid of substrate microarrays. ChemBioChem 7:310–320
Seo JH, Adachi K, Lee BK, Kang DG, Kim YK, Kim KR, Lee HY, Kawai T, Cha HJ (2007) Facile and rapid direct gold surface immobilization with controlled orientation for carbohydrates. Bioconjugate Chem 18:2197–2201
Sharon N, Lis H (2004) History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14:53R–62R
Shin I (2007) Carbohydarte microarray for high-throughput analysis of carbohydrate protein interaction. In: Bewley CA (ed) Protein-carbohydrate interactions in infectious diseases. RCS, Cambridge, pp 221–247
Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y (2006) Avian flu: influenza virus receptors in the human airway. Nature 440:435–436
Skehel JJ, Wiley DC (2000) Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69:531–569
Smith EA, Thomas WD, Kiessling LL, Corn RM (2003) Surface plasmon resonance imaging studies of protein-carbohydrate interactions. J Am Chem Soc 125:6140–6148
Souteyrand E, Chen C, Cloarec J-P, Nesme X, Simonet P, Navarro I, Martin J-R (2000) Comparaison between the electrochemical and optoelectrochemical impedance measurement for the detection of DNA hybridization. Appl Biochem Biotechnol 89:246–251
Souteyrand E, Cloarec J-P, Martin J-R, Wilson C, Lawrence I, Mikkelsen S, Lawrence MF (1997) Direct detection of the hybridization of specific DNA sequences by field effect. J Phys Chem 101:2980–2985
Sperandio M (2006) Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J 273:4377–4389
Stevens J, Blixt O, Glaser L, Taubenberger JK, Palese P, Paulson JC, Wilson IA (2006) Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J Mol Biol 355:1143–1155
Stoll MS, Feizi T, Loveless RW, Chai W, Lawson AM, Yuen C-T (2000) Fluorescent neoglycolipids improved probes for oligosaccharide ligand discovery. Eur J Biochem 267:1795–1804
Su J, Mrksich M (2002) Using mass spectrometry to characterize self-assembled monolayers presenting peptides, proteins, and carbohydrates. Ang Chem Inter Ed Engl 41:4715–4718
Tamborrini M, Werz DB, Frey J, Pluschke G, Seeberger PH (2006) Anti-carbohydrate antibodies for the detection of anthrax spores. Ang Chem Inter Ed Engl 45:6581–6582
Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA (2003) DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 197:823–829
Taylor PR, Gordon S, Martinez-Pomares L (2005) The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol 26:104–110
Thoegersen H, Lemieux RU (1982) Further justification of the exo-anomeric effect. Conformational analysis based on nuclear magnetic resonance. Can J Chem 60:44–57
Tornoe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1, 2, 3]-triazoles by regiospecific copper(I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67:3057–3064
Tsatas D, Kanagasundaram V, Kaye A, Novak U (2002) EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J Clin Neurosci 9:282–288
Turnbull WB, Stoddart JF (2002) Design and synthesis of glycodendrimers. Rev Mol Biotechnol 90:231–255
Updike SJ, Hicks GP (1967) The enzyme electrode. Nature 214:986–988
Van Kerhof JC, Bergveld P, Schasfoort RBM (1993) Development of an ISFET based heparin sensor using the ion-step measuring method. Biosens Bioelectron 8:463–472
Vankayalapati H, Jiang S, Singh G (2002). Glycosylation based on glycosyl phosphates as glycosyl donors. Synlett 16–25
Varki A (1993) Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3:93–130
Varki A, Cummings R, Esko J, Freeze H, Hart GW, Marth J (1999) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, New York
Veeneman GH (1998) Chemical synthesis of O-glycosides. In: Boons G-J (ed) Carbohydrate chemistry. Blackie Academic & Professional, New York, pp 98–174
Vroman L (1962) Effect of adsorbed proteins on the wettability of hydrophilic and hydrophobic solids. Nature 196:476–477
Wacker R, Schröder H, Niemeyer CM (2004) Performance of antibody microarrays fabricated by either DNA-directed immobilization, direct spotting, or streptavidin–biotin attachment: a comparative study. Anal Biochem 330:281–287
Wanderley JL, Moreira MEC, Benjamin A, Bonomo AC, Barcinski MA (2006) Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol 176:1834–1839
Wang D (2003) Carbohydrate microarrays. Proteomics 3:2167–2175
Wang D, Liu S, Trummer BJ, Deng C, Wang A (2002) Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat Biotechnol 20:275–281
Werz DB, Seeberger PH (2005a) Carbohydrates as the next frontier in pharmaceutical research. Chemistry 11:3194–3206
Werz DB, Ranzinger R, Herget S, Adibekian A, Von Der Lieth C-W, Seeberger PH (2007) Exploring the structural diversity of mammalian carbohydrates (glycospace) by statistical databank analysis. Chem Biol 2:685–691
Werz DB, Seeberger PH (2005b) Total synthesis of antigen Bacillus anthracis tetrasaccharide—creation of an anthrax vaccine candidate. Ang Chem Inter Ed Engl 44:6315–6318
Whitfield DM, Douglas SP (1996) Glycosylation reactions: present status future directions. Glycoconj J 13:5–17
Wink T, Van Zuilen SJ, Bult A, Van Bennekom WP (1997) Self-assembled monolayers for biosensors. Analyst 122:43R–50R
Yamada KM, Araki M (2001) Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci 114:2375–2382
Yanagisawa M, Taga TKN, Ariga T, Yu RK (2005) Characterization of glycoconjugate antigens in mouse embryonic neural precursor cells. J Neurochem 95:1311–1320
Yanagisawa M, Yu RK (2007) The expression and functions of glycoconjugates in neural stem cells. Glycobiology 17:57R–74R
Yanagishita M, Hascall VC (1992) Cell-surface heparan-sulfate proteoglycans. J Biol Chem 267:9451–9454
Yates EA, Terry CJ, Rees C, Rudd TR, Duchesne L, Skidmore MA, Lévy R, Thanh NTK, Nichols RJ, Clarke DT, Fernig DG (2006) Protein–GAG interactions: new surface-based techniques, spectroscopies and nanotechnology probes. Biochem Soc Trans 34:427–430
Yeung C, Leckband D (1997) Molecular level characterization of microenvironmental influences on the properties of immobilized proteins. Langmuir 13:6746–6754
Young NM, Gidney MAJ, Gudmundsson BME, Mackenzie CR, To R, Watson DC, Bundle DR (1999) Molecular basis for the lack of mimicry of Brucella polysaccharide antigens by Ab2g antibodies. Mol Immunol 36:339–347
Yu H, Chokhawala HA, Huang S, Chen X (2006) One-pot three-enzyme chemoenzymatic approach to the synthesis of sialosides containing natural and non-natural functionalities. Nat Protocols 1:2485–2492
Yu RK, Bieberich E, Xia T, Zeng GC (2004) Regulation of ganglioside biosynthesis in the nervous system. J Lipid Res 45:783–793
Yu RK, Yanagisawa M (2006) Glycobiology of neural stem cells. CNS Neurol Disord Drug Targets 5:415–423
Zhang SC (2001) Defining glial cells during CNS development. Nat Rev Neurosci 2:840–843
Zhang Y, Luo S, Tang Y, Yu L, Hou K-Y, Cheng J-P, Zeng X, Wang PG (2006) Carbohydrate-protein interactions by “clicked” carbohydrate self-assembled monolayers. Anal Chem 78:2001–2008
Zhang Z, Wong C-H (2000) Glycosylation methods: use of phosphites. In: Ernst B, Hart GW, Sinay P (eds) Carbohydrates in chemistry and biology. Wiley-VCH, Weinheim, pp 117–134
Zhou X, Zhou J (2006) Oligosaccharide microarrays fabricated on aminooxyacetyl functionalized glass surface for characterization of carbohydrate–protein interaction. Biosens, Bioelectron
Zou W, Mackenzie R, Therien L, Hirama T, Yang Q, Gidney MA, Jennings HJ (1999) Conformational epitope of the type III group B Streptococcus capsular polysaccharide. J Immunol 163:820–825
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Chevolot, Y., Vidal, S., Laurenceau, E., Morvan, F., Vasseur, JJ., Souteyrand, E. (2010). Carbohydrates as Recognition Receptors in Biosensing Applications. In: Zourob, M. (eds) Recognition Receptors in Biosensors. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-0919-0_7
Download citation
DOI: https://doi.org/10.1007/978-1-4419-0919-0_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-0918-3
Online ISBN: 978-1-4419-0919-0
eBook Packages: EngineeringEngineering (R0)