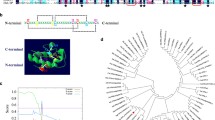
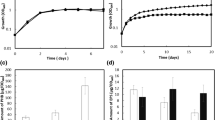
An osmotic stress, such as excessive salinity or drought, has deleterious effects on the establishment and maintenance of symbiosis between Sinorhizobium meliloti and its host plant alfalfa. Bacteria protect themselves against high external osmolarity by accumulating osmoprotective organic compounds in their cytoplasm, the so-called compatible solutes. Although glycine betaine (GB) is one of the most effective osmoprotectant used by bacteria, proline betaine (PB) is the major betaine produced by alfalfa. PB is secreted by germinating seedlings of many Medicago species and is found in roots, nodules, and bacteroids. S. meliloti has the capacity to use betaines as compatible solutes but also as carbon and nitrogen sources. Three different transport systems (two ABC transporters and one BCCT transporter) are involved in the uptake of PB and GB in S. meliloti. BetX, a periplasmic binding protein encoded by a gene located in a PB catabolic locus, has been characterized. Although the betX gene does not belong to an ABC transporter operon, BetX contributes to the binding and uptake of PB and GB. Further, BetX is not associated with the already characterised betaine ABC transporters and the protein-membrane complex with which it is associated is unknown. Expression analysis of a betX-lacZ fusion revealed induction by PB, mono-methyl-proline (MMP, an intermediate in PB catabolism), and salt stress, suggesting that BetX has a catabolic and osmoprotection role. BetR, a TetR-like regulator is the betX expression repressor in the absence of ligand, and the repression is abolished by addition of substrate (MMP or PB). However, betX induction by osmotic stress is independent of BetR, suggesting an additional level of regulation. A betX mutant shows a defect in growth capacity but osmoprotection by PB and GB was unchanged due to the presence of other betaine transporters in S. meliloti. Nodulation capacity and nitrogen-fixation activity of a betX mutant was not affected.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Springer Science + Business Media B.V.
About this paper
Cite this paper
Sagot, B., Alloing, G., Hérouart, D., Le Rudulier, D., Dupont, L. (2008). Identification of BetX, a Periplasmic Protein Involved in Binding and Uptake of Proline Betaine and Glycine Betaine in Sinorhizobium meliloti. In: Dakora, F.D., Chimphango, S.B.M., Valentine, A.J., Elmerich, C., Newton, W.E. (eds) Biological Nitrogen Fixation: Towards Poverty Alleviation through Sustainable Agriculture. Current Plant Science and Biotechnology in Agriculture, vol 42. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-8252-8_101
Download citation
DOI: https://doi.org/10.1007/978-1-4020-8252-8_101
Publisher Name: Springer, Dordrecht
Print ISBN: 978-1-4020-8251-1
Online ISBN: 978-1-4020-8252-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)