Abstract
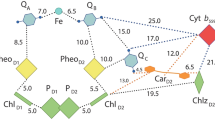
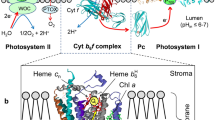
Three Prosthetic Groups, Heme CN, Chl A, And β-Carotene Were Identified In Crystal Structures Of The Cytochrome B 6 F Complex In The Thermophilic Cyanobacterium, M. Laminosus (Kurisu Et Al. 2003) And The Green Alga, C. Reinhardtii (Stroebel Et Al. 2003). The Functions Of These Groups Are Still Not Understood. A Native Structure Of The Cytochrome B 6 F Complex From The Thermophilic Cyanobacterium, M. Laminosus, Was Obtained From Crystals Grown With Divalent Cadmium (Pdb Accession: 2E74; (Yamashita Et Al. 2007) ). One Cd2+ Binding Site Bridges His143 Of Cytochrome F And The Acidic Residue, Glu75, Of Cyt B 6; (Ii) A Second Site Has Three Identified Ligands, Asp58 (Subunit Iv), Glu3 (Petg Subunit) And Glu4 (Petm Subunit). Binding Sites Of Quinone Analogue Inhibitors Map The Transfer Pathway Of The Lipophilic Quinone Across The Complex. Two Sites Were Found For The Chromone Ring Of The Tridecyl-Stigmatellin (Tds) Quinone Analogue Inhibitor, One Near The P-Side [2Fe-2S] Cluster (Pdb: 2E76). A Second Tds Site Faces The Quinone Exchange Cavity As An Axial Ligand Of Heme C N. A Similar Binding Site As An Axial Ligand To Heme C N Was Found For The N-Side Quinone Analogue Inhibitor, Nqno (Pdb: 2E75). Binding Of These Inhibitors Required Their Addition Before That Of The Lipid Used To Facilitate Crystallization. Binding Of Nqno And Tds As Axial Ligands To Heme C N Implies That C N Utilizes Plastoquinone As A Natural Ligand, Thus Defining An N-Side Electron Transfer Compleand Pq In Thex Consisting Of Hemes B N, C N, And Pq In The Reduction Pathway Of Pq In The Cavity. Strong Coupling Of Hemes Bn And Cn Suggests A Mechanism For 2 Electron Reduction Of Pq, Thus Avoiding The Generation Of Plastosemiquionone And Reactive Oxygen Species. The Nqno Binding Site Explains Several Experimental Observations Associated With Its Inhibitory Action: A Negative Shift In The Heme C N EM (Alric Et Al. 2005), Increased Amplitude Of Light-Induced Reduction Of Heme B N (Jones And Whitmarsh 1988; Furbacher Et Al. 1989), And G Value Shifts In The Epr Spectrum Attributed To Interaction Between Hemes C N And B N (Zatsman Et Al. 2006; Baymann Et Al. 2007). These Structures Suggest Pathways For H+ Uptake And Potential Site(S) Of Ferredoxin Binding.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Alric J, Pierre Y, Picot D, Lavergne J, Rappaport F (2005) Spectral and redox characterization of the heme Ci of the cytochrome b6 f complex. Proc Natl Acad Sci USA 102:15860-15865.
Baymann F, Giusti F, Picot D, Nitschke W (2007) The ci/bH moiety in the b6 f complex studied by EPR: A pair of strongly interacting hemes. Proc Natl Acad Sci USA 104:519-524.
Cramer WA, Zhang H, Yan J, Kurisu G, Smith JL (2006) Trans-membrane traffic in the cytochrome b6 f complex. Annu Rev Biochem 75:769-790.
Furbacher PN, Girvin ME, Cramer WA (1989). On the question of interheme electron transfer in the chloroplast cytochrome b6 in situ. Biochemistry 28:8990-8998.
Joliot P, Joliot A (2005) Quantification of cyclic and linear flows in plants. Proc Natl Acad Sci USA 102:4913-4918.
Jones R, Whitmarsh J (1988) Inhibition of electron transfer and electrogenic reaction in the cytochrome b/f complex by 2-n-nonyl-4-hydroxyquinoline N-oxide (NQNO) and 2,5-dibromo-3methyl-6-isopropyl-p-benzoquinone (DBMIB). Biochim Biophys Acta 933:258-268.
Kurisu G, Zhang H, Smith JL, Cramer WA (2003) Structure of the cytochrome b6 f complex of oxygenic photosynthesis: Tuning the cavity. Science 302:1009-1014.
Stroebel D, Choquet Y, Popot J-L, Picot D (2003) An atypical heam in the cytochrome b6 f complex. Nature 426:413-418.
Whitelegge JP, Zhang H, Taylor R, Cramer WA (2002) Full subunit coverage liquid chromatography electrospray-ionization mass spectrometry (LCMS+) of an oligomeric membrane protein complex: the cytochrome b6 f complex from spinach and the cyanobacterium, M. laminosus. Mol Cell Proteomics 1:816-827.
Yamashita E, Zhang H, Cramer, WA (2007) Structure of the cytochrome b6 f complex: Quinone analogue inhibitors as ligands of heme cn. J Mol Biol 370:39-52.
Yu J, Le Brun NE (1998) Studies of the cytochrome subunits of menaquinone: Cytochrome c reductabase (bc complex) of Bacillus subtilis. J Biol Chem 273:8860-8866.
Zatsman AI, Zhang H, Gunderson WA, Cramer WA, Hendrich M (2006) Heme-heme interactions in the cytochrome b6 f complex: EPR spectroscopy and correlation with structure. J Am Chem Soc 128:14246-14247.
Zhang H, Whitelegge JP, Cramer WA (2001) Ferredoxin: NADP+ oxidoreductase is a subunit of the chloroplast cytochrome b6 f complex. J Biol Chem 276:38159-38165.
Author information
Authors and Affiliations
Editor information
Rights and permissions
Copyright information
© 2008 Springer Science + Business Media, B.V.
About this paper
Cite this paper
Yamashita, E., Zhang, H., Baniulis, D., Cramer, W.A. (2008). Structure of the Cytochrome b 6 f Complex: n-Side Donor Pathway to the Plastoquinone Pool. In: Allen, J.F., Gantt, E., Golbeck, J.H., Osmond, B. (eds) Photosynthesis. Energy from the Sun. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-6709-9_128
Download citation
DOI: https://doi.org/10.1007/978-1-4020-6709-9_128
Publisher Name: Springer, Dordrecht
Print ISBN: 978-1-4020-6707-5
Online ISBN: 978-1-4020-6709-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)