Abstract
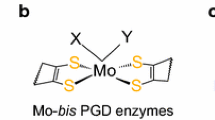
Knowledge of the ligand environment of molybdenum in its enzymes is, to say the least, scanty. Such evidence as there is, based on comparisons between the e.p.r. parameters of, for example, xanthine oxidase and molybdenum complexes of sulphur ligands (see table 23, page 216), is thought to indicate molybdenum-sulphur binding. Most attention has been concentrated on cysteine as the obvious candidate for the role of a sulphur-containing biological ligand. Interest has also been shown in the potential alternative binding site for molybdenum, namely a flavin moiety, on the grounds that several of the molybdenum enzymes are metaloflavoproteins.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Copyright information
© 1975 Palgrave Macmillan, a division of Macmillan Publishers Limited
About this chapter
Cite this chapter
Mcauliffe, C.A. (1975). Model Studies. In: McAuliffe, C.A. (eds) Techniques and Topics in Bioinorganic Chemistry. Aspects of Inorganic Chemistry. Palgrave Macmillan, London. https://doi.org/10.1007/978-1-349-02253-3_15
Download citation
DOI: https://doi.org/10.1007/978-1-349-02253-3_15
Publisher Name: Palgrave Macmillan, London
Print ISBN: 978-1-349-02255-7
Online ISBN: 978-1-349-02253-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)