Abstract
Contact with the skin is inevitable or desirable for daily life products such as cosmetics, hair dyes, perfumes, drugs, household products, and industrial and agricultural products. Whereas the majority of these products are harmless, a number can become metabolized and/or activate the immunological defense via innate and adaptive mechanisms resulting in sensitization and allergic contact dermatitis upon following exposures to the same substance. Therefore, strict safety (hazard) assessment of actives and ingredients in products and drugs applied to the skin is essential to determine I) whether the chemical is a potential sensitizer and if so II) what is the safe concentration for human exposure to prevent sensitization from occurring. Ex vivo skin is a valuable model for skin penetration studies but due to logistical and viability limitations the development of in vitro alternatives is required. The aim of this review is to give a clear overview of the organotypic in vitro skin models (reconstructed human epidermis, reconstructed human skin, immune competent skin models incorporating Langerhans Cells and T-cells, skin-on-chip) that are currently commercially available or which are being used in a laboratory research setting for hazard assessment of potential sensitizers and for investigating the mechanisms (sensitization key events 1–4) related to allergic contact dermatitis. The limitations of the models, their current applications, and their future potential in replacing animals in allergy-related science are discussed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
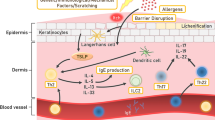
Approximately 15–20% of the general population have allergic contact dermatitis (ACD) resulting from environmental chemical exposure, making this skin disease a major health problem. ACD is a delayed-type hypersensitivity immune reaction mediated by T-cells, resulting from repeated exposure of the skin to an allergen (Peiser et al. 2012). Development occurs in two phases: in the first (induction) phase, exposure to a chemical allergen causes immunological priming known as skin sensitization. The second (elicitation) phase is triggered if a sensitized person is again exposed to the same allergen (or a cross-reactive allergen). Therefore, prevention of ACD should be prioritized, warranting extensive efforts into understanding the cellular mechanisms of sensitization in order to identify sensitizers, predict the potency of a sensitizer, and most importantly to predict the safe (no response) concentration of a potential sensitizer. The first part in the process leading to sensitization is the penetration of a chemical hapten through the Stratum corneum of the skin into the viable layers of the underlying epidermis (Fig. 1, key event 1). Here, the chemical will activate keratinocytes to secrete cytokines, e.g., IL1a, IL-18 (key event 2) attracting dendritic cells (DCs). After exposure to an allergen or an irritant, Langerhans cells (LCs) residing in the epidermis migrate toward the dermis. During sensitization, but not irritation, haptens penetrating the skin either directly activate LC or form a hapten–carrier protein complex that is taken up by skin-resident LC. These hapten-loaded LCs will change into a mature phenotype by upregulating their surface maturation markers such as CXCR4, CD86, and CD83, start increasing their CXCL 8 secretion, and begin their migration from the epidermis to the dermis. In the dermis, they further continue to mature and now also start upregulating CCR7 which enables them together with CXCR4 to migrate toward the lymph nodes (key event 3).
Diagram showing adverse outcome pathway for sensitization. (Key event 1) The penetration of a chemical hapten through the skin’s S. corneum into the viable layers of the underlying epidermis; (Key event 2) Activation of keratinocytes resulting in cytokine secretion, e.g., IL1a, IL-18, TNF-a; (Key event 3) Langerhans cell (LC) activation (migration: upregulation of CCR7 (CCL19, CCL21) and CXCR4 (CXCL12) and maturation: upregulation of CD83 and CD86) directly or by a hapten–carrier protein complex); and (Key event 4) Presentation of the antigen by matured LC to antigen-responsive T-lymphocytes in the draining lymph nodes which stimulate proliferation and maturation of T-lymphocytes into primed effector and memory T-lymphocytes are shown
The central event in immune sensitization is the presentation of antigen by DC to antigen-responsive T-cells in the local lymph node which results in T-cell priming (memory) (key event 4). This process is now thought to be orchestrated by the activation and maturation state of DC and their cytokine and chemokine products, and also by factors released by other cell types, including keratinocytes and fibroblasts.
In the past, animals were used to determine whether or not a chemical was a potential sensitizer. The murine local lymph node assay (LLNA) and the guinea pig Buehler test were the most frequently used tests (Api et al. 2015). However, these animal models were subject to ethical considerations and gave a number of false positives and negatives most probably due to differences between human and animal skin physiology and immunity (Seok et al. 2013; Mak et al. 2014). Since the complete European marketing ban in 2013 for risk assessment of cosmetic products which contain ingredients that have been tested on animals, there has been an urgent need to develop alternative methods to identify chemical sensitizers. Because of the high complexity of skin sensitization biology, as documented in the OECD adverse outcome pathway (AOP), only a combination of test methods covering key events 1–4 are expected to lead to a test strategy that will enable the industry to conduct safety assessment of chemicals regarding sensitization potential and potency without the use of animals (Rovida et al. 2015; OECD 2012) (Fig. 1).
Therefore, a major worldwide objective is to develop and provide alternatives to animal testing for the prediction of the skin-sensitizing potential of chemicals within test strategies. The most promising in vitro methods available at this time are the direct peptide reactivity assay (DPRA) (Gerberick et al. 2004), KeratinoSens™ (Delaine et al. 2011; Andreas et al. 2011; Natsch et al. 2010), the Myeloid U937 skin sensitization test (MUSST) (Ade et al. 2006), and the cell line activation test h-CLAT (Ashikaga et al. 2006). However, major limitations have already been identified in current assays which use conventional submerged skin culture. These include solubility problems in particular hydrophobic compounds such as oils and surfactants, and how to test mixtures. These limitations may be overcome by developing skin models which more closely represent human skin physiology. Since a major route of penetration of substances applied to the skin is via the S. corneum of a differentiated epidermis, there is increasing interest in developing organotypic skin models for allergenicity testing. This review describes the current state of the art for organotypic skin models which are being developed for hazard assessment of potential sensitizers. An overview of the use of ex vivo skin for allergenicity testing is described, followed by the progress on developing cultured organotypic models of increasing complexity ranging from reconstructed human epidermis to immune competent skin models with integrated Langerhans cells or T-cells. The common feature of these models is that they are all cultured at the air–liquid interface which promotes epidermal differentiation and stratification, thus enabling chemicals to be applied topically to the S. corneum, mimicking human exposure, and enabling exposure of hydrophobic compounds.
2 Ex Vivo Skin
Freshly excised skin obtained from standard surgical procedures is a valuable source of intact human skin for allergy research (Rustemeyer et al. 2003; Jacobs et al. 2004, 2006; Lehe et al. 2003; Pistoor et al. 1996; Schmook et al. 2001). Ex vivo skin has a relatively intact barrier function most suitable for penetration studies and contains LC which can become activated and migrate from the epidermis into the dermis upon chemical exposure (Fig. 2). Explant skin cultures have been used to study the characteristics of chemical-induced migration of LC either as full thickness human organotypic skin explant cultures (hOSEC) (Rustemeyer et al. 2003; Lehe et al. 2003; Pistoor et al. 1996; Jacobs et al. 2002) or as a partial thickness epidermal explant culture (Ouwehand et al. 2008). A more accelerated migration of LC out of the epidermis occurs upon treatment with contact allergens compared to non-sensitizers (Lehe et al. 2003). Organotypic skin explant cultures (with methyl-green pyronine labeled KC RNA?) derived from human skin, porcine skin, or rabbit skin were able to predict the irritant hazard potential of 22 chemicals with 100, 95, and 93% accuracy, respectively (Jacobs et al. 2002). The major problem with implementing ex vivo skin models outside of the field of basic research is the logistics of supply of fresh skin to the laboratory and the short viability period of the skin (<24 h), particularly if functional LC maturation and migration readouts are required. Furthermore, the skin is subject to donor variability due to the undefined numbers and types of cells present making it very difficult to make into a standardized assay.
Histology (hematoxylin–eosin staining) of (a) native skin (biopsy), (b) reconstructed human epidermis (RhE) on a polycarbonate filter, (c) reconstructed epidermis on fibroblast-populated dermis (RhS), and (d) An HLA-DR immunohistochemical staining of MUTZ-LC integrated in reconstructed epidermis on fibroblast-populated collagen hydrogel (RhS-LC) (stratum corneum was lost during immunohistochemical staining procedure)
3 Reconstructed Human Epidermis (RhE)
RhE is the simplest form of organotypic skin model with an epidermal barrier function. RhE is constructed by seeding keratinocytes onto a porous membrane (transwell). After several days of submerged culture, the RhE is exposed to the air from above with the culture medium below. This method of culture stimulates the basal keratinocytes to differentiate and migrate upward to form a stratified epidermis with S. corneum (Fig. 2). The method is easily standardized and this has resulted in a number of commercial RhE now being available (Table 1). The EpiSkin™, EpiDerm™, SKinEthic™RhE, and EpiCs® RhE models have already been validated assays for skin irritation and skin corrosion testing according to the OECD test guidelines 439 and 432, respectively (Cottrez et al. 2015, 2016; Saito et al. 2013; Andres et al. 2017; Gibbs et al. 2013). RhE can be used to investigate key sensitization events 1 and 2 in which penetration of a chemical hapten through the S. corneum into the viable layers of the epidermis is the first step of the sensitization process. Keratinocytes respond by secreting cytokines such as IL-1 α, TNF-α, and IL-18 (Fig. 1). The prediction (label) and quantification (potency, characterization) of the sensitization hazard of various sensitizers and non-sensitizers have been tested in a number of studies using different biomarker readouts. In the SENS-IS and EpiSensA assays, changes in gene expression are analyzed upon topical exposure with a chemical (Table 1). The SENS-IS assay has been studied most extensively using the EpiSkin™ model. In a ring study with three participating laboratories, 150 chemicals were tested by analyzing the expression of a carefully selected panel of 65 genes (Cottrez et al. 2015, 2016). This study highlighted the transferability and robustness (repeatability) of the SENS-IS assay to detect sensitizers with a high predictivity and 100% reproducibility between laboratories. The sensitivity, specificity, and accuracy of this assay were all well above 90% compared to the LLNA, guinea pig, and human data based on literature sources obtained by Basketter et al. (2014), suggesting it to be a serious alternative to in vivo sensitization testing. It could be argued that the use of such a broad set of genes will increase expense and complicate implementation in a broad setting. The EpiSensA assay is also based on gene analysis (Saito et al. 2013, 2017). However, only the predictive performance of five genes, all related to cellular stress, is examined. Three out of five genes, namely ATF3, DNAJB4, and GCLM, but not HSPA6 and HSPH1 showed a high accuracy, 100, 93.8, and 87.5, respectively, when tested with 16 chemicals suggesting that they could be useful markers for skin sensitization. Most probably because of the smaller set of gene biomarkers used, the overall sensitivity, specificity, and accuracy were slightly lower compared to the SENS-IS assay. The assay was developed making use of the MaTtek EpiDerm™ model (Saito et al. 2013). Later, the assay was also performed in the LabCyte EPI-MODEL24 SIT. IL-8 was added to the set of genes that were analyzed with the goal of decreasing false negatives (Saito et al. 2017). A total of 72 chemicals (43 hydrophilic chemicals and 29 lipophilic chemicals including 11 pre/pro-haptens) were evaluated this time. The sensitivity, specificity, and accuracy were 93, 100, and 93% for lipophilic chemicals and 96, 75, and 88% for hydrophilic chemicals. These values are higher than values obtained from existing validated in vitro tests (DPRA, h-CLAT, and KeratinoSens) (Saito et al. 2017) which did not distinguish between hydrophilic and lipophilic chemicals. Also, most probably different panels of test chemicals were used making direct comparisons difficult. Five sensitizers (OXA, Benzoyl peroxide, benzyl cinnamate, lilial, and tridecane) were positive only for the induction of IL-8. Three of these five sensitizers were lipophilic, showing a high capability of the EpiSensA assay to detect lipophilic chemicals and thereby overcoming one of the major limitations of the existing validated in vitro tests. Also, all of the 11 tested pre/pro-haptens, including 6 hydrophilic pre/pro-haptens (Isoeugenol, ethylene diamine, diethylene triamine, resorcinol, cinnamic alcohol, and eugenol) that often showed false-negative results, could be detected in the EpiSenzA assay explaining its relatively high predictivity.
The RhE IL-18 assay is based on the ability of contact sensitizers but not respiratory sensitizers or contact irritants to be able to increase intracellular production and release IL-18 (Gibbs et al. 2013). The assay was developed by combining the NCTC 544 keratinocyte IL-18 assay which could label a sensitizer (Corsini et al. 2009, 2013; Galbiati et al. 2011) with the RhE potency assay which assesses sensitizer potency (characterization) based on the irritant property of the chemical (Teunis et al. 2013; dos Santos et al. 2011; Spiekstra et al. 2009). The resulting assay was an RhE assay based on a single biomarker (IL-18) which was easily transferable from the Vrije Universiteit medical center (VUmc) in-house RhE model to commercially available RhE (SkinEthic™ RhE, EpiDerm™, and EpiCS®). Therefore, the RhE IL-18 assay is a relatively simple and robust method to assess a sensitizer label as well as potency (Gibbs et al. 2013). Using the VUmc RhE, chemicals were labeled (YES/NO) as sensitizer if a threshold of more than fivefold IL-18 release was reached. This threshold needs to be set for each different RhE model. The potency of the chemical, also known as the in vitro estimation of expected sensitization induction level, was assessed by interpolating in vitro EC50 (chemical concentration required to reduce viability by 50%) and IL-18 SI2 (chemical concentration required to increase IL-18 release by twofold) with LLNA EC3 and human NOEL values from standard reference curves generated using DNCB (extreme) and benzocaine (weak) (Galbiati et al. 2017; Gibbs 2017). Notably, whereas a good prediction was observed when traditional test panel chemicals were tested, when the assay was challenged with metal salts representative of leachables from medical devices, it was found that these metals which are very difficult to test in vitro and in vivo also fell outside of the applicability domain of this assay (Gibbs et al. 2013; Galbiati et al. 2017; Gibbs 2017). In addition to interpolating in vitro EC50 and IL-18 SI2 with LLNA EC3 and human NOEL values, a simple binary prediction model was developed for assessing sensitizer potency based on the irritant potential of the chemical (EC50 value and/or IL-1alpha release) or IL-18 SI2 (Gibbs 2017; Teunis et al. 2014). A ring study with four laboratories showed 77% accuracy with the binary prediction model for sensitizer potency (EC50 ≥ 7 mg/ml = weak to moderate sensitizer and EC50 < 7 mg/ml = strong to extreme sensitizer) (Teunis et al. 2014). In all laboratories, human RhE EC50 data showed better correlation to human data than to mouse LLNA-EC3 data. A low intra- and inter-experiment variability between laboratories and the different RhE models was observed.
Another promising assay is the SenCeeTox assay (McKim et al. 2012). EpiDerm™ and the SkinEthic™ RhE models were used to categorize chemical sensitizers by combining solubility, chemical reactivity, cytotoxicity, and activation of the Nrf2/ARE pathway. The expressions of eight Nrf2/ARE, one AhR/XRE, and two Nrf1/MRE controlled genes were measured using qRT-PCR. The fold induction at six exposure concentrations of a training set of 11 chemical sensitizers (representing extreme/strong-, moderate-, weak-, and non-sensitizing potency categories) was combined with glutathione (GSH) reactivity and cytotoxicity (MTT assay) data to determine the sensitization potential of the compounds and to establish the sensitivity of the two RhE models. Thereafter, a set of seven low-solubility chemicals and extracts used in the manufacture of medical devices were assessed. The ability of the assay to accurately place the compounds in one of the four potency categories was 71%. In addition to the RhE assays described above, several non-commercialized RhE are being used to develop assays that might be used in the future for sensitization testing of chemicals. For example, the Leiden epidermal model and the N/TERT epidermal model have also been used to test sensitization by measuring the Keap1-Nrf2-ARA activation pathway but only one sensitizer and one non-sensitizer have been tested to date (Alloul-Ramdhani et al. 2014). A larger panel of chemicals needs to be tested in order to determine whether the Leiden epidermal model and N/TERT epidermal model will be a suitable tool for chemical labeling. Also, a new RhE model has been presented as an open-source protocol by the University of São Paulo (Pedrosa et al. 2017). This UPS-RhE in-house model showed 85.7% specificity, 100% sensitivity, and 92.3% accuracy with a high within-laboratory reproducibility (92.3%) when thirteen chemicals were tested for their skin irritation potential according to the EpiSkin™ protocol (OECD TG 439) (Pedrosa et al. 2017).
Taken together, a number of commercially available and in-house RhE are being used to develop very promising assays which are currently entering different phases of validation. RhE is stable during transport making them extremely interesting for laboratories without the in-house know-how to construct and culture RhE. When using different types of RhE within an assay, the prediction model thresholds need to be calibrated beforehand. For example, some differences in the induction level of marker genes were observed in the EpiSenza assay between the LabCyte EPI-MODEL SIT24 and the EpiDerm™ model. The induction levels of ATF3 and DNJAB4 in the LabCyte EPI-MODEL SIT24 were slightly lower than in the EpiDerm™ model. Also, differences were observed between the IL-18 stimulation indexes in the RhE IL-18 assay when comparing the results obtained from different types of RhE (Gibbs et al. 2013). These differences are most probably due to different barrier properties of the S. corneum (Kano et al. 2011; Ponec et al. 2002), affecting the penetration of the topically applied chemicals and the metabolic activity within the different RhE due to the use of different culture media. RhE has the advantage over conventional submerged cultures in that they have a S. corneum to enable hydrophobic and chemicals of poor solubility to be tested using relevant vehicles which are used in in vivo studies. Furthermore, they release keratinocyte-derived (pro)inflammatory cytokines, are metabolically competent to a certain extent and do have a barrier function although these properties are not yet as well developed as those found in healthy native skin (Netzlaff et al. 2005). However, they still have limitations in that they only incorporate one cell type (keratinocytes) and do not have a dermal compartment which would make the model more representative on human skin. This is important since it is the in vitro cross talk between keratinocytes and fibroblasts which drives the inflammatory cytokine response in skin models lacking immune cells (Spiekstra et al. 2005, 2007).
4 Bilayered Reconstructed Human Skin (RhS)
Bilayered reconstructed skin models are constructed by seeding keratinocytes on a fibroblast-populated dermal matrix, e.g., a collagen hydrogel, collagen–elastin matrix, and donor dermis (Spiekstra et al. 2005, 2007; van den Broek et al. 2012; Gibbs et al. 2006). As with RhE, after several days of submerged culture, RhS is lifted to the air–liquid interface so that the keratinocytes differentiate to form a stratified epithelium with S. corneum (Fig. 2). As with RhE, the epidermis of RhS addresses chemical penetration (key event 1) and keratinocyte activation (key event 2) (Fig. 1). In RhS, interaction between fibroblasts and keratinocytes results in the formation of the basal membrane (El Ghalbzouri et al. 2005). In particular, fibroblasts are needed for dermis extracellular matrix secretion and optimal localization of dermal–epidermal junction components such as type VII collagen and laminin V (Marionnet et al. 2006). Importantly, keratinocytes upon contact with a chemical produce IL-1α which triggers dermal fibroblasts to produce a cascade of cytokines and chemokines, thus initiating the inflammatory response which in vivo results in LC migration into the dermis and immune cell invasion into the skin (Spiekstra et al. 2005, 2007; Ouwehand et al. 2011a, b). There are currently three commercially available RhS and a number of in-house models (Table 2). The EpidermFT™ has been used to determine the skin irritation potential of surfactants by assessment of the release of the primary cytokine interleukin IL-1α after exposure to 46 commercial skin cleansers (containing 224 nonionic or anionic surfactant-containing formulations) (Walters et al. 2016). The IL-1α release measured in vitro was compared to clinical TEWL (transepidermal water loss) measurements and showed good correlation (R2 = 0,66). Another commercially available RhS is the Phenion® FT model. The barrier property (key event 1) of the Phenion® FT was compared with ex vivo pig skin after topical application of testosterone, caffeine, nicotine, and benzoic acids. The Phenion® FT model turned out to be more permeable than pig skin but its barrier properties were comparable to those of RhE (EpiDermFT, SkinEthic™ RhE, and EpiSkin™) (Ackermann et al. 2010). Also, the StrataTest® showed consistent IL-1α release between batches after exposure to SDS (Rasmussen et al. 2010). The organotypic skin culture (ORG) in-house model of the University of Antioquia has been used for testing corrosive and irritation potency of a small panel of 11 substances by measuring general pro-inflammatory cytokine release and cell viability (MTT assay) (Morales et al. 2016). One out of four substances tested for corrosion, SDS, was incorrectly classified as corrosive and two out of three surfactants, Triton-×100 and Tween 20, were incorrectly classified as irritants. A possible explanation for this could be the exposure time to the chemical which was based on the protocols used for the EpiDermFT model, resulting in damage to the fibrin matrix. Also, the high levels of basal pro-inflammatory cytokine release in this model could be related to the use of the fibrin matrix (fibrin induces a secretory phenotype in fibroblasts, leading to pro-inflammatory cross talk with keratinocytes (Martinez et al. 2006)). The VUmc in-house RhS was used to investigate the alarm signals after exposure to a chemical by measuring key cytokines that initiate the infiltration of immune cells such as immature dendritic cells, T-cells, B-cells, and neutrophils into the skin (key event 2). It was shown that the exposure to an allergen (nickel sulfate and potassium dichromate) as well as to an irritant (SDS), with or without the presence of IL-1α or TNF-α neutralizing antibodies, results in an IL-1α and TNF-α dependent increase in CCL20 and CXCL8 secretion and IL-1α and TNF-α independent CCL27 secretion. This data suggests that skin-residential keratinocytes and fibroblasts respond to allergen as well as irritant exposure by releasing mediators that initiate immune cell infiltration. Whether this may facilitate an ACD or ICD reaction depends further on the properties of the chemical and how it interacts with immune cells, e.g., LC (Spiekstra et al. 2005; Kosten et al. 2015). Limited sensitization testing has been performed on these RhSs so far, which may be attributed to difficulties with stable transportation hindering commercialization or more complex methodology required for constructing in-house models. Whereas RhE and RhS have found an important niche in allergy research and sensitizer identification, a major limitation in these models is that they lack integrated immune cells which play a pivotal role in all human skin disease including allergy.
5 Immune Competent Skin Models
RhE and RhS models are starting to progress to include immune cells and thereby include in a single model key events 1, 2, and 3 (Fig. 1). Current commercially available RhE and RhS all lack immune cells. However, more complex in-house models with integrated LC and/or T-cells are being developed (Table 3). The first model to be developed was an RhE with integrated CD34+ cord blood progenitor-derived LC, which was used to study the reactivity of LC to topically applied allergens (Facy et al. 2005). The LC adopted an activated morphology (higher Langerin staining in the body and shorter dendrites), and the epidermis showed increased IL-1β and CD86 mRNA expression when exposed to sensitizers compared to irritants. This RhE-LC model developed by L’Oréal showed some donor variation with the majority of the donors responding to the sensitizers (Table 3). The major limitations, however, with this model were (I) the dermis compartment was not present and therefore no LC migration occurred and (II) it was very logistically complicated to construct due to the dependence of cultured primary keratinocytes and cord blood-derived LC.
In order to overcome the limitations of the RhE-LC model containing cord blood-derived LC, VUmc developed an RhS with integrated MUTZ-3-derived LC (Fig. 2). MUTZ-3 is an acute myeloid leukemia-derived human cell line with CD34+ proliferating progenitor cells which can be differentiated into LC (MUTZ-3 LC) in a cytokine-dependent fashion. MUTZ-3 LCs closely resemble their native counterparts, both phenotypically and functionally (Kosten et al. 2015; Masterson et al. 2002; Santegoets et al. 2008; dos Santos et al. 2009). By incorporating MUTZ-LC into an RhS (reconstructed epidermis on fibroblast-populated collagen hydrogel), a unique model was developed which (I) enabled the distinct mechanisms of migration of LC into the dermis to be investigated in a standardized manner (key event 3) (Ouwehand et al. 2010, 2011; Kosten et al. 2015) and (II) overcame the complicated logistics of using primary cell-derived LC. Using the RhS-containing functional MUTZ-LC, the only assay until now has been developed which can distinguish sensitizers from irritants based on the different mechanisms of LC migration and phenotypic plasticity. Migration of sensitizer-exposed maturing CXCR4+, CD86+ MUTZ-LC, into the dermis and consequent increase in CCR7 expression is blocked with neutralizing antibodies to CXCL12, whereas migration of irritant-exposed non-maturing MUTZ-LC is blocked by neutralizing antibodies to CCL5. Within the dermis, the irritant-exposed MUTZ-LC undergoes a phenotypic switch to a macrophage-like cells (CD1A−/CD14+/CD68+) under the influence of IL-10 (Ouwehand et al. 2011; Kosten et al. 2015; de Gruijl et al. 2006). Indeed, the model was able to correctly label three surfactants (SDS, Tween 80, and Triton ×100) as true irritants, whereas these surfactants tend to score as false positives in current in vitro and animal models. Most importantly, it is the incorporation of fibroblasts into the RhS which permits this LC migration due to their secretion of CXCL12 and CCL5 upon chemical exposure which provides the chemotactic gradient for LC. Furthermore, by reconstructing human oral mucosa in the same way as RhS, different migratory mechanisms of mucosa LC can now be compared with that of skin upon sensitizer exposure (Kosten et al. 2016). In a recent comparative study, monocyte-derived LC (Mo-LC) were compared with MUTZ-3-derived LC incorporated in RhS using cytokine secretion and mRNA as a readout for LC phenotype (Bock et al. 2017). Whereas both types of LC showed phenotypic changes upon chemical exposure, the limitation of using primary Mo-LC in RhS does add a hurdle for further widespread implementation. Also, blood-derived LC are influenced by donor variability which is not the case for cell line-derived LC. However, like CD34+-derived LC, Mo-DC do represent donor-specific biological variances which reflect biological diversity in the in vivo situation. To what extent the functionality of the different types of LC-like cells and donor variation affects their interaction with T-cells (key event 4) still needs to be elucidated.
Skin models incorporating T-cells are in the very early phase of development. Van den Bogaard et al. developed a model which mimics psoriasis by enabling CD4+T-cells, activated with anti-CD3/CD28 monoclonal antibody-coated beads, to migrate into the dermis of reconstructed skin (RhE on acellular dermis in a transwell culture system) (van den Bogaard et al. 2014) (Table 3). The reconstructed skin did not contain fibroblasts, and therefore no direct cell-to-cell contact between the T-cells and the keratinocytes occurred, thus enabling allogenic cells to be cultured together without cytotoxic effects. Before incorporation into the model, the T-cells were reported to be able to produce INF-γ, TNF-α, IL-17, and IL-22. At day two after initiating migration, gene expression analysis still showed relatively abundant INF-γ expression compared to IL-17, IL-22, and TNF-α. Also, pro-inflammatory cytokine and chemokine production by keratinocytes (IL-6, IL-8, IL-23, and CXCL-10) was highest at day 2. It was found that direct contact between keratinocytes and T-cells was not necessary for cross talk since soluble factors produced by T-cells were the main stimulus for the inflammatory phenotype. Since no model has yet incorporated both LC and T-cells, currently no in vitro skin model exists which can be used to study all four key events of the sensitization process.
6 Skin-on-a-Chip
Current commercially available or in-house skin models are based on static culture systems. Novel microphysiological systems are being developed, creating the possibility of culturing human skin models in a systemically controlled microenvironment in which homeostasis can be created and maintained (van den Broek et al. 2017; Watson et al. 2017; Ahadian 2017). The perfusion of the systems is expected to introduce shear stress, clear secreted products, increase the barrier function, create biomolecular gradients, and permit interaction with distant cells and may possibly even enable the influx and outward migration of immune cells from and to the lymph nodes. In addition, the maintenance and testing period of (commercially) available skin equivalents may possibly be prolonged by using bioreactor platforms. Several simplistic models for substance penetration in skin have been developed. For example, a model has been described in which epidermal, dermal, and endothelial human cells were co-cultured in a microfluidic device separated by porous membranes that allowed communication between the different cell type monolayers (Wufuer et al. 2016). Other models describe cultured monolayers of HaCat-KC cells, co-cultured with U937 cells or just human epidermal keratinocytes alone in a microfluidic device to assess the irritation potential and toxicity of chemical compounds (Wufuer et al. 2016; Ramadan and Ting 2016). A number of skin-on-a-chip models have been demonstrated to be promising for future substance testing (Table 4). Percutaneous penetration in RhS has been demonstrated where microfluidic channels (gravity driven) were used to collect the penetrated substances (FAM-tagged oligonucleotides). This model showed that the barrier function remained consistent for over 3 weeks of culturing (Abaci et al. 2015). The model was exposed to a clinically relevant concentration of the anticancer drug Doxorubicin via the microfluidics, thus mimicking systemic application. Later, physiological relevance of the model was improved by the addition of perfusable vascularization using induced pluripotent stem cell (iPSC)-derived endothelial cells and 3D printing technology (Abaci et al. 2016). Other skin-on-a-chip models have also introduced vasculature, e.g., Lee et al. introduced fluidic channels using PDMS (polydimethylsiloxane) and HUVEC in RhS with HaCat-KC (Lee et al. 2017). Mori et al. developed a perfusable skin-on-a-chip model with HUVEC-lined nylon wires within the dermal compartment, mimicking the blood vessel–tissue barrier and also preventing ECM contraction (Mori et al. 2017). In this model, the percutaneous absorption of caffeine and ISDN was measured in the medium collected from beneath the skin equivalent or from the vascular channels. Another very promising model has been described in which the scaffold is constructed from decellularized porcine jejunum (BioVaSc matrix) with intact vasculature placed in a bioreactor system (Groeber et al. 2016). This model enables the interaction of cellular and noncellular compartments of the bloodstream with different layers of tissues and might be an excellent tool for immunological research and in particular to study the migration of lymphocytes in allergic contact dermatitis. Taken together, skin-on-a-chip models are expected to have added value for substance testing in allergenicity research. However, until now, only proof of concept has been established and biomarker analysis still needs to be defined. Technology areas which require attention include sensor integration and user-friendliness. Furthermore, biological optimization, such as extracellular matrix modification to reduce gel contraction and medium composition changes for an improved culture period, is still required.
7 Conclusions and Future Perspectives
In addition to single-organ skin models, multi-organ platforms are also under development in order to accurately predict drug toxicity, systemic absorption, and metabolism of drugs in, e.g., the skin, small intestine, liver, and kidneys. A four-organ chip including a preformed skin model and human intestine has been co-cultured with liver lobules and human proximal tubule epithelial cells (kidney) by Maschmeyer et al. (Maschmeyer 2015; Maschmeyer et al. 2013, 2015). Atac et al. (2013) are developing a multi-organ platform in which the commercially available EpiDermFT model is cultured in combination with subcutaneous tissue in a microfluid device together with single-hair follicular units. Hair shafts are an easy route for chemical compounds to penetrate the skin which makes such a model very interesting for allergenicity testing.
In conclusion, reconstructed human skin models for allergy research and identifying contact sensitizers are rapidly advancing and are already replacing the use of animals in many areas of substance testing and research. RhE has enabled the testing of hydrophobic chemicals and chemicals of poor solubility. They are metabolically competent to a certain extent and have a barrier function. They are easily standardized which has already resulted in a number of commercially available RhE and have already been validated for skin irritation and skin corrosion testing. They are limited because they only incorporate keratinocytes. RhS allows us to study the crosstalk between keratinocytes and fibroblasts, which drives the inflammatory cytokine response. Commercialization of RhS is hindered due to difficulties with stable transportation. Most RhE and RhS lack integrated immune cells. Immune competent models with integrated LC or T-cells are being developed to include key events 1, 2, and 3 (Fig. 1) in a single model. So far, no model has incorporated both LC and T-cells. Skin-on-a-chip has introduced the possibility to culture skin models in perfusable microphysiological culture systems in which homeostasis can be controlled and maintained. The different levels of complexity of the models ranging from RhE to skin-on-chip will enable a suitable model to be selected to fit each different research question.
Abbreviations
- ACD :
-
Allergic Contact Dermatitis
- AOP :
-
Adverse Outcome Pathway
- DC :
-
Dendritic Cell
- DNCB :
-
2,4-Dinitrochlorobenzene
- DPRA :
-
Direct Peptide Reactivity Assay
- EC3 :
-
Estimated Concentration of a substance expected to produce a stimulation index of 3
- EC50 :
-
Half maximal Effective Concentration
- ECM :
-
Extra Cellular Matrix
- FAM :
-
Fluorescein Amidite
- GSH :
-
Glutathione
- HaCaT :
-
Spontaneously transformed aneuploid immortal keratinocyte cell line
- h-CLAT :
-
Human Cell Line Activation Test
- hOSEC :
-
Human Organotypic Skin Explant Cultures
- HUVEC :
-
Human Umbilical Vein Endothelial Cell
- ICD :
-
Irritant Contact Dermatitis
- ISDN :
-
Isosorbide Dinitrate
- LC :
-
Langerhans Cell
- LEM :
-
Leiden Epidermal Model
- LLNA :
-
Local Lymph Node Assay
- MO-LC :
-
Monocyte Derived Langerhans Cell
- MTT :
-
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay
- MUSST :
-
Myeloid U936 Skin Sensitization Test
- MUTZ-LC :
-
Acute Myelomonocytic Leukemia Cell (Mutz-3)-derived Langerhans Cell
- NEM :
-
N/TERT Epidermal Model
- NOEL :
-
No Observed Effect Level
- OECD :
-
Organization for Economic Co-operation and Development
- ORG :
-
Organotypic Skin Culture
- OXA :
-
Oxazolone
- PDMS :
-
Polydimethylsiloxane
- RhE :
-
Reconstructed Human Epidermis
- RhS :
-
Bilayered Reconstructed Human Skin
- SDS :
-
Sodium Dodecyl Sulfate
- SI :
-
Stimulation Index
- TEWL :
-
Transepidermal Water Loss
- UPS-RhE :
-
Reconstructed Human Epidermis developed by University of São Paulo
- VUmc :
-
Vrije Universiteit Medical Center
References
Abaci HE et al (2015) Pumpless microfluidic platform for drug testing on human skin equivalents. Lab Chip 15(3):882–888
Abaci HE et al (2016) Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv Healthcare Mat 5(14):1800–1807
Ackermann K et al (2010) The Phenion full-thickness skin model for percutaneous absorption testing. Skin Pharmacol Physiol 23(2):105–112
Ade N et al (2006) Activation of U937 cells by contact sensitizers: CD86 expression is independent of apoptosis. J Immunotoxicol 3(4):189–197
Ahadian S, et al (2017) Organ-on-a-chip platforms: a convergence of advanced materials, cells, and microscale technologies. Adv Healthc Mater
Alloul-Ramdhani M, Tensen CP, El Ghalbzouri A (2014) Performance of the N/TERT epidermal model for skin sensitizer identification via Nrf2-Keap1-ARE pathway activation. Toxicol In Vitro 28(5):982–989
Andreas N et al (2011) The intra- and inter-laboratory reproducibility and predictivity of the KeratinoSens assay to predict skin sensitizers in vitro: results of a ring-study in five laboratories. Toxicol In Vitro 25(3):733–744
Andres E et al (2017) Preliminary performance data of the RHE/IL-18 assay performed on SkinEthic RHE for the identification of contact sensitizers. Int J Cosmet Sci 39(2):121–132
Api AM, Basketter D, Lalko J (2015) Correlation between experimental human and murine skin sensitization induction thresholds. Cutaneous Ocular Toxicol 34(4):298–302
Ashikaga T et al (2006) Development of an in vitro skin sensitization test using human cell lines: the human cell line activation test (h-CLAT) I. Optimization of the h-CLAT protocol. Toxicol In Vitro 20(5):767–773
Atac B et al (2013) Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 13(18):3555–3561
Basketter DA et al (2014) Categorization of chemicals according to their relative human skin sensitizing potency. Dermatitis 25(1):11–21
Bock S et al (2017) Characterization of reconstructed human skin containing Langerhans cells to monitor molecular events in skin sensitization. Toxicol In Vitro 46:77–85
Corsini E et al (2009) Use of IL-18 production in a human keratinocyte cell line to discriminate contact sensitizers from irritants and low molecular weight respiratory allergens. Toxicol In Vitro 23(5):789–796
Corsini E et al (2013) NCTC 2544 and IL-18 production: a tool for the identification of contact allergens. Toxicol In Vitro 27(3):1127–1134
Cottrez F et al (2015) Genes specifically modulated in sensitized skins allow the detection of sensitizers in a reconstructed human skin model. Development of the SENS-IS assay. Toxicol In Vitro 29(4):787–802
Cottrez F et al (2016) SENS-IS, a 3D reconstituted epidermis based model for quantifying chemical sensitization potency: reproducibility and predictivity results from an inter-laboratory study. Toxicol In Vitro 32:248–260
de Gruijl TD et al (2006) A postmigrational switch among skin-derived dendritic cells to a macrophage-like phenotype is predetermined by the intracutaneous cytokine balance. J Immunol 176(12):7232–7242
Delaine T et al (2011) Structure-Activity Relationship between the in vivo skin sensitizing potency of analogues of phenyl glycidyl ether and the induction of Nrf2-dependent luciferase activity in the keratinosens in vitro assay. Chem Res Toxicol 24(8):1312–1318
dos Santos GG et al (2009) Progress on the development of human in vitro dendritic cell based assays for assessment of the sensitizing potential of a compound. Toxicol Appl Pharmacol 236(3):372–382
dos Santos GG et al (2011) A potential in vitro epidermal equivalent assay to determine sensitizer potency. Toxicol In Vitro 25(1):347–357
El Ghalbzouri A et al (2005) Basement membrane reconstruction in human skin equivalents is regulated by fibroblasts and/or exogenously activated keratinocytes. J Invest Dermatol 124(1):79–86
Facy V et al (2005) Reactivity of Langerhans cells in human reconstructed epidermis to known allergens and UV radiation. Toxicol In Vitro 19(6):787–795
Galbiati V et al (2011) Further development of the NCTC 2544 IL-18 assay to identify in vitro contact allergens. Toxicol In Vitro 25(3):724–732
Galbiati V et al (2017) Development of an in vitro method to estimate the sensitization induction level of contact allergens. Toxicol Lett 271:1–11
Gerberick GF et al (2004) Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci 81(2):332–343
Gibbs S, et al (2017) Assessment of metal sensitizer potency with the reconstructed human epidermis IL-18 assay. Toxicology
Gibbs S et al (2006) Autologous full-thickness skin substitute for healing chronic wounds. Br J Dermatol 155(2):267–274
Gibbs S et al (2013) An epidermal equivalent assay for identification and ranking potency of contact sensitizers. Toxicol Appl Pharmacol 272(2):529–541
Groeber F et al (2016) A first vascularized skin equivalent for as an alternative to animal experimentation. Altex 33(4):415–422
Jacobs JJ et al (2002) An in vitro model for detecting skin irritants: methyl green-pyronine staining of human skin explant cultures. Toxicol In Vitro 16(5):581–588
Jacobs JJL et al (2004) Assessment of contact allergens by dissociation of irritant and sensitizing properties. Toxicol In Vitro 18(5):681–690
Jacobs JJL et al (2006) Skin irritants and contact sensitizers induce Langerhans cell migration and maturation at irritant concentration. Exp Dermatol 15(6):432–440
Kano S et al (2011) Comparison of several reconstructed cultured human skin models by microscopic observation: their usefulness as an alternative membrane for skin in drug permeation experiments. AATEX 16(2):51–58
Kosten IJ et al (2015a) Gingiva equivalents secrete negligible amounts of key chemokines involved in langerhans cell migration compared to skin equivalents. J Immunol Res 2015:627125
Kosten IJ et al (2015b) MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicol Appl Pharmacol 287(1):35–42
Kosten IJ et al (2016) MUTZ-3 Langerhans cell maturation and CXCL12 independent migration in reconstructed human gingiva. Altex 33(4):423–434
Lee S et al (2017) Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed Microdevices 19(2):22
Lehe CL et al (2003) A two-centre evaluation of the human organotypic skin explant culture model for screening contact allergens. Altern Lab Anim 31(6):553–561
Mak IW, Evaniew N, Ghert M (2014) Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 6(2):114–118
Marionnet C et al (2006) Interactions between fibroblasts and keratinocytes in morphogenesis of dermal epidermal junction in a model of reconstructed skin. J Invest Dermatol 126(5):971–979
Martinez V et al (2006) Evaluation of eye and skin irritation of arginine-derivative surfactants using different in vitro endpoints as alternatives to the in vivo assays. Toxicol Lett 164(3):259–267
Maschmeyer I, et al (2015) Chip-based human liver-intestine and liver-skin co-cultures—a first step toward systemic repeated dose substance testing in vitro. Eur J Pharm Biopharm 95(Pt A):77–87
Maschmeyer I et al (2015) A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 15(12):2688–2699
Masterson AJ et al (2002) MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood 100(2):701–703
McKim JM, Keller DJ, Gorski JR (2012) An in vitro method for detecting chemical sensitization using human reconstructed skin models and its applicability to cosmetic, pharmaceutical, and medical device safety testing. Cutan Ocular Toxicol 31(4):292–305
Morales M et al (2016) Evaluation of fibrin-based dermal-epidermal organotypic cultures for in vitro skin corrosion and irritation testing of chemicals according to OECD TG 431 and 439. Toxicol In Vitro 36:89–96
Mori N, Morimoto Y, Takeuchi S (2017) Skin integrated with perfusable vascular channels on a chip. Biomaterials 116:48–56
Natsch A et al (2010) Chemical basis for the extreme skin sensitization potency of (E)-4-(ethoxymethylene)-2-phenyloxazol-5(4H)-one. Chem Res Toxicol 23(12):1913–1920
Netzlaff F et al (2005) The human epidermis models EpiSkin, SkinEthic and EpiDerm: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm 60(2):167–178
OECD (2012) OECD series on testing and assessment no. 168: the adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins. Part 1: Scientific evidence. Part 2: Use of the AOP to develop chemical categories and integrated assessment and testing approaches. OECD, Paris
Ouwehand K et al (2008) CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis. Eur J Immunol 38(11):3050–3059
Ouwehand K et al (2010) Comparison of a novel CXCL12/CCL5 dependent migration assay with CXCL8 secretion and CD86 expression for distinguishing sensitizers from non-sensitizers using MUTZ-3 Langerhans cells. Toxicol In Vitro 24(2):578–585
Ouwehand K et al (2011a) Technical advance: langerhans cells derived from a human cell line in a full-thickness skin equivalent undergo allergen-induced maturation and migration. J Leukoc Biol 90(5):1027–1033
Ouwehand K et al (2011b) Irritant-induced migration of Langerhans cells coincides with an IL-10-dependent switch to a macrophage-like phenotype. J Invest Dermatol 131(2):418–425
Pedrosa TDN et al (2017) A new reconstructed human epidermis for in vitro skin irritation testing. Toxicol In Vitro 42:31–37
Peiser M et al (2012) Allergic contact dermatitis: epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell Mol Life Sci 69(5):763–781
Pistoor FHM et al (1996) Novel predictive assay for contact allergens using human skin explant cultures. Am J Pathol 149(1):337–343
Ponec M et al (2002) Characterization of reconstructed skin models. Skin Pharmacol Appl Skin Physiol 15(Suppl 1):4–17
Ramadan Q, Ting FC (2016) In vitro micro-physiological immune-competent model of the human skin. Lab Chip 16(10):1899–1908
Rasmussen C et al (2010) The StrataTest (R) human skin model, a consistent in vitro alternative for toxicological testing. Toxicol In Vitro 24(7):2021–2029
Rovida C et al (2015) Integrated Testing Strategies (ITS) for safety assessment. Altex 32(1):25–40
Rustemeyer T et al (2003) Comparison of two in vitro dendritic cell maturation models for screening contact sensitizers using a panel of methacrylates. Exp Dermatol 12(5):682–691
Saito K et al (2013) Development of a new in vitro skin sensitization assay (Epidermal Sensitization Assay; EpiSensA) using reconstructed human epidermis. Toxicol In Vitro 27(8):2213–2224
Saito K et al (2017) An in vitro skin sensitization assay termed EpiSensA for broad sets of chemicals including lipophilic chemicals and pre/pro-haptens. Toxicol In Vitro 40:11–25
Santegoets SJ et al (2008) Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J Leukoc Biol 84(6):1364–1373
Schmook FP, Meingassner JG, Billich A (2001) Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int J Pharm 215(1–2):51–56
Seok J et al (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110(9):3507–3512
Spiekstra SW et al (2005) Induction of cytokine (interleukin-1alpha and tumor necrosis factor-alpha) and chemokine (CCL20, CCL27, and CXCL8) alarm signals after allergen and irritant exposure. Exp Dermatol 14(2):109–116
Spiekstra SW et al (2007) Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen 15(5):708–717
Spiekstra SW et al (2009) Potential method to determine irritant potency in vitro—comparison of two reconstructed epidermal culture models with different barrier competency. Toxicol In Vitro 23(2):349–355
Teunis M et al (2013) Transfer of a two-tiered keratinocyte assay: IL-18 production by NCTC2544 to determine the skin sensitizing capacity and epidermal equivalent assay to determine sensitizer potency. Toxicol In Vitro 27(3):1135–1150
Teunis MAT et al (2014) International ring trial of the epidermal equivalent sensitizer potency assay: reproducibility and predictive capacity. Altex-Altern Animal Experimentation 31(3):251–268
van den Bogaard EH et al (2014) Crosstalk between Keratinocytes and T Cells in a 3D Microenvironment: a model to study inflammatory skin diseases. J Invest Dermatol 134(3):719–727
van den Broek LJ et al (2012) Development, validation and testing of a human tissue engineered hypertrophic scar model. Altex 29(4):389–402
van den Broek LJ et al (2017) Progress and future prospectives in skin-on-chip development with emphasis on the use of different cell types and technical challenges. Stem Cell Rev 13(3):418–429
Wagner I et al (2013) A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 13(18):3538–3547
Walters RM et al (2016) In vitro assessment of skin irritation potential of surfactant-based formulations by using a 3-D skin reconstructed tissue model and cytokine response. Atla-Altern Lab Animals 44(6):523–532
Watson DE, Hunziker R, Wikswo JP (2017) Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology. Exp Biol Med 242(16):1559–1572
Wufuer M et al (2016) Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci Rep 6:37471
Acknowledgements
The authors have no conflicts of interest to declare. This manuscript was supported in part by EuroStars project (TESHI 8855).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Rodrigues Neves, C., Gibbs, S. (2018). Progress on Reconstructed Human Skin Models for Allergy Research and Identifying Contact Sensitizers. In: Bagnoli, F., Rappuoli, R. (eds) Three Dimensional Human Organotypic Models for Biomedical Research. Current Topics in Microbiology and Immunology, vol 430. Springer, Cham. https://doi.org/10.1007/82_2018_88
Download citation
DOI: https://doi.org/10.1007/82_2018_88
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62451-4
Online ISBN: 978-3-030-62452-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)