Abstract
Histophilus somni is responsible for sporadic disease worldwide in cattle and, to a lesser extent, in small ruminants, bighorn sheep (Ovis canadensis), and North American bison (Bison bison). The importance of H. somni diseases can be attributed to improved clinical and laboratory recognition, combined with the growth in intensive management practices for cattle. Although outbreaks of bovine histophilosis can occur year-round, in northern and southern hemispheres, it is most frequent in late fall and early winter. Weather, stress, dietary changes, and comingling of cattle are likely to be major triggers for outbreaks. The most frequent clinical expressions of histophilosis include undifferentiated fever, fibrinosuppurative pneumonia, encephalitis-leptomeningitis, necrotizing myocarditis, and diffuse pleuritis. Neurological disease occurs either as thrombotic meningoencephalitis (TME) or as suppurative meningitis with ventriculitis. Acute myocarditis is characteristically necrotizing and generally involves one or both papillary muscles in the left ventricular myocardium. Biofilm-like aggregates of bacteria occur in capillaries and veins in myocardium, in the central nervous system, and on endocardial surfaces. H. somni is a component of bovine respiratory disease (BRD) complex. In our experience, it is most commonly diagnosed in subacute-to-chronic polymicrobial pulmonary infections in combination with Mannheimia haemolytica, Trueperella pyogenes, Pasteurella multocida, or Mycoplasma bovis. Other, less common forms of H. somni disease present as polyarthritis/tenosynovitis, abortion with placentitis and fetal septicemia, epididymitis-orchitis, and ocular infections. It is likely that H. somni is under-recognized clinically and diagnostically. Most state and provincial laboratories in North America rely on bacterial isolation to confirm infection. The use of more sensitive detection methods on field cases of histophilosis will help resolve the pathogenesis of H. somni in natural outbreaks, and whether the disease is as common elsewhere as it is in Canada.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Papillary Muscle
- Bovine Viral Diarrhea Virus
- Bronchiolitis Obliterans
- Bighorn Sheep
- Bovine Respiratory Syncytial Virus
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Research into the biology of Histophilus somni moved on apace in the last 20 years in the laboratories of Corbeil, Inzana, Czuprynski, Tegtmeier, and others (Yarnall et al. 1988; Inzana et al. 1992; Tegtmeier et al. 2000; Sylte et al. 2001; Sandal et al. 2007; Corbeil 2007; Sandal and Inzana 2010; Siddaramappa et al. 2011). In considerable contrast, new or corroborating information regarding the natural ecology of histophilosis in production settings is limited, particularly in the USA. What novel data we possess about clinical disease was developed by a small cadre of clinicians, epidemiologists, microbiologists, and pathologists in Canada and, to a lesser extent, Denmark (Humphrey et al. 1982; Harris and Janzen 1989; Gogolewski et al. 1989; Van Donkersgoed et al. 1990; Schuh and Harland 1991; Van Donkersgoed et al. 1995; Angen et al. 1998; Booker et al. 1999; Tegtmeier et al. 1999b, 2000; Haines et al. 2001, 2004; Clark 2005). Diseases due to H. somni are a diagnostic challenge due to the agent’s status as both a commensal and a pathogen, its tendency to occur in combination with other agents, and its fastidious growth characteristics (Quinn et al. 2007). It is a neglected truism that research hypotheses about pathogens should be anchored in field work on the natural disease. This is particularly true for H. somni.
Multiple knowledge gaps remain about bovine histophilosis. There is the practical aspect of veterinarians attempting to advise clients dealing with clinical histophilosis in their herds and flocks. One of the first questions asked about the disease was: Where in the host does vascular invasion by H. somni originate (Kennedy et al. 1960)? Half a century after Kennedy and colleagues posed that question, we still lack an answer (Maxie and Youssef 2007). Does it slip between pulmonary pneumocytes, digest underlying basement membrane, as suggested by Corbeil and colleagues (Zekarias et al. 2010; Agnes et al. 2013), and pass into the capillary beds of the lungs? What fraction of undifferentiated fever in fattening cattle is attributable to histophilosis? Why is it so difficult to experimentally reproduce the diffuse pleuritis that is one common clinical expression of histophilosis? How and why does intravascular infection localize with such extraordinary numbers of bacteria in papillary muscles of the left ventricular myocardium? Does formation of biofilms in brain, heart, and elsewhere limit the practical significance of in vitro antimicrobial sensitivity of isolates in specific outbreaks? How effective are commercial vaccines under feedlot conditions and which products, if any, does it make economic sense to use? If thrombotic meningoencephalitis (TME) indeed faded in importance since the 1980s as part of the H. somni disease complex (HSDC) (Orr 1992), what drove the process? A recent analysis of the literature sought to define basic aspects of responses by cattle to experimental challenge with infectious agents contributing to the bovine respiratory disease (BRD) complex (Grissett et al. 2015). The authors wished to characterize basic parameters such as time of exposure to onset of clinical signs, time to peak disease outbreak, time for signs to resolve, minimum time to shedding, time to maximum shedding, time for shedding to resolve, and time to seroconversion. Of eight major BRD agents examined, one stood apart. H. somni lacked the published studies of natural and experimental disease that met the authors’ modest inclusion criteria.
This chapter is written by two diagnosticians based in a western US diagnostic laboratory. We attempt to summarize our understanding of histophilosis as a natural disease. It is informed in part by laboratory accessions from field outbreaks over the past 25 years.
Histophilosis was documented as a disease in feedlot cattle in the 1950s (Griner et al. 1956). Kennedy et al. (1960) identified the causative agent as Haemophilus-like. The name Haemophilus somnus was proposed by Bailie in 1969. The agent was renamed H. somni in 2003, and a new genus was established in the family Pasteurellaceae as a result (Angen et al. 2003). H. somni incorporates two species of previously uncertain placement that were isolated from sheep: ‘Histophilus ovis’ and ‘Haemophilus agni.’ H. somni-like agents were isolated from diseased sheep as early as the 1920s, where a septicemic disease was described in late fall and early winter (Mitchell 1925). H. somni has since been associated with multiple clinicopathological syndromes in cattle, most of them presumed sequelae to septicemia. Major manifestations are fever, pneumonia, pleuritis, meningoencephalitis-myelitis, necrotizing myocarditis, and pericarditis. Two or more of these manifestations can occur in individual animals (Van Donkersgoed et al. 1990). Other diseases due to H. somni are abortion, conjunctivitis, arthritis-tenosynovitis, and reproductive diseases of the male and female reproductive tracts. Lesions may occur in other sites, typically as a facet of septicemia: nephritis, endophthalmitis, myositis, laryngitis, otitis media, and intestinal necrosis.
It is likely that histophilosis continues to be underdiagnosed in commercial herds and flocks. This is due to the ease with which small fatal lesions can be overlooked at necropsy, particularly in the heart, and due to difficulties with laboratory isolation of H. somni, especially from the lungs, when other pathogenic organisms are concurrently present (Moisan and Fitzgerald 1995; Haines et al. 2004). In addition, H. somni is susceptible to most antibiotics, so most treated animals would be culture-negative.
2 Clinical Diseases
2.1 Cattle
Although the incidence of thrombotic meningoencephalitis-myelitis (TME) appears to have declined in the past 20 years (Orr 1992; Haines et al. 2004), for many food animal veterinarians, the impression persists that TME remains the main manifestation of infection. If that were true, H. somni could be relegated to the status of an interesting, but relatively minor, pathogen. TME is sporadic in feedlots, backgrounding operations, and cow–calf herds. It occurs, relatively rarely, as a disease in dairy operations and among beef cattle on grass (Panciera et al. 1968; Saunders and Janzen 1980; Saunders et al. 1980; de Lahunta and Divers 2008). TME affected only 11 % of feedlots over one two-year investigation period (Little 1986). Average mortality due to TME was 0.1 % of the population at risk (Little 1986). A recent survey of diseases in US feedlot established that neurological disease of all kinds (including TME) affected 1.1 % of all feedlot cattle. To put this in perspective, 16.2 % of feedlot cattle were affected with respiratory disease (USDA 2011). It is more useful to consider H. somni as the cause of a disease complex in which many cattle are infected and only a few develop clinical symptoms. Some animals develop brief clinical signs and then carry H. somni in the lung for weeks, as demonstrated in one small study of experimental pneumonia (Gogolewski et al. 1989). The economic impact of subclinical histophilosis is largely unexplored, in spite of its likely importance (Kennedy et al. 1960). An experienced food animal veterinarian remarked recently, in the context of BRD complex, that subclinical disease is the monster we don’t see (Griffin 2014). This is due to the practical difficulties of attempting to follow specific cohorts of cattle through slaughter for sampling and testing, and matching those findings to those in individual animals, treated and untreated at the feed yard. Histophilosis is a component of BRD, which is the single most important cause of morbidity, mortality, and economic loss in stocker and feedlot cattle in North America (Loneragan et al. 2001; Gagea et al. 2006; Woolums 2015). Bacterial pneumonia of all causes (including H. somni) is second only to diarrhea as the leading cause of economic loss in dairy calves (Panciera and Confer 2010). H. somni was identified as the third most important bacterial pathogen found in BRD in one Midwestern study (1994–2002), with Mannheimia haemolytica ranked first (46.3 % of isolations), followed by Pasteurella multocida (34.7 %), and H. somni (19.0 %) (Welsh et al. 2004). BRD tends to peak around two weeks after animals arrive in feedlots, with 15–45 % of all incoming calves requiring treatment and death occurring in 1–5 % (Kelly and Janzen 1986).
In North America, clinical histophilosis is most commonly seen in stocker cattle (135–250 kg weaned calves), animals aged 6–12 months in feedlots, and young dairy calves (Griner et al. 1956; Panciera et al. 1968; Saunders et al. 1980; Bryson et al. 1990; Orr 1992; Moisan and Fitzgerald 1995; de Lahunta and Divers 2008; Francoz et al. 2015). In some parts of Europe, histophilosis is considered one of the most important causes of respiratory disease in young calves (Tegtmeier et al. 1995). Disease occurs year–round, but, in Northern Hemispheres, most clinical cases occur between October and January (Panciera et al. 1968; Orr 1992). In Wyoming, the bovine histophilosis season occurs in the fall when spring-born calves are comingled. This is associated with an abrupt drop in temperature in September–October and stress due to transportation, handling, vaccination, dietary change, and sorting. Clinical histophilosis is particularly associated with Western and Midwestern states and provinces of the USA and Canada. Similar weather-related stress in late fall and early winter in Southern Hemisphere countries coincides with the peak in histophilosis cases (pers. comm., Carlos Margineda).
2.1.1 Histophilus somni Disease Complex (HSDC) and Respiratory Disease
The HSDC encompasses septicemia and associated complications: undifferentiated fever, pneumonia, pleuritis, myocarditis, thrombotic meningoencephalitis-myelitis, tenosynovitis, and otitis media (Nation et al. 1983; Harris and Janzen 1989; Clark 2005). Signs of respiratory disease are typical of BRD: tachypnea, cough, nasal discharge, and depression. Some animals with pneumonia are found dead—this is the most common history associated with animals that have severe fibrinous pleuritis at necropsy (Clark 2005). Concurrent infections, particularly with viruses (bovine respiratory syncytial virus, bovine parainfluenza virus 3, bovine herpesvirus 1, bovine coronavirus, and bovine viral diarrhea virus), may predispose cattle to pulmonary histophilosis (Woolums 2015). Such agents are generally not detected in H. somni-infected cattle at the time of death. For reasons related to specific virulence factors, H. somni can persist in lungs even when clinical signs are minimal (Gogolewski et al. 1989). Since H. somni alone causes pneumonia experimentally (Gogolewski et al. 1987a, b, 1988; Gogolewski et al. 1989; Geertsema et al. 2011), it is difficult to determine whether viral infection precedes histophilosis in cases where viruses are not detected at death.
The most comprehensive field studies of histophilosis have been performed in Canada. HSDC accounts for up to 40 % of all mortalities at some Canadian feedlots (Van Donkersgoed et al. 1994a, b), with most clinical cases recognized two-to-nine weeks after introduction (Van Donkersgoed et al. 1990). Serological studies suggest that animals are infected and seroconvert within 14–21 days of feedlot entry. A sequence of clinical manifestations at different time points has been reported (Van Donkersgoed et al. 1990). The clinical onset of fatal pneumonia of all causes, including histophilosis, has a median of 12 days after arrival (range 1–30 days). Other clinical syndromes occur somewhat later: overt septicemia (median time of onset 17 days; range 13–19), polyarthritis (18 days; range 5–41); pleuritis (22 days; range 11–37); myocarditis (22 days; range 3–36); and TME (17 days; range 19–29) (Van Donkersgoed et al. 1990). A subsequent feedlot study found that most cases of H. somni myocarditis occur around 60 days, with a peak in acute bronchopneumonia cases at 25 days (Gagea et al. 2006). A logical inference is that HSDC starts as a disease of the lower respiratory tract, and it is from here that septicemia originates (Clark 2005). Experimental studies established that H. somni can pass between alveolar epithelial cells and induce alveolar epithelial cells to secrete matrix metalloproteinases, digesting basement membrane collagen (Zekarias et al. 2010, Agnes et al. 2013).
2.1.2 Central Nervous System Disease
Thrombotic meningoencephalitis-myelitis (TME) due to H. somni is largely a disease of older calves and yearlings (Griner et al. 1956; Fecteau and George 2004). Originally called thromboembolic meningoencephalitis (Kennedy et al. 1960), it is now recognized that endothelial damage and thrombosis, rather than embolism, is central to the pathogenesis of the disease (Maxie and Youssef 2007). It is rare to see TME in animals less than 4 months of age, but cases are reported in neonatal calves, some as young as 2 days old (Headley et al. 2013; Saunders et al. 1980). The disease is particularly associated with feedlots, where losses may be high and continue over several weeks (Descarga et al. 2002). TME does not behave like a contagious disease, and during an outbreak, individual cases occur sporadically in separate pens throughout a feed yard. Some strains of H. somni that are isolated from the reproductive tract of healthy cattle are capable of inducing suppurative encephalitis following experimental intracisternal inoculation (Kwiecien and Little 1992). Clinical signs are typical of acute meningoencephalitis: depression, fever and blindness with or without seizures, coma, and sudden death (De Lahunta and Divers 2008). The closed-to-semi-closed eyes in many affected cattle gave rise to the common phrase ‘sleeper syndrome’ and the species name in H. somni. Fever is generally present (Fecteau and George 2004). Otitis is a feature in some animals (McEwen and Hulland 1985). Animals may be so obtunded that it is not possible to tell whether they are blind, or instead are severely depressed (Harris and Janzen 1989). Lameness with a stiff gait, ataxia, and paresis is common. In extensive ranch operations, animals on grass are likely to be found dead. Untreated animals rarely live longer than 24–48 h (De Lahunta and Divers 2008). Only animals detected early in the clinical course (i.e., while still ambulatory) are likely to survive the following treatment. In Wyoming, it is generally impractical to subject animals with neurological signs to detailed neurological or ophthalmological examination, or to antemortem diagnostics such as collection and assessment of cerebrospinal fluid. Ancillary testing may be helpful, since hemorrhagic retinal infarcts have been reported in 20 % of cases, and meningeal exudate is generally abundant (Nayar et al. 1977; Little 1986; Orr 1992; Fecteau and George 2004). There is a consensus that the incidence of TME declined in the 1980s, following its recognition in the mid-1950s (Harris and Janzen 1989). In the 1960s, TME was the most common infectious neurological disease seen in US feedlots. It accounted for 60.5 % of all cases in Kansas in which a diagnosis of encephalic disease was made, compared to the second most common disease (polioencephalomalacia), which was responsible for 16.9 % (Bailie 1969). A proportion of animals with neurological histophilosis present with paresis due to the major lesion(s) occurring in the spinal cord.
2.1.3 Myocarditis and Sudden Death
Myocarditis-infarction is the most common form of H. somni infection that results in sudden death. TME, acute pneumonia, and diffuse pleuritis can also cause sudden death. The importance of myocarditis in histophilosis was recognized in the 1980s when a series of reports from Canada noted the association between this lesion and infection with H. somni (Janzen 1987; Guichon et al. 1988; Harris and Janzen 1989; Schuh and Harland 1991; Haines et al. 2004). Clients have reported seeing animals walk across a pen and drop dead without any premonitory signs—often one necrotic papillary muscle is found to be acutely ruptured. Chronic forms of myocardial histophilosis occur, leading to lethargy, ill thrift, and depression. This is less common in our experience.
Between October 2008 and May 2009, we monitored the occurrence of fatal histophilosis on one backgrounding operation and a large feedlot in southeast Wyoming. This was after the attending veterinarian (Dr. R. Hunter, Wheatland) alerted the Wyoming State Veterinary Laboratory to annually recurring necrotizing myocarditis. Our initial response was skepticism, since H. somni myocarditis was rarely diagnosed in mailed-in accessions. Like other diagnosticians, we assumed that papillary myocarditis was a peculiarity of feedlots in western Canada. Subsequent study confirmed the veterinarian’s impression. Total morbidity and mortality rates (all causes) over 8 months (September–May) among 4612 cattle in the backgrounding operation were 15.9 % (734 animals) and 0.34 % (16 animals), respectively. The unusually low mortality was attributed to low-stress handling. Morbidity and mortality rates in 4199 cattle in the feedlot during the same period were 34.1 % (1433 animals) and 4.5 % (187 animals), respectively. Necrotizing myocarditis accounted for 18.8 and 8.0 % of all deaths in the background operation and feedlot, respectively. No effort was made to assess what proportion of BRD cases contained H somni, with or without other agents. All cases of myocarditis examined at the laboratory were due to histophilosis, as established by culture and immunohistochemistry (IHC).
2.1.4 Other Manifestations of HSDC
H. somni is often found in the joints of animals with HSDC. Typically, arthritis-synovitis occurs following episodes of undifferentiated fever and TME. There is polyarthritis with firm swellings of joints, and animals display stiffness, lameness, and knuckling (Harris and Janzen 1989). Soft tissue around affected joints may be edematous. The atlanto-occipital joint is often affected (Maxie and Youssef 2007). H. somni can be isolated in pure culture from the joints of untreated animals. Otitis may coincide with the peak hemophilosis season in feedlot cattle (November–December). Morbidity is generally low, but in some herds, it reaches 10 % (Nation et al. 1983). Affected animals have a copious serous discharge from one or both external ear canals. Discharge drains from the pinna, moistening the hair coat and forming frozen balls of exudate. Affected cattle are febrile. In affected herds, there may be concurrent respiratory disease or TME (McEwen and Hulland 1985). H. somni can be obtained in pure culture from such cases. Routine short-term antibiotic treatment is effective. In some animals, infection tracks along the vestibulocochlear nerve and cause suppurative leptomeningitis. H somni is an occasional cause of conjunctivitis (Lamont and Hunt 1982).
2.1.5 Reproductive Tract Infection, Accessory Sex Glands, and Disease
Infection of the male and female reproductive tracts is an important aspect of infection with H. somni. Many clinically healthy bulls, and a smaller proportion of cows/heifers, harbor the bacterium in their reproductive tracts (61 and 15 %, respectively) for extended periods (Kwiecien and Little 1991; Corbeil et al. 1986). One early study reported that commensal infection was most common in young bulls with detection of H. somni in descending order in the following: preputial orifice > preputial cavity > urinary bladder > accessory sex glands > ampulla of ductus deferens (Humphrey et al. 1982). Infection in bulls has minimal clinical effects, apart from causing infrequent infertility and poor semen quality. Shedding of the organism in urine or discharges is one source of environmental contamination (Yaeger and Holler 2007).
In addition to commensal infection, endometritis accompanied by vaginitis-cervicitis (dirty cow syndrome) occurs due to H. somni, principally in Australia, Europe, Canada, and Africa. This is associated with early returns to service and infertility (Van Dreumel and Kierstead 1975; Saunders and Janzen 1980; Stephens et al. 1986; Corbeil et al. 1986). H. somnus is commonly isolated from the diseased female bovine reproductive tract in pure or mixed cultures (Kwiecien and Little 1991). Infection is characterized by purulent vaginal discharge and, in some herds, granular vaginitis-cervicitis following breeding (Chladek 1975; Saunders and Janzen 1980; Last et al. 2001; van der Burgt 2008; Headley et al. 2013; Bano et al. 2011). However, reproductive tract syndromes, other than sporadic abortion, have not been noted by some veterinary pathologists (Clark 2005). In a review of abortion cases investigated by the Washington Animal Disease Diagnostic Laboratory, 9 cases due to H. somni were detected over 9 months (Corbeil et al. 1986). H. somni was responsible for 0.23 % of all bovine abortions in one large survey in the American Midwest (Kirkbride 1993a). It ranked as the 11th most common cause of bacterial abortion and comprised 1.6 % (21/1299) of all cases in which a bacterial agent was detected. Laboratory-confirmed cases of spontaneous abortion typically occur in late gestation, reflecting in part the likelihood of an owner finding such larger fetuses. Experimental studies show that abortion may occur at any time during pregnancy and that blood-borne infection is an efficient route in causing placental infection (Corbeil et al. 1986). Use of vaginal–endometrial and preputial washes, which for our laboratory tend to be underused as a diagnostic tool to investigate infertility, can help establish the involvement of H. somni in some episodes (van der Burgt 2008). A review of the role of the bacterium in causing herd infertility considered the role of this pathogen tenuous (Yaeger and Holler 2007). Mastitis due to H. somni is seen on rare occasion and was reproduced experimentally (Hazlett et al. 1983).
2.1.6 Treatment and Control
There are three approaches to control anticipated outbreaks of histophilosis in cattle: vaccination, mass treatment with antimicrobial agents, and vaccination for other agents of the BRD complex that predispose to respiratory disease.
There are 26 USDA-approved H. somni vaccines manufactured by 6 companies for the American market. They are heavily used, with 69.7 % of all large feedlots in the US vaccinating cattle against H. somni (USDA 2011). Surprisingly, their ability to confer immunity in production settings is modest or unknown (Little 1986; Van Donkersgoed et al. 1994a, b; Booker et al. 1999; Bowland and Shewen 2000; Geertsema et al. 2011; Larson and Step 2012; Taylor and Confer 2014). Exceptions are bacterins that confer immunity against TEM following experimental intravenous or intracisternal challenge (Williams et al. 1978; Stephens et al. 1982, 1984). In experimental settings, vaccination reduces the severity of clinical respiratory disease following challenge. The proprietary data generated by companies have been sufficient to persuade regulatory agencies to grant product licenses (Hall et al. 1977; Saunders and Janzen 1980; Amstutz et al. 1981; Morter et al. 1982; Morter and Amstutz 1983; Groom and Little 1988; Ribble et al. 1988; Silva and Little 1990; Stephens 1990).
The capacity of commercial products to reduce losses due to TEM is one possible explanation for the apparent shift in clinical expression of histophilosis from neurological (1960–late 1980s) to respiratory, cardiac, and septicemic disease (late 1980s–to date). Problems associated with H. somni products include loss of protective surface antigens during manufacture, induction of IgE-specific antibody responses, and anaphylactic/endotoxic reactions (Ellis and Yong 1997; Geertsema et al. 2011). Experiences with the latter are responsible for the reluctance of some clinicians to use H. somni bacterins, even in herds where histophilosis is likely each fall. Incorporation of protective components, such as recombinant IbpA subunits, might enhance the efficacy of commercial products (Zekarias et al. 2011; Lo et al. 2012).
An alternative to vaccination is metaphylactic treatment in anticipation of disease outbreaks, along with prompt individual treatment of sick cattle (Van Donkersgoed et al. 1994a). In a recent survey, it was found that 92.6 % of large feedlots (>8000 head) in the USA used mass treatment with antibiotics when a BRD outbreak was anticipated in lightweight calves (<320 kg)(USDA 2011). Many antibiotics have good in vitro activity against bacterial pathogens of BRD, including H. somni (Van Donkersgoed et al. 2009; Wollums 2015). Post-arrival metaphylaxis with long-acting oxytetracycline does not reduce the risk of hemophilosis mortality, but minimizes total losses due to BRD (Van Donkersgoed et al. 1994a, b; Van Donkersgoed et al. 2009). Tilmicosin (Micotil®), tulathromycin (Draxxin®), ceftiofur (Naxcel®, Excenel®, Excede®), enrofloxacin, trimethoprim–sulfadoxine, and florfenicol (with and without nonsteroidal anti-inflammatories such as flunixin meglumine) are used to suppress the occurrence of HSDC. However, there is concern about later problems that may result due to antibiotic resistance after metaphylaxis. H. somni-associated endometritis responds well to intrauterine treatment with antibiotics such as oxytetracycline, sexual rest, and switching from natural service to artificial insemination (Last et al. 2001; Yaeger and Holler 2007).
The third approach is to vaccinate against other agents of the BRD complex. Published information on the effectiveness of vaccines for histophilosis is limited. A recent meta-analysis suggested that the risk of BRD morbidity in vaccinated animals is not statistically different from that in controls. Such analyses are constrained by the small number of published vaccine studies that deal with respiratory disease and H. somni (Larson and Step 2012). It is likely that there are benefits to vaccinating feedlot cattle against infectious cofactors for H. somni infection (Mannheimia haemolytica; Pasteurella multocida; bovine respiratory syncytial virus; bovine herpesvirus 1). The magnitude of this effect is unknown (Larson and Step 2012; Wollums 2015).
2.2 Small Domestic Ruminants
H. somni (now incorporating Haemophilus agni and Haemophilus ovis) can be a pathogen or a commensal bacterium of the genital tract of sheep, with the highest prevalence in ram lambs aged 12–20 weeks (Walker and LeaMaster 1986). Histophilosis is a considerably less common disease in sheep than cattle, manifesting primarily as lameness, septicemia, and epididymitis-orchitis (Kennedy et al. 1958; Poonacha and Donahue 1984; Lundberg 1986). Other presentations are abortion, generalized pyemia, metritis, mastitis, and TME. Histophilosis tends to affect lambs, with polyarthritis in younger (1–4 weeks) and septicemia in older animals (4–7 months) (Philbey et al. 1991). Disease can occur as outbreaks. Morbidity is 1–10 %, though more commonly at the lower end of the scale (0.5–1.5 %). Mortality approaches 90 % in untreated symptomatic lambs. Sheep are either found dead or they expire within hours of developing clinical signs of fever, depression, recumbency, and cyanosis. Neurological disease occurs in some episodes, affecting both lambs and adults. As in cattle, CNS disease may occur as TME, or as suppurative leptomeningitis-ventriculitis-choroiditis (Philbey et al. 1991). The means of transmission is unknown. Septicemia can be induced experimentally by intranasal and intravenous inoculation (Rahaley 1978a, b). Suppurative leptomeningitis-ventriculitis without vasculitis can be induced by intracisternal inoculation of both septicemic and one of two preputial strains tested (Lees et al. 1994). As with cattle, stress and crowding are presumed to be precipitating factors.
Epididymitis-orchitis is a multifactorial bacterial disease in 6–15 month-old rams. H. somni is one of several causative agents that infect the epididymis and testis (Lees et al. 1990; Ridler and Sargison 2007). Disease has been reported in New Zealand, Australia, Canada, South America, and South Africa. The sheep at highest risk are vigorous rams in intensive systems and on high planes of nutrition. The disease is occasionally fatal with fever due to epididymitis-orchitis. Chronic epididymitis is more common and tends to affect older animals. In one experimental study using isolates from various sites and species, the most pathogenic was from a natural outbreak of ovine epididymitis-orchitis (Díaz-Aparicio et al. 2009). The main differential diagnosis in Wyoming is brucellosis due to Brucella ovis, as the clinical presentations are similar. At least two multiplex PCR assays have been developed to identify H. somni, as well as B. ovis and Actinobacillus seminis, in semen and urine (Saunders et al. 2007; Moustacas et al. 2013). Outbreaks of H. somni-induced vulvitis-vaginitis occur during the breeding seasons. Disease is transmissible venereally to rams, which develop ulcerations on the glans penis (Ball et al. 1991). H. somni has also been isolated from the vaginal fluid of apparently asymptomatic ewes (Higgins et al. 1981).
H. somni is a commensal in goats. It has been isolated from the vagina and prepuce, but not from nasal passages. On the basis of partial 16S rRNA sequences, three caprine isolates identical to an ovine septicemic isolate were assumed to have been derived from sheep (Jánosi et al. 2009). H. somni has not been associated with disease in goats to date.
2.3 Bighorn Sheep
H. somni has been isolated from bighorn lambs (Ovis canadensis canadensis) with respiratory disease and in free-ranging herds with poor lamb recruitment (Ward et al. 2006; Miller 2001). Bronchopneumonia is a population-limiting disease in bighorns, and its major infectious components are considered to be Mannheimia haemolytica, Bibersteinia trehalosi, and Mycoplasma ovipneumoniae (George et al. 2008; Besser et al. 2014). H. somni appears to be a minor component in natural outbreaks. As in domestic sheep, it is harbored in the reproductive tract, particularly the vagina (Ward et al. 2006). Isolates from bighorn sheep are similar to those from domestic sheep, although there are some differences in cultural characteristics (Ward et al. 2006).
2.4 Bison
Commercial bison (Bison bison) are susceptible to the same feedlot stressors as domestic cattle. Like cattle, they are predisposed to BRD due to pasteurellosis and mycoplasmosis, and to pulmonary histophilosis following weather and comingling stress in feedlots (Dyer and Ward 1998; Dyer 2001). Strains of H. somni that are almost identical to those from cattle have been isolated from tonsils of bison (Ward et al. 1999a, b). Sudden death is the most common indication of histophilosis in bison (Berezowski, undated). H. somni is most often a pathogen in recently weaned bison calves. Animals are found recumbent, depressed, blind, and—shortly before death—convulsing. Affected bison calves generally die within 24–48 h of clinical onset.
3 Pathology
3.1 Cattle
3.1.1 Respiratory Disease in Cattle
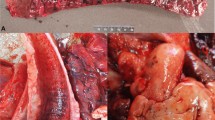
Acute pulmonary lesions of histophilosis take several forms. One is acute fibrinosuppurative bronchopneumonia (Fig. 1a). Grossly, these changes resemble those of acute pasteurellosis due to P. multocida. In our experience and that of others (Griffin 2012), it is not possible to draw a definitive conclusion about the likely involvement of H. somni based on the gross appearance of lungs. These lobular lesions are bilateral and affect cranial–ventral portions of the lungs. Affected parenchyma is consolidated and gray to red-gray, with intraluminal exudate in small airways. Areas of consolidation contain multiple small abscesses, arising in and effacing bronchioles (Andrews et al. 1985; Tegtmeier et al. 1995). Mediastinal and tracheobronchial lymph nodes are mildly edematous. In a small proportion of cases of acute histophilosis, gross lesions resemble those of acute mannheimiosis, with similarly florid bronchopneumonia and pleuritis. Pleuritis, when present in acute pulmonary disease due to H. somni, can occur as a solitary lesion, with varying volumes of exudate in the pleural sacs (Andrews et al. 1985) (Fig. 1b). Severe acute fibrinous pleuritis occurring 30–90 days after arrival in feedlots is the most common manifestation of histophilosis in feedlots in western Canada (Clark 2005). Many animals die acutely with few premonitory signs. It is noteworthy that most acute cases lack lesions of concurrent pneumonia (Fig. 2a, b).
a, b Two major forms of acute pneumonia in H. somni. a Classical anteroventral fibrinosuppurative bronchopneumonia; grossly such lungs resemble those infected with Pasteurella multocida. Image credit Dr. Bruce Anderson, Noah’s Arkive. b Fibrinosuppurative bronchopneumonia with severe associated pleuritis. Image credit Dr. Ted Clark, Calgary, AB
a, b Fibrinous pleuritis with septal inflammation. a Gross appearance of affected lung. b Histopathological appearance of a. Inflammation extends into septal areas, associated with septal edema and lymphangitis. Red = aggregates of H. somni antigen. Pulmonary parenchyma is free of inflammation. Immunohistochemistry preparation (IHC). Photo credit a, b Dr. Ted Clark, Calgary, AB
Microscopically, as first noted by Gogolewski et al. (1987), acute pulmonary lesions are characterized by a distinctive pattern of degeneration in leukocytes, which lack distinct cytoplasmic margins and form ballooned, homogenized nuclei (Fig. 3a–d). These may be early neutrophil extracellular traps (NETs), which have been shown to occur in leukocytes following in vivo exposure to H. somni (Brinkmann and Zychlinsky 2007; Hellenbrand et al. 2013) (Fig. 3c). These changes may be distinct from nuclear streaming (‘oat cells’), which are a pronounced feature of mannheimiosis (Fig. 3d). Foci of coagulative necrosis, and intralesional colonies of Gram-negative coccobacilli, are present (Fig. 4a, b) (Jackson et al. 1987; Potgieter et al. 1988; Gagea et al. 2006). Vasculitis and thrombosis, a prominent feature of TME, develop in a proportion of acutely affected lungs (Fig. 4a) (Andrews et al. 1985). This is characteristic of experimental pneumonia at 24 h after inoculation (Gogolewski et al. 1987). In calves dying with pleuritis alone, there is severe septal edema and interlobular thrombo-lymphangitis associated with the presence of H. somni antigen (Clark 2005). Histologically, the picture is of a purulent bronchiolitis and bronchopneumonia. Fibrin-rich exudate floods alveolar spaces. Necrosis affects bronchiolar epithelium and muscle, leading to bronchiolitis obliterans in survivors. Epithelial ulceration is evident as early as 3.5–7.0 h post-infection (Tegtmeier et al. 1999a, b). Necrotic foci, originating in distal airways, are scattered throughout affected (anteroventral) portions of the lung. H. somni is found in areas of acute inflammation and in foci of necrosis (Gogolewski et al. 1987; Bryson et al. 1990; Tegtmeier et al. 1995). Bacterial antigen is present as extracellular aggregates in bronchi/bronchioles and in necrotic foci (Fig. 4b). Bacterial antigen is also present in apparently intracellular locations in viable margins around such foci, but it is not possible to identify which inflammatory cells are involved. Bacteria can be seen in the cytoplasm of neutrophils and pulmonary alveolar macrophages as early 3.5 and 7.0 h after intrabronchial challenge, but were not seen in pneumocytes or in pulmonary intravascular macrophages (Tegtmeier et al. 1999a, b). Occlusive thrombosis affects septal lymphatics. Florid and widespread necrotizing phlebitis, such as found in the meninges in TME, is in our experience uncommon in acutely affected lungs in natural histophilosis.
a–d Microscopic appearance of acute pulmonary histophilosis. a Acute bronchopneumonia with focal necrosis of bronchiolar wall (arrow). Alveoli are flooded with fibrin-rich fluid: natural infection. Hematoxylin and eosin (HE stain). b Acute necrosis in pulmonary lobule. Large aggregates of H. somni antigen are in the necrotic tissue, invested by a zone of degenerating leukocytes in which smaller amounts of presumably intracellular H somni antigen are present: natural infection (IHC). c Necrosis of leukocytes, with the formation of swollen, homogenous nuclei suggestive of neutrophil extracellular traps (NETs) in alveoli; 2 days post-challenge: experimental infection. d Necrosis of leukocytes, with the formation of ‘oat cells’ and streaming of chromatin: natural infection
a, b Microscopic appearance of chronic pulmonary histophilosis: natural infection. a Two common features are necrosis, in many instances arising in destroyed distal airways, and lymphangitis with fibrin thrombi undergoing fibrous organization (L). Portion of a focal area of necrosis illustrated in b. HE stain. b Necrotic focus with intralesional H. somni antigen (IHC). In the absence of the immunohistochemistry, such foci can be assumed to be due to Mycoplasma bovis
Polymicrobial bronchopneumonia, in which H. somni is one component, is common, particularly after infection has been established for several weeks, and antibiotic treatments have been used (Schiefer et al. 1978; Andrews et al. 1985; Corbeil et al. 1986; Gagea et al. 2006; Booker et al. 2008). Up to 60 % of cattle with pneumonia in which H. somni is detected at necropsy have one or more other respiratory pathogens (Booker et al. 2008). Such lungs have suppurative bronchopneumonia with bronchiectasis and bronchiolitis obliterans. Common coinfecting agents are Pasteurella multocida, Mannheimia haemolytica, Mycoplasma bovis, Trueperella pyogenes, and/or Bibersteinia trehalosi (Schiefer et al. 1978; Panciera and Confer 2010). A common microscopic finding is the presence of multifocal necrosis, which can be confused with Pyogranulomatous caseonecrotic foci that typify chronic pneumonia due to Mycoplasma bovis (Caswell et al. 2010). It is noteworthy that M. bovis and H. somni are often found together in calves with chronic pneumonia (Booker et al. 2008). The presence of bacteria in necrotic foci may partly explain the ability of H. somni to persist chronically in lungs. Pneumonia due to M. bovis and H. somni has many common features, such as feedlot diseases that occur early in the feeding period, concurrent arthritis, tenosynovitis and otitis, tendency to chronicity following unsuccessful antibiotic treatment, and an anteroventral distribution. It is useful to establish by IHC whether necrotic foci contain one or both agents (Fig. 4). A third and relatively common pulmonary lesion of histophilosis is interstitial pneumonia due to septicemia/endotoxemia. Such pneumonia is characterized by intra-alveolar edema and hemorrhage (Caswell and Williams 2007; O’Toole et al. 2009) and is often seen when myocardial lesions are present (Janzen 1987), and some contribution may be made by vascular changes secondary to left-sided heart failure. Affected lungs are heavy due to edema and red as a result of hemorrhage and congestion. The etiological agent may be misinterpreted as evidence of primary viral pneumonia and should prompt prosectors to examine the heart closely for focal myocarditis.
3.1.2 CNS Disease in Cattle
The classical lesion of TME is moderate-to-severe acute multifocal hemorrhage with necrosis throughout the brain (Stephens et al. 1981). It is a useful necropsy practice to sample cerebrospinal fluid by syringe at the cisterna magna when dealing with neurological cases (Nietfeld 2012). In TME, such fluid is cloudy and blood-tinged, and can be used for cytospin cytology and bacterial culture to expedite diagnosis. Individual red-brown infarcts are 1–30 mm in diameter and vary from 1 to 3 to >50. Few or no grossly evident lesions may be present in the brain and spinal cord when animals are promptly treated with antibiotics (Clark 2005). The extent of lesions is best revealed by slicing brain at 1-cm intervals after it has fixed in formalin for 48 h. We occasionally see cases where the primary or sole lesions are in the spinal cord. Chronic TME, with the formation of mature abscesses, is rare in our experience. Presumably, most cattle with neurological histophilosis either die acutely, or recover following prompt treatment. Nevertheless, particularly in herds where histophilosis is active, any ‘brainer’ animal with long-standing neurological signs and intracerebral abscesses should be considered a histophilosis suspect until proven otherwise. Suppurative leptomeningitis, ventriculitis, and choroiditis that lack the textbook lesions of necrohemorrhage and vasculitis in brain can be a facet of histophilosis (McEwen and Hulland 1985). Examination of the globes after fixation is helpful diagnostically, since the high proportion of TME-affected cattle have keratitis, retinal edema (‘cotton spots’), and hemorrhage (Dukes 1971).
Histologically, septic thrombosis affects capillaries, post-capillary venules, and veins in TME. H. somni forms intravascular aggregates resembling biofilms. In very early cases, as noted by Kennedy et al. (1960), dense aggregates of bacteria plug capillaries. Bacterial adherence to endothelium allows H. somni to breach the blood–brain barrier, resulting in characteristic angiocentric microabscesses. There is usually some degree of vasculitis, primarily phlebitis, affecting vessels that contain intraluminal H. somni. It is noteworthy that the seminal report of TME in 1956 noted this association, although it was interpreted at the time as bacterial phagocytosis by microvascular endothelium (Griner et al. 1956). On the basis of in vitro studies, an intracellular location is unlikely (Czuprynski et al. 2004; Behling-Kelly et al. 2006). Although ultrastructural studies of TME are limited, it is likely that the cardinal intravascular event is microvascular plugging by bacteria that results in apoptosis of endothelium (Sylte et al. 2001).
3.1.3 Myocardial and Vascular Disease in Cattle
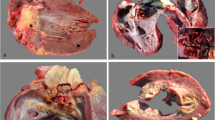
Cardiac lesions associated with histophilosis were noted briefly in the first account of TME. Of 21 animals with TME, 10 had cardiovascular lesions, including myocardial infarction, myocarditis, and pericarditis (Griner et al. 1956). It was not until the mid-1980s that the importance of cardiac lesions was recognized as one important cause of sudden ‘pen deaths’ (Janzen 1987; Harris and Janzen 1989; Schuh and Harland 1991). Such lesions generally affect one or both papillary muscles of the left ventricular myocardium (Fig. 5). These may be the only major changes found postmortem. Where concurrent lesions are present, they may involve the brain, spinal cord, and meninges. In our experience, florid pneumonia is uncommon when animals die of necrotizing myocarditis due to H. somni, suggesting that myocarditis is a sequel to silent septicemia rather than concurrent pneumonia. The endocardium overlying affected papillary muscles is often discolored (Fig. 6a–d). It is a good practice to incise papillary muscles in ‘sudden death’ calves in search of 1–3 cm areas of purple hemorrhage (acute) to tan (subacute) discoloration that typify early cases. Rarely, disseminated small lesions are evident throughout the myocardium (Fig. 6b). In a proportion of cases, such disseminated myocarditis may only be evident microscopically. Subacute and chronic lesions are fibrotic with multiple small pockets of exudate. Chronic lesions have a well-developed fibrous capsule and may incorporate a necrotic sequestrum (Fig. 6d). Inconsistent findings are ruptured chordae tendineae, endocardial rupture, adherent intraventricular thrombosis, valvular endocarditis, fibrinous endocarditis, and pericarditis. Bacteria on endocardial surfaces, and to a lesser extent in necrotic myocardium, form large, biofilm-like aggregates. Similar aggregates are seen following experimental infection (Sandal et al. 2009; Elswaifi et al. 2012). Other opportunistic or pathogenic bacteria (E. coli, Pseudomonas spp., Streptococcus spp., and Mycoplasma bovis) can cause suppurative or necrotizing myocarditis, but these are associated with randomly distributed lesions. These bacteria do not tend to target papillary muscles, unlike H. somni.
a–d Gross images of cardiac histophilosis. a Endocardial hemorrhage overlying necrosis in papillary muscle of left ventricle, typical of acute necrotizing myocarditis. b Acute disseminated myocardial necrosis throughout all portions of the heart. c Typical acute lesion in papillary muscle of left ventricular myocardium. Such lesions are typically 1–3 cm. d Chronic abscess in one of two papillary muscles, with a smaller lesion in interventricular septum. Rupture of such sequestra at the endocardial surface causes acute death
As the term TME suggests, particularly when disease is acute, bacteria are found in blood vessels, primarily capillaries, venules, and veins (Fig. 7a–e). Biofilm-like aggregates form in vessels in cardiac papillary muscles and elsewhere in the heart. Large colonies up to 400 μm thick form on endocardial surfaces, including valves (Fig. 7d). In older, more subacute lesions, there is necrosis or apoptosis of endothelium and necrosis of perivascular cardiocytes, with varying degrees of myocarditis. The resultant lesion—angiocentric myocarditis with intralesional bacteria—characterizes histophilosis microscopically. It should be corroborated by IHC. Ultrastructurally, bacterial communities are closely associated with degenerating endothelial cells. Endothelial cells contract, so that bacteria rest on denuded vascular basement membranes. Why H. somni tends to lodge in vessels in one or both papillary muscles of the left ventricle is unclear. These muscles are susceptible to hypoxia due to a dependence on subendocardial perfusion since most coronary blood flow occurs during diastole, unlike more superficial parts of the ventricular myocardium. Variation in the anatomy of individual animals’ hearts (presence of a ‘papillary muscle artery’; few or no anastomotic connections with the extrapapillary subendocardial plexus; balance between class A and class B arterial network) may predispose them to bacterial infection and infarction (Teixeira Filho et al. 2001; Ranganathan and Burch 1969).
a–e Biofilm-like formation in vivo in field cases of bovine histophilosis. a Thrombotic encephalitis (TME) with large (asterisk) and small colonies (arrow) of H. somni in close apposition to endothelium. There is early perivascular inflammation and hemorrhage. Brain. HE stain. b TME with H. somni stained by IHC (red) in capillaries, some of which appear occluded. Brain. c Adherence of H. somni to endothelium in beaded patterns (arrows). Brain. IHC. d Ulcerated endocardium with thick layer of H. somni in fibrin layer adherent to inflamed myocardium. Heart. HE stain. e Layer of H. somni adherent to venous endothelium, forming an almost continuous film of bacteria. Many capillaries are plugged with H. somni. Heart. HE stain
It is sometimes implied or stated in reviews of H. somni that vasculitis is a systemic feature of histophilosis. Our impression is that vasculitis is a prominent feature of TME, and microvascular and venous lesions are common in acute and peracute cardiac histophilosis. However, vasculitis in other tissues, such as in the lung, is relatively modest and probably cannot be used as a marker for likely H. somni infection.
3.1.4 Other Tissue Lesions in Cattle
Abortion due to H. somni is associated with necrotizing placentitis involving cotyledons and intercotyledonary placenta, combined with medial arteritis (van Dreumel and Kierstead 1975; Miller et al. 1983a, b; Widders et al. 1986). Embryonic loss has also been attributed to histophilosis (Ruegg et al. 1988; Kaneene et al. 1986a, b). Unlike the situation with pneumonia, abortion is generally associated with the isolation of H. somni alone (Corbeil et al. 1986). Placental lesions may be sufficiently severe to be evident grossly. Systemic lesions vary in the fetus but, when present, include angiocentric meningoencephalitis with vasculitis and generalized vascular lesions consistent with bacterial septicemia and disseminated intravascular coagulation (Van Dreumel and Kierstead 1975; Miller et al. 1983b; Widders et al. 1986; Headley et al. 2013, 2015). There have been few recent morphological studies of the lesions associated with H. somni in the female reproductive tract and none using IHC. Infected tracts may be free of lesions, or have mucopurulent vaginitis with adenitis of the major vestibular gland (Miller et al. 1983a).
A neglected aspect of histophilosis is the potential importance of paired tonsillar tissues comprising Waldeyer’s ring. The crypts of bovine palatine tonsils, like those of bison (Ward et al. 1999a, b), contain H. somni embedded in exudate, often in an inspissated, partly mineralized form (Fig. 8a–d). Some bacteria are in the cytoplasm of mononuclear cells in tonsillar lymphoid aggregates. This suggests one extrapulmonary mechanism whereby boluses of bacteria might enter the circulation, initiating septicemia. Laryngitis is an intermittent feature in histophilosis. Some pathologists wonder whether this too might be a portal of entry, since bilateral ulcerative or erosive laryngitis with thrombophlebitis is not uncommon in cattle dying of TME (T. Clark, personal communication). We have not found consistent lesions in the larynges of cattle dying of acute histophilosis, including TME.
a–d Palatine tonsil. a Gross image of inspissated white exudate in crypts of palatine tonsil of calf. Box = area comparable to that illustrated microscopically in b. b Lumen of tonsillar crypt filled with partly mineralized exudate. Most H. somni-positive antigen is present in the lumen of the crypt. There are smaller amounts of antigen in cells in crypt wall. IHC. c Abundant extracellular bacterial antigen (purple material) in crypt lumen; bacteria stained positively for H. somni in adjacent section. HE stain. d Intracytoplasmic H. somni-positive antigen in cells, presumably macrophages, in crypt wall (arrow). IHC
3.2 Small Ruminants
The principal findings at necropsy of fattening lambs with generalized histophilosis are those typical of septicemia—disseminated petechial hemorrhages, particularly in liver and skeletal muscle and, in longer-surviving lambs, nascent microabscesses. Multifocal hepatic necrosis is a helpful if not invariable finding. Lesions in other organs such as the kidney and myocardium consist of pale 0.5–2.0-mm-diameter foci (Rahaley 1978a, b; Orr et al. 1992). Lambs surviving for >1 day typically develop CNS disease and polyarthritis with periarticular inflammation. The histological lesions are those of disseminated septicemia with necrosis, vasculitis, and thrombosis, most evident in liver and skeletal muscle (Kennedy et al. 1958; Rahaley 1978a). H. somni occasionally causes outbreaks of perinatal death in lambs (Rahaley and White 1977). Pregnant ewes at 65–80 days’ gestation that are challenged intravenously abort due to acute placentitis (Rahaley 1978b). It is likely that septicemia with preferential localization in the uterus and placenta is the basis for late-term and perinatal losses in lambs. Spontaneous abortion due to H. somni appears to be rare in sheep (Kirkbride 1993b), but has been reported as a result of polymicrobial infection that includes H. somni (Hajtós 1987). CNS disease in sheep, as in cattle, occurs in two forms: a TME-like syndrome, essentially indistinguishable from the bovine disease, and suppurative leptomeningitis-ventriculitis-choroiditis, particularly in lambs that survive an initial episode of septicemia (Kennedy et al. 1958; Philbey et al. 1991; Romero et al. 2013). The distinctive relationship that occurs in cattle between microvascular endothelium and flat adherent colonies of H. somni can also be seen microscopically in the brains of sheep (Cassidy et al. 1997). Lesions consistent with a prior episode of septicemia in non-CNS tissues are often found (Philbey et al. 1991). Stress due to transportation and close confinement or crowding appears to contribute to the disease process. There are also similarities to the pneumonia found in cattle. H. somni is recovered from pneumonic lungs in mixed growth with pathogens such as T. pyogenes and M. haemolytica (Philbey et al. 1991).
Uncomplicated epididymitis-orchitis is rarely a cause of death. It is unusual for samples from this infection to be submitted for histology unless a regulated disease such as ovine brucellosis is suspected. Lesions are of bacterial orchitis and epididymitis, with marked testicular swelling (acute disease) or induration and atrophy (chronic disease), along with spermatic granulomas. No vaccine is currently available in the USA. Control is by including antibiotics in the feed (typically chlortetracycline) (Kimberling 1988).
3.3 Bighorn Sheep
As noted above, bacterial pneumonia in bighorn sheep is an important polymicrobial epizootic disease that limits the expansion of the species in its natural habitat in western North America (Post 1962). In the only investigation published to date on a possible role for H. somni, four of 12 sheep had immunohistochemical evidence of the bacterium in pneumonic lungs (Ward et al. 2006). It is assumed that H. somni is a minor component in epizootics.
3.4 Bison
H. somni was found by IHC in a retrospective study of 7 of 21 bison with terminal pneumonia. A subsequent investigation found that some of the virulence factors for histophilosis in cattle, such as IbpA, are present in H. somni isolated from the palatine tonsils of healthy, commercially managed bison (Ward et al. 1999a, b; Zekarias et al. 2011). Pneumonia due to a complex of microorganisms is common and important in ranched bison, particularly when they are finished at feed yards. It is unclear at present what proportion of bison with pneumonia have detectable H. somni in lesions. Leptomeningitis and hemorrhagic infarcts in the brain and spinal cord, presumably TME-like, have also been found in bison (Berezowski, undated).
4 Laboratory Diagnosis and Antimicrobial Resistance Testing
The most appropriate animals to select for necropsy are those with early clinical signs typical of the syndrome confronting the practitioner. Ideally, animals should be untreated by antibiotics. When treated, details of any antimicrobials used, including duration of administration, should be provided to laboratory personnel. There is a natural reluctance for owners to kill animals early in the clinical course and for them to prefer to submit chronic ‘lunger’ animals that were treated repeatedly. Unfortunately, laboratory results from long-standing cases are difficult to interpret due to the likelihood of finding multiple pathogens. Several excellent articles provide general approaches to field necropsies of cattle and on diagnostic sample submission (Caswell et al. 2012; Cooper and Brodersen 2010; Griffin 2012). Most necropsy tissues examined at our laboratory are generated by field necropsies done by veterinarians. The best laboratory accessions come with a detailed clinical history, uncontaminated fresh samples, and digital images of gross lesions (Griffin 2012).
Due to the varied clinical presentations of histophilosis, veterinarians should submit multiple fresh and fixed tissue samples. If H. somni is one of the pathogens that is suspected, optimal samples come from lung, heart, brain, and synovial fluid, as well as any other tissues with gross lesions. Appropriate samples from the cranial vault are one or more pieces of grossly discolored cerebral tissue; swabs of exudate in leptomeninges or ventricles; and CSF collected postmortem by needle and syringe before the head is disarticulated. If a veterinarian suspects TME and is unable collect tissues aseptically under field conditions, one or more heads should be submitted intact to the laboratory. This allows skulls to be opened and samples taken for culture using sterile instruments in a controlled environment. Lung samples should be taken at the junction of affected and unaffected parenchyma. Pulmonary tissue with abnormal structural changes (lesioned) can be problematic because of bacterial overgrowth by organisms other than H. somni, particularly in subacute-to-chronic pneumonias (Corbeil et al. 1986; Tegtmeier et al. 2000). Pasteurella multocida and Mannheimia haemolytica propagate quickly and overgrow H. somni. Like others, we find that IHC of formalin-fixed tissue is useful in that it gives a sense of how much H. somni antigen is present and its relationship to lesions and therefore its likely importance (Haines et al. 2004; Clark 2005). Lesions in the heart, typically in anterior or posterior papillary muscle of left ventricle, are good candidates for culture. It is rare that confounding bacteria, such as Mycoplasma bovis, are detected at this site (Haines et al. 2004).
For culture, if only one fresh sample is taken per tissue, it is better to submit pieces of tissue rather than a swab. Lesioned tissue generates the largest volume of bacteria and increases the odds of a successful culture. An appropriate sample size is 5.0 × 5.0 × 2.5 cm (2 × 2 × 1 in.). If the sample is fluid, 1 ml should be collected and transferred to a red-top tube before shipment to the laboratory. Where arthritis is the main presentation, it is useful to ask the practitioner to saw long bones proximal and distal to the joint, and submit the affected joint with capsule intact for laboratory sampling. The value of using nasopharyngeal swabs from live cattle is limited and so is rarely used; there is some evidence that H. somni preferentially colonizes the lower respiratory tract rather than nasal mucosa in calves with pulmonary disease (Corbeil 2007). Some animals are nasopharyngeal carriers of nonpathogenic H. somni. Therefore, isolation of H somni from such swabs may not indicate that it is the primary pathogen found in the lower respiratory tract. Trans-tracheal washes circumvent this problem, but these are almost never used by our clients. Isolation of H. somni from the vagina in cases of herd infertility needs to be interpreted with caution. A role is plausible when there is heavy bacterial growth, combined with post-breeding endometritis, vaginitis, and a purulent vaginal discharge (Yaeger and Holler 2007).
Isolation of H. somni by culture is less sensitive than PCR for H. somni (Tegtmeier et al. 2000; Bell et al. 2014). Multiplex PCR assays have been developed for H. somni in lung and in sheep semen (Tegtmeier et al. 2000; Saunders et al. 2007; Moustacas et al. 2013). Quantitative PCR, whether multiplex or in an individual assay, is a sensitive way to detect H. somni. Several North American laboratories using an H. somni PCR bundle it with probes for other respiratory pathogens. Detecting H. somni in samples by PCR is valuable, but no antimicrobial information is generated unless bacterial isolation is successful. Culture has the advantage of providing the laboratory isolates for use in antimicrobial susceptibility assays, MALDI-TOF mass spectroscopic characterization, other comparative analyses, and epidemiological studies (Tegtmeier et al. 2000; Portis et al. 2012; Kuhnert et al. 2012; Frey and Kuhnert 2015). Antimicrobial susceptibility assays for H. somni include disk diffusion (Kirby-Bauer, Edisk) and broth microdilution (D’Amours et al. 2011; Goldspink et al. 2015). H. somni is susceptible to multiple antimicrobial classes in vitro. Susceptibility to individual antimicrobials includes ceftiofur, penicillin, enrofloxacin, and florfenicol. Resistance to tetracyclines is emerging in the USA and Australia (D’Amours et al. 2011; Portis et al. 2012).
H. somni can be isolated from the upper respiratory tract, semen, vagina, and preputial mucosa. Bovine and ovine H. somni isolates can be differentiated using PCR amplification and restriction enzyme digests of the rpoB gene (Tanaka et al. 2005). At present, it is difficult to readily distinguish pathogenic from nonpathogenic isolates of H. somni in a conventional diagnostic laboratory. This requires inoculating cattle and the involvement of a H. somni specialist. When pneumonia is chronic and multiple pathogens are isolated, it would be helpful to clients to determine which agents are primary and which are secondary. This is not usually possible. IHC increases the overall rate of positive diagnosis of pulmonary and cardiac histophilosis, particularly in tissues from antibiotic-treated animals (Tegtmeier et al. 1995; Bryson et al. 1990).
Given the pitfalls of attempting to grow a fastidious bacterium like H. somni from tissue or swabs from tissues with varying degrees of autolysis, sometimes in the presence of other pathogens, and following antibiotic treatment, one approach to diagnosis is a combination of IHC and PCR when H. somni is suspected. Surprisingly, only one (California) of 33 state/provincial laboratories in the USA and Canada is listed on the AAVLD PCR Web site as offering a PCR test for H. somni (Graham 2015). The most commonly used protocol is based on one developed in Denmark, which detects the 16S rRNA gene of H. somni (Angen et al., 1998). In addition to the California laboratory, which now uses a culture MALDI-TOF MS approach, three state laboratories in the USA (Kansas, Iowa, and Nebraska) have deployed or are implementing a PCR test for H. somni.
A tentative identification of H. somni is based on the growth of small tan/yellow-white colonies on blood agar plates incubated in 10 % CO2, with no or very little growth in air. A final identification is based on Gram staining (pleomorphic Gram-negative bacilli or coccobacilli, 1 μm × 1–3 μm in size), oxidase-positive, catalase-negative, and variable indole production, fermentation of glucose, and nitrate reduction (Markey et al. 2013). H. somni is often isolated from lungs along with other bacterial pathogens such as P. multocida or M. haemolytica. Identification of H. somni based on culture alone is likely to underestimate the incidence of disease. In one Danish study that compared culture with IHC, strong immunostaining was seen in 15 of 17 culture-positive cases, as well as in 20 pneumonic lungs from which H. somni was not isolated, although Histophilus-compatible lesions were present (Tegtmeier et al. 1995). Similar findings were obtained in a retrospective study of H. somni in bison, comparing isolation with IHC (Dyer 2001). These results suggest that laboratories relying on culture alone for an etiological diagnosis will underestimate the presence of H. somni in pneumonic lungs.
Microagglutination assays have been used to monitor populations of cattle for infection, particularly in the early feedlot period. Unfortunately, many clinically healthy cattle have high serological titers (Widders et al. 1986). Currently, the use of serological assays for diagnostic purposes, based on antibodies to whole H. somni, is of limited value; moreover, such assays are not widely available. Their value is small due to H. somni inhabiting mucosal sites in asymptomatic cattle, because of the possibility of subclinical infections, cross-reactive antibodies with other bacteria, or the confounding effects of vaccination (Kania et al. 1990; Corbeil 2007; Pan et al. 2014). The recent development of an enzyme-linked immunosorbent assay (ELISA) using antibodies to H. somni exopolysaccharide (EPS) (described in a later chapter), a major component of the H. somni biofilm matrix, holds promise of having an application in diagnostic settings (Pan et al. 2014). Exopolysaccharide is generated only when H. somni forms biofilm or is under stress conditions, so serological responses are less likely in healthy and recently vaccinated cattle. A disadvantage of the EPS ELISA is that EPS is a relatively weak immunogen, and serological responses can be short-lived (6–10 weeks post-infection). An ELISA has also been developed to the virulence factor IbpA, particularly its A5 subunit, which is more immunogenic. The serological response of infected calves, as detected by the A5 IbpA ELISA, tends to be more prolonged (up to 10 weeks post-infection) (Lo et al. 2012). The diagnostic value of the EPS and IbpA ELISAs in confirming natural outbreaks of H. somni remains to be established.
Once a laboratory diagnosis of HSDC is made, it is helpful to alert veterinarians that continuing sporadic illness may continue for weeks or months, particularly when the initial case is seen in fall–early winter.
5 Summary
We mentioned in the introduction that a disparity exists between the perceived significance of histophilosis in Canada, where it is high, versus elsewhere in the world, not least in the USA, where it is considerably lower. It is possible that its documented importance in Canada is related to regional factors, such as strain differences, feedlot practices, or harsh climate. What strikes us is the excellence of Canadian investigational work since the mid-1980s, when Dr. Peter Little’s group was first active, which helped clarify the importance of histophilosis in the feedlot industry. Comparable field studies have not been performed in the USA. Such studies are unfashionable and expensive, albeit critical for a large and concentrated animal industry (Kelly and Janzen 1986). There may be a tendency to group all bacterial agents of BRD complex under one rubric and assume that they behave similarly in response to stress, clinical recognition, treatment, and vaccination (Griffin et al. 2010). Yet the overall yearly bovine mortality rates have not changed appreciably, and the risk of death attributable to respiratory tract disorders has increased compared to the risk of death in 1994 (Loneragan et al. 2001). Additional field studies seem essential to assess the importance of the disease to the cattle industry in the USA.
Abbreviations
- BRD:
-
Bovine respiratory disease
- CNS:
-
Central nervous system
- HSDC:
-
H somni disease complex
- IHC:
-
Immunohistochemistry
- MALDI-TOF MS:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- NET:
-
Neutrophil extracellular trap
- PCR:
-
Polymerase chain reaction
- TME:
-
Thrombotic meningoencephalitis
References
Agnes JT, Zekarias B, Shao M, Anderson ML, Gershwin LJ, Corbeil LB (2013) Bovine respiratory syncytial virus and Histophilus somni interaction at the alveolar barrier. Infect Immun 81:2592–2597
Amstutz HE, Horstman LA, Morter RL (1981) Clinical evaluation of the efficacy of Haemophilus somnus and Pasteurella sp. bacterins. Bov Pract 16:106–108
Andrews JJ, Anderson TD, Slife LN, Stevenson GW (1985) Microscopic lesions associated with the isolation of Haemophilus somnus from pneumonic bovine lungs. Vet Pathol 22:131–136
Angen Ø, Ahrens P, Tegtmeier C (1998) Development of a PCR test for identification of Haemophilus somnus in pure and mixed cultures. Vet Microbiol 63:39–48
Angen Ø, Ahrens P, Kuhnert P, Christensen H, Mutters R (2003) Proposal of Histophilus somni gen. nov., sp. nov. for the three species incertae sedis ‘Haemophilus somnus’, ‘Haemophilus agni’ and ‘Histophilus ovis’. Int J Syst Evol Microbiol 53:1449–1456
Bailie WE (1969) Characterization of Haemophilus somnus, a new species, a microorganism isolated from infectious thromboembolic meningoencephalitis of cattle. PhD thesis, Kansas State University, Manhattan, KS
Ball HJ, Kennedy S, Ellis WA (1991) Experimental reproduction of ovine vulvitis with bacteria of the haemophilus/histophilus group. Res Vet Sci 50:81–85
Bano L, Bonci M, Drigo I, Tonon E, Mazzolini E, Carminato A, Granato A, Ceglie L, Natale A, Zanette G, Agnoletti F (2011) Recurrent detection of Histophilus somni in the genital tract of dairy cattle with reproductive failures in Italy. Large Anim Review 17:171–176
Behling-Kelly E, Vonderheid J, Kim KS (2006) Roles of cellular activation and sulfated glycans in Haemophilus somnus adherence to bovine brain microvascular endothelial cells. Infect Immun 74:5311–5318
Bell CJ, Blackburn P, Elliott M, Patterson TI, Ellison S, Lahuerta-Marin A, Ball HJ (2014) Investigation of polymerase chain reaction assays to improve detection of bacterial involvement in bovine respiratory disease. J Vet Diagn Invest 26:631–634
Berezowski J: Hemophilosis. In: Diseases of bison. Specialized Livestock Health and Production, Western College of Veterinary Medicine, Saskatoon, Canada. http://www.usask.ca/wcvm/herdmed/specialstock/bison/bisondis.html#Hemophilosis
Besser TE, Cassirer EF, Potter KA, Lahmers K, Oaks JL, Shanthalingam S, Srikumaran S, Foreyt WJ (2014) Epizootic pneumonia of bighorn sheep following experimental exposure to Mycoplasma ovipneumoniae. PLoS One 10, 9(10):e110039
Booker CW, Guichon PT, Jim GK, Schunicht OC, Harland RJ, Morley PS (1999) Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can Vet J 40:40–48
Booker CW, Abutarbush SM, Morley PS, Jim GK, Pittman TJ, Schunicht OC, Perrett T, Wildman BK, Fenton RK, Guichon PT, Janzen ED (2008) Microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in Western Canada. Can Vet J 49:473–481
Bowland SL, Shewen PE (2000) Bovine respiratory disease: commercial vaccines currently available in Canada. Can Vet J 41:33–48
Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5:577–582
Bryson DG, Ball HJ, McAliskey M, McConnell W, McCullough SJ (1990) Pathological, immunocytochemical and microbiological findings in calf pneumonias associated with Haemophilus somnus infection. J Comp Pathol 103:433–445
Cassidy JP, McDowell SW, Reilly GA, McConnell WJ, Forster F, Lawler D (1997) Thrombotic meningoencephalitis associated with Histophilus ovis infection in lambs in Europe. Vet Rec 140:193–195
Caswell JL, Williams KJ (2007) Respiratory system. In: Maxie MG (ed) Jubb, Kennedy, and Palmer’s pathology of domestic animals, vol 2, 5th edn. Saunders Elsevier, Edinburgh, p 567
Caswell JL, Bateman KG, Cai HY, Castillo-Alcala F (2010) Mycoplasma bovis in respiratory disease of feedlot cattle. Vet Clin North Am Food Anim Pract 26:365–379
Caswell JL, Hewson J, Slavić D, DeLay J, Bateman K (2012) Laboratory and postmortem diagnosis of bovine respiratory disease. Vet Clin North Am Food Anim Pract 28:419–441
Chladek DW (1975) Bovine abortion associated with Haemophilus somnus. Am J Vet Res 36:1041
Clark T (2005) Histophilus somni—unique features, pathogenesis and lesions update. Proc Am Assoc Bov Pract 38:68–71
Cooper VL, Brodersen BW (2010) Respiratory disease diagnostics of cattle. Vet Clin North Am Food Anim Pract 26:409–416
Corbeil LB (2007) Histophilus somni host-parasite relationships. Anim Health Res Rev 8:151–160
Corbeil LB, Widders PR, Gogolewski R, Arthur J, Inzana TJ, Ward AC (1986) Haemophilus somnus: bovine reproductive and respiratory disease. Can Vet J 27:90–93
Czuprynski CJ, Leite F, Sylte M, Kuckleburg C, Schultz R, Inzana T, Behling-Kelly E, Corbeil L (2004) Complexities of the pathogenesis of Mannheimia haemolytica and Haemophilus somnus infections: challenges and potential opportunities for prevention? Anim Health Res Rev 5:277–282
D’Amours GH1, Ward TI, Mulvey MR (2011) Genetic diversity and tetracycline resistance genes of Histophilus somni. Vet Microbiol 150:362–372
De Lahunta A, Divers TJ (2008) Thrombotic meningoencephalitis. In: Rebhun’s Diseases of Dairy Cattle, pp. 508–510, 520. Saunders Elsevier 2nd edition
Descarga CO, Piscitelli HG, Zielinski GC (2002) Thromboembolic meningoencephalitis due to Haemophilus somnus in feedlot cattle in Argentina. Vet Rec 150:817
Díaz-Aparicio E, Tenorio-Gutiérrez VR, Arellano-Reynoso B (2009) Pathogenicity of different strains of Histophilus somni in the experimental induction of ovine epididymitis. Can J Vet Res 73:157–160
Dukes TW (1971) The ocular lesions in thromboembolic meningoencephalitis (ITEME) of cattle. Can Vet J 12:180–182
Dyer NW (2001) Haemophilus somnus bronchopneumonia in American bison (Bison bison). J Vet Diagn Invest 13:419–421
Dyer NW, Ward ACS (1998) Pneumonic pasteurellosis associated with Pasteurella haemolytica serotype A6 in American bison. J Vet Diagn Invest 10:360–362
Ellis JA, Yong C (1997) Systemic adverse reactions in young Simmental calves following administration of a combination vaccine. Can Vet J 38:45–47
Elswaifi SF, Scarratt WF, Inzana TJ (2012) The role of lipooligosaccharide phosphorylcholine in colonization and pathogenesis of Histophilus somni in cattle. Vet Res 43:49
Fecteau G, George LW (2004) Bacterial meningitis and encephalitis in ruminants. Vet Clin North Am Food Anim Pract 20:363–377
Filho AT, Roll C, Soares CL, Carambula SF (2001) The blood supply of the papillary muscles of the left ventricle in the Hereford cattle. Italian J Anat Embryol 106:293–298
Francoz D, Buczinski S, Bélanger AM, Forté G, Labrecque O, Tremblay D, Wellemans V, Dubuc J (2015) Respiratory pathogens in Québec dairy calves and their relationship with clinical status, lung consolidation, and average daily gain. J Vet Int Med 29:381–387
Frey J, Kuhnert P (2015) Identification of animal Pasteurellaceae by MALDI-TOF mass spectrometry. Methods Mol Biol 1247:235–243
Gagea MI, Bateman KG, van Dreumel T, McEwen BJ, Carman S, Archambault M, Shanahan RA, Caswell JL (2006) Diseases and pathogens associated with mortality in Ontario beef feedlots. J Vet Diagn Invest 18:18–28
Geertsema RS, Zekarias B, Scheuch L la F, Worby C, Russo R, Gershwin LJ, Herdman DS, Lo K, Corbeil LB (2011) IbpA DR2 subunit immunization protects calves against Histophilus somni pneumonia. Vaccine 29:4805–4812
George JL, Martin DJ, Lukacs PM, Miller MW (2008) Epidemic pasteurellosis in a bighorn sheep population coinciding with the appearance of a domestic sheep. J Wildl Dis 44:388–403
Gogolewski RP, Leathers CW, Liggitt HD, Corbeil LB (1987a) Experimental Haemophilus somnus pneumonia in calves and immunoperoxidase localization of bacteria. Vet Pathol 24:250–256
Gogolewski RP, Kania SA, Inzana TJ, Widders PR, Liggitt HD, Corbeil LB (1987b) Protective ability and specificity of convalescent serum from calves with Haemophilus somnus pneumonia. Infect Immun 55:1403–1411
Gogolewski RP, Kania SA, Liggitt HD, Corbeil LB (1988) Protective ability of antibodies against 78- and 40-kilodalton outer membrane antigens of Haemophilus somnus. Infect Immun 56:2307–2316
Gogolewski RP, Schaefer DC, Wasson SK, Corbeil RR, Corbeil LB (1989) Pulmonary persistence of Haemophilus somnus in the presence of specific antibody. J Clin Microbiol 27:1767–1774
Goldspink LK, Mollinger JL, Barnes TS, Groves M, Mahony TJ, Gibson JS (2015) Antimicrobial susceptibility of Histophilus somni isolated from clinically affected cattle in Australia. Vet J 203:239–243
Graham T (2015) PCR Database. South Dakota State University. http://pcr.sdstate.org/
Griffin D (2012) Field necropsy of cattle and diagnostic sample submission. Vet Clin North Am Food Animal Pract 28:391–405
Griffin D (2014) The monster we don’t see: subclinical BRD in beef cattle. Anim Health Res Rev 15:138–141
Griffin D, Chengappa MM, Kuszak J, McVey DS (2010) Bacterial pathogens of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract 26:381–394
Griner LA, Jensen R, Brown WW (1956) Infectious embolic meningoencephalitis in cattle. J Am Vet Med Assoc 129:417–421
Grissett GP, White BJ, Larson RL (2015) Structured literature review of responses of cattle to viral and bacterial pathogens causing bovine respiratory disease complex. J Vet Intern Med 29:770–780
Groom SC, Little PB (1988) Effects of vaccination of calves against induced Haemophilus somnus pneumonia. Am J Vet Res 49:793–800
Guichon PT, Pritchard J, Jim GK (1988) Haemophilus somnus myocarditis in a feedlot steer. Can Vet J 29:1012–1013
Haines DM, Martin KM, Clark EG, Jim GK, Janzen ED (2001) The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis. Can Vet J 42:857–860
Haines DM, Moline KM, Sargent RA, Campbell JR, Myers DJ, Doig PA (2004) Immunohistochemical study of Hemophilus somnus, Mycoplasma bovis, Mannheimia hemolytica and bovine viral diarrhea virus in death losses due to myocarditis in feedlot cattle. Can Vet J 45:231–234
Hajtós I (1987) Isolation of Actinobacillus seminis and Histophilus ovis strains from aborted ovine fetuses in Hungary. Acta Vet Hung 35:415–425
Hall RF, Williams JM, Smith GL (1977) Field evaluation of Haemophilus somnus bacterin. Vet Med Small Anim Clin 72:1368–1370
Harris FW, Janzen ED (1989) The Haemophilus somnus disease complex (Hemophilosis): a review. Can Vet J 30:816–822
Hazlett MJ, Little PB, Barnum DA (1983) Experimental production of mastitis with Haemophilus somnus in the lactating bovine mammary gland. Can Vet J 24:135–136
Headley SA, Oliveira VH, Figueira GF (2013) Histophilus somni-induced infections in cattle from southern Brazil. Trop Anim Health Prod 45:1579–1588
Headley SA, Voltarelli D, de Oliveira VHS, Bronkhorst DE, Alfieri AF, Filho LC, Okano W, Alfieri AA (2015) Association of Histophilus somni with spontaneous abortions in dairy cattle herds from Brazil. Trop Anim Health Prod 47:403–413
Hellenbrand KM, Forsythe KM, Rivera-Rivas JJ, Czuprynski CJ, Aulik NA (2013) Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb Pathog 54:67–75
Higgins R, Godbout-Delasalle F, Messiers S, Couture Y, Lamothe P (1981) Isolation of Histophilus ovis from vaginal discharge in ewes in Canada. Can Vet J 22:395–396
Humphrey JD, Little PB, Stephens LR, Barnum DA, Doig PA, Thorsen J (1982) Prevalence and distribution of Haemophilus somnus in the male bovine reproductive tract. Am J Vet Res 43:791–795
Inzana TJ, Gogolewski RP, Corbeil LB (1992) Phenotypic phase variation in Haemophilus somnus lipooligosaccharide during bovine pneumonia and after in vitro passage. Infect Immun 60:2943–2951
Jackson JA, Andrews JJ, Hargis JW (1987) Experimental Haemophilus somnus pneumonia in calves. Vet Pathol 24:129–134
Jánosi K, Hajtó I, Makrai L, Gyuranecz M, Varga J, Fodor L (2009) First isolation of Histophilus somni from goats. Vet Microbiol 133:383–386
Janzen ED (1987) Myocardial lesions caused by Haemophilus somnus in cattle. Can Vet J 28:208
Kaneene JB, Coe PH, Gibson CD, Yamini B, Marinez RO, Morrow DA (1986a) The role of Haemophilus somnus in early embryonic death. I. The effect of the organism on embryos by day 8 postbreeding. Theriogenology 26:189–198
Kaneene JB, Coe PH, Gibson CD, Yamini B, Morrow DA, Marinez RO (1986b) The role of Haemophilus somnus in bovine early embryonic death. III. The effect of the organism on embryos by day 21 postbreeding. Theriogenology 27:737–749
Kania SA, Gogolewski RP, Corbeil LB (1990) Isolation and characterization of a 78-kilodalton outer membrane protein of Haemophilus somnus. Infect Immun 58:237–244
Kelly AP, Janzen ED (1986) A review of morbidity and mortality rates and disease occurrence in North American feedlot cattle. Can Vet J 27:496–500
Kennedy PC, Frazier LM, Theilen GH, Biberstein EL (1958) A septicemic disease of lambs caused by Haemophilus agni (new species). Am J Vet Res 19:645–654
Kennedy PC, Biberstein EL, Howarth JA, Frazier LM, Dungworth DL (1960) Infectious meningo-encephalitis in cattle, caused by a Haemophilus-like organism. Am J Vet Res 21:403–409
Kimberling CV (1988) Epididymo-orchitis. Jensen and Swift’s Diseases of Sheep, 3rd edn. Lea and Febiger, Philadelphia, pp 11–13
Kirkbride CA (1993a) Bacterial agents detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Invest 5:64–68
Kirkbride CA (1993b) Diagnoses in 1784 ovine abortions and stillbirths. J Vet Diagn Invest 5:398–402
Kuhnert P, Bisgaard M, Korczak BM, Schwendener S, Christensen H, Frey J (2012) Identification of animal Pasteurellaceae by MALDI-TOF mass spectrometry. J Microbiol Methods 89:1–7
Kwiecien JM, Little PB (1991) Haemophilus somnus and reproductive disease in the cow: A review. Can Vet J 32:595–601
Kwiecien JM, Little PB (1992) Isolation of pathogenic strains of Haemophilus somnus from the female bovine reproductive tract. Can J Vet Res 56:127–134
Lamont HH, Hunt BW (1982) Haemophilus somnus and conjunctivitis. Vet Rec. 111:21
Larson RL, Step DL (2012) Evidence-based effectiveness of vaccination against Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni in feedlot cattle for mitigating the incidence and effect of bovine respiratory disease complex. Vet Clin N America, Food Animal Practice 28:97–106
Last RD, Macfarlane MD, Jarvis CJ (2001) Isolation of Haemophilus somnus from dairy cattle in kwaZulu-Natal. An emerging cause of ‘dirty cow syndrome’ and infertility? J S Afr Vet Assoc 72:95
Lees VW, Meek AH, Rosendal S (1990) Epidemiology of Haemophilus somnus in young rams. Can J Vet Res 54:331–336
Lees VW, Yates WD, Corbeil LB (1994) Ovine Haemophilus somnus: experimental intracisternal infection and antigenic comparison with bovine Haemophilus somnus. Can J Vet Res 58:202–210
Little PB (1986) The Haemophilus somnus complex. In: Howard JL (ed) Current veterinary therapy 3. Food animal practice. WB Saunders Company, Philadelphia, pp 546–548
Lo KL, Kimball RA, Lehmann J, Gershwin LJ, Worby C, Corbeil LB (2012) Antibody responses of calves to Histophilus somni recombinant IbpA subunits. Comp Immunol Microbiol Infect Dis 35:453–459
Loneragan GH, Dargatz DA, Morley PS, Smith MA (2001) Trends in mortality ratios among cattle in US feedlots. J Am Vet Med Assoc 219:1122–1127
Lundberg MS (1986) Isolation of Haemophilus agni from six Alberta ram lambs with septicemia. Can Vet J 27:501–503
Markey B, Leonard F, Archambault M, Cullinane A, Maguire D (2013) Clinical Veterinary Microbiology, 2nd edn. Mosby Elsevier, Edinburgh, pp 349–354
Maxie MG, Youssef S (2007) Nervous system. In: Maxie G (ed) Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, vol 1, 5th edn. Saunders, Philadelphia, pp 408–411
McEwen SA, Hulland TJ (1985) Haemophilus somnus-induced otitis and meningitis in a heifer. Can Vet J 26:7–8
Miller MW (2001) Pasteurellosis. In: Williams ES, Barker IK (eds) Infectious diseases of wild mammals, 3rd edn. Iowa State University Press, Ames, pp 330–339
Miller RB, Barnum DA, McEntee KE (1983a) Hemophilus somnus in the reproductive tracts of slaughtered cows: location and frequency of isolations and lesions. Vet Pathol 20:515–521
Miller RB, Van Camp SD, Barnum DA (1983b) The effects of intra-amniotic inoculation of Hemophilus somnus on the bovine fetus and dam. Vet Pathol 20:574–583
Miller DS, Weiser GC, Ward ACS, Drew ML, Chapman PL (2012) Pasteurellaceae isolated from bighorn sheep (Ovis canadensis) from Idaho, Oregon, and Wyoming. Am J Vet Res 73:1024–1028
Mitchell CA (1925) Haemophilus ovis (nov. spec.) as the cause of a specific disease in sheep. J Am Vet Med Assoc 68:8–18
Moisan PG, Fitzgerald SD (1995) Haemophilus somnus myocarditis in feedlot cattle. Agri-Practice 16:21–24
Morter RL, Amstutz HE (1983) Evaluation the efficacy of a Haemophilus somnus bacterin in a controlled field trial. Bov Practitioner 18:82–83
Morter R, Amstutz H, Crandell R (1982) Clinical evaluation of prophylactic regimens for bovine respiratory disease. Bovine Pract 17:56
Moustacas VS, Silva TMA, Costa LF, Xavier MN, Carvalho CA Jr, Costa ÉA, Paixão TA, Santos RL (2013) Species-specific multiplex PCR for the diagnosis of Brucella ovis, Actinobacillus seminis, and Histophilus somni infection in ram. BMC Vet Res 9:51
Nation PN, Frelier PF, Gifford GA, Carnat BD (1983) Otitis in feedlot cattle. Can Vet J 24:238
Nayar PS, Ward GE, Saunders JR, MacWilliams P (1977) Diagnostic procedures in experimental Hemophilus somnus infection in cattle. Can Vet J 18:159–163
Nietfeld JC (2012) Neuropathology and diagnostics in food animals. Vet Clin North Am Food Anim Pract 28:515–534
O’Toole D, Allen T, Hunter R, Corbeil LB (2009) Diagnostic exercise: Myocarditis due to Histophilus somni in feedlot and backgrounded cattle. Vet Pathol 46:1015–1017
Orr JP (1992) Haemophilus somnus infection: a retrospective analysis of cattle necropsied at the Western College of Veterinary Medicine from 1970 to 1990. Can Vet J 33:719–722
Orr J, Chirino-Trejo M, Haines D, Schwab M, Moisan P, Ebbott D, Uhleg T (1992) Alberta. Thrombotic encephalitis, myocarditis, and pneumonia in lambs. Can Vet J 33:277
Pan Y, Fisher T, Olk C, Inzana TJ (2014) Detection of antibodies to the biofilm exopolysaccharide of Histophilus somni following infection in cattle by enzyme-linked immunosorbent assay. Clin Vaccine Immunol 21:1463–1467
Panciera RJ, Confer AW (2010) Pathogenesis and pathology of bovine pneumonia. Vet Clin North Am Food Anim Pract 26:191–214
Panciera RJ, Dahlgren RR, Rinker HB (1968) Observations on septicemia of cattle caused by a Haemophilus-like organism. Pathol Vet 5:212–226
Philbey AW, Glastonbury JR, Rothwell JT, Links IJ, Searson JE (1991) Meningoencephalitis and other conditions associated with Histophilus ovis infection in sheep. Aust Vet J 68:387–390
Poonacha KB, Donahue JM (1984) Haemophilus ovis infection in lambs. Vet Med 79:541–542
Portis E, Lindeman C, Johansen L, Stoltman G (2012) A ten-year (2000–2009) study of antimicrobial susceptibility of bacteria that cause bovine respiratory disease complex—Mannheimia haemolytica, Pasteurella multocida and Histophilus somni —in the United States and Canada. J Vet Diagn Invest 24:932–944
Post G (1962) Pasteurellosis of Rocky Mountain bighorn sheep (Ovis canadensis canadensis). J Wildl Dis 23:1–14
Potgieter LND, Helman RG, Greene W, Breider MA, Thurber ET, Peetz RH (1988) Experimental bovine respiratory tract disease with Haemophilus somnus. Vet Pathol 25:124–130
Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, FitzPatrick ES (2007) Histophilus somni, Haemophilus parasuis, and Avibacterium paragallinarum. Veterinary Microbiology and Microbial Disease, 2nd edn. Wiley-Blackwell, Oxford, pp 314–320
Rahaley RS (1978a) Pathology of experimental Histophilus ovis infection in sheep. I Lambs Vet Pathol 15:631–637
Rahaley RS (1978b) Pathology of experimental Histophilus ovis infection in sheep. II. Pregnant ewes. Vet Pathol 15:746–752
Rahaley RS, White WE (1977) Histophilus ovis infection in sheep in western Victoria. Aust Vet J 53:124–127
Ranganathan N, Burch GE (1969) Gross morphology and arterial supply of the papillary muscles of the left ventricle of man. Am Heart J 77:506–516
Ribble CS, Jim GK, Janzen ED (1988) Efficacy of immunization of feedlot calves with a commercial Haemophilus somnus bacterin. J Vet Res 52:191–198
Ridler AL, Sargison ND (2007) New Zealand. In: Aitken ID (ed) Diseases of Sheep, 4th edn, Blackwell Publishing Inc, Oxford, p 508
Romero A, Quinteros C, Marinho P, O’Tool D, Dutra F (2013) Thrombotic meningoencephalitis (TME) by Histophilus somni in sheep in Uruguay. Veterinaria (Montevideo) 49:38–44
Ruegg PL, Marteniuk JV, Kaneene JB (1988) Reproductive difficulties in cattle with antibody titers to Haemophilus somnus. J Am Vet Med Assoc 193:941–942
Sandal I, Inzana TJ (2010) A genomic window into the virulence of Histophilus somni. Trends Microbiol 18:90–99
Sandal I, Hong W, Swords WE, Inzana TJ (2007) Characterization and comparison of biofilm development by pathogenic and commensal isolates of Histophilus somni. J Bacteriol 189:8179–8185
Sandal I, Shao JQ, Annadata S, Apicella MA, Boye M, Jensen TK, Saunders GK, Inzana TJ (2009) Histophilus somni biofilm formation in cardiopulmonary tissue of the bovine host following respiratory challenge. Microbes Infect 11:254–263
Saunders JR, Janzen ED (1980) Haemophilus somnus infections. II. A Canadian field trial of a commercial bacterin: clinical and serological results. Can Vet J 21:219–224
Saunders JR, Thiessen WA, Janzen ED (1980) Haemophilus somnus infections. I. A ten year (1969–1978) retrospective study of losses in cattle herds in Western Canada. Can Vet J 21:119–123
Saunders VF, Reddacliff LA, Berg T, Hornitzky M (2007) Multiplex PCR for the detection of Brucella ovis, Actinobacillus seminis and Histophilus somni in ram semen. Aust Vet J 85:72–77
Schiefer B, Ward GE, Moffatt RE (1978) Correlation of microbiological and histological findings in bovine fibrinous pneumonia. Vet Pathol 15:313–332
Schuh J, Harland R (1991) Haemophilus somnus myocarditis versus thrombotic meningoencephalitis in western Canada. Can Vet J 32:439
Siddaramappa S, Challacombe JF, Duncan AJ, Gillaspy AF, Carson M, Gipson J, Orvis J, Zaitshik J, Barnes G, Bruce D, Chertkov O, Detter JC, Han CS, Tapia R, Thompson LS, Dyer DW, Inzana TJ (2011) Horizontal gene transfer in Histophilus somni and its role in the evolution of pathogenic strain 2336, as determined by comparative genomic analyses. BMC Genom 12:570
Silva SV, Little PB (1990) The protective effect of vaccination against experimental pneumonia in cattle with Haemophilus somnus outer membrane antigens and interference by lipopolysaccharide. Can J Vet Res 54:326–330
Stephens LR (1990) Positive and negative aspects of host immune response to Haemophilus, Actinobacillus and Pasteurella. Can J Vet Res 54(Suppl):S41–S44
Stephens LR, Little PB, Wilkie BN, Barnum DA (1981) Infectious thromboembolic meningoencephalitis in cattle: a review. J Am Vet Med Assoc 178:378–384
Stephens LR, Little PB, Humphrey JD, Wilkie BN, Barnum DA (1982) Vaccination of cattle against experimentally induced thromboembolic meningoencephalitis with a Haemophilus somnus bacterin. Am J Vet Res 43:1339–1342
Stephens LR, Little PB, Wilkie BN, Barnum DA (1984) Isolation of Haemophilus somnus antigens and their use as vaccines for prevention of bovine thromboembolic meningoencephalitis. Am J Vet Res 45:234–239
Stephens LR, Slee KJ, Poulton P, Larcombe M, Kosior E (1986) Investigation of purulent vaginal discharge in cows with particular reference to Haemophilus somnus. Aust Vet J 63:182–185
Sylte MJ, Corbeil LB, Inzana TJ, Czuprynski CJ (2001) Haemophilus somnus induces apoptosis in bovine endothelial cells in vitro. Infect Immun 69:1650–1656
Tanaka A, Hoshinoo K, Hoshino T, Tagawa Y (2005) Differentiation between bovine and ovine strains of Histophilus somni based on the sequences of 16S rDNA and rpoB gene. J Vet Med Sci 67:255–262
Taylor JD, Confer AW (2014) Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Bibersteinia trehalosi. In: Smith B (ed) Large Animal Internal Medicine, 5th edn. Elsevier, St. Louis, pp 1477–1481
Tegtmeier C, Jensen NE, Jensen HE (1995) Development of a peroxidase-antiperoxidase (PAP) technique for the identification of Haemophilus somnus in pneumonic calf lungs in Denmark. APMIS 103:540–547
Tegtmeier C, Bloch B, Jensen NE, Jensen HE (1999a) Initial lung lesions in two calves experimentally infected with Haemophilus somnus. Zentralbl Veterinarmed B 46:517–523
Tegtmeier C, Uttenthal A, Friis NF, Jensen NE, Jensen HE (1999b) Pathological and microbiological studies on pneumonic lungs from Danish calves. Zentralbl Veterinarmed B 46:693–700
Tegtmeier C, Angen Ø, Ahrens P (2000) Comparison of bacterial cultivation, PCR, in situ hybridization and immunohistochemistry as tools for diagnosis of Haemophilus somnus pneumonia in cattle. Vet Microbiol 76:385–394
Teixeira Filho A, Roll C, Soares CL, Carambula SF (2001) The blood supply of the papillary muscles of the left ventricle in the Hereford cattle. Ital J Anat Embryol 106:293–298
USDA (2011) Feedlot 2011. Part IV: Health and health management on U.S. Feedlots with a capacity of 1,000 or More Head. USDA–APHIS–VS–CEAH–NAHMS. Fort Collins, CO
van der Burgt GM (2008) An investigation into fertility problems in a dairy herd possibly associated with Histophilus somni (previously Haemophilus somnus). Cattle Pract 16:86–90
Van Donkersgoed J, Janzen ED, Harland RJ (1990) Epidemiological features of calf mortality due to hemophilosis in a large feedlot. Can Vet J 31:821–825
Van Donkersgoed J, Janzen ED, Potter AA, Harland RJ (1994a) The occurrence of Haemophilus somnus in feedlot calves and its control by postarrival prophylactic mass medication. Can Vet J 35:573–580
Van Donkersgoed J, Potter AA, Mollison B, Harland RJ (1994b) The effect of a combined Pasteurella haemolytica and Haemophilus somnus vaccine and a modified-live bovine respiratory syncytial virus vaccine against enzootic pneumonia in young beef calves. Can Vet J 35:239–241
Van Donkersgoed J, Guenther C, Evans BN, Potter AA, Harland RJ (1995) Effects of various vaccination protocols on passive and active immunity to Pasteurella haemolytica and Haemophilus somnus in beef calves. Can Vet J 36:424–429
Van Donkersgoed J, Berg J, Hendrick S (2009) A comparison of florfenicol-flunixin meglumine versus tulathromycin for the treatment of undifferentiated fever in fall-placed feedlot calves. Vet Ther 10:78–85
Van Dreumel AA, Kierstead M (1975) Abortion associated with Hemophilus somnus infection in a bovine fetus. Can Vet J 16:367–370
Walker RL, LeaMaster BR (1986) Prevalence of Histophilus ovis and Actinobacillus seminis in the genital tract of sheep. Am J Vet Res 47:1928–1930
Ward AC, Dyer NW, Corbeil LB (1999a) Characterization of putative Haemophilus somnus isolates from tonsils of American bison (Bison bison). Can J Vet Res 63:166–169
Ward AC, Dyer NW, Fenwick BW (1999b) Pasteurellaceae isolated from tonsillar samples of commercially-reared American bison (Bison bison). Can J Vet Res 63:161–165
Ward ACS, Weiser GC, Anderson BC, Cummings PJ, Arnold KF, Corbeil LB (2006) Haemophilus somnus (Histophilus somni) in bighorn sheep. Can J Vet Res 70:34–42
Welsh RD, Dye LB, Payton ME, Confer AW (2004) Isolation and antimicrobial susceptibilities of bacterial pathogens from bovine pneumonia: 1994–2002. J Vet Diagn Invest 16:426–431
Widders PR, Paisley LG, Gogolewski RP, Evermann JF, Smith JW, Corbeil LB (1986) Experimental abortion and the systemic immune response to “Haemophilus somnus” in cattle. Infect Immun 54:555–560
Williams JM, Smith GL, Murdock FM (1978) Immunogenicity of a Haemophilus somnus bacterin in cattle. Am J Vet Res 39:1756–1762
Wollums AR (2015) The bronchopneumonias (respiratory disease complex of cattle, sheep, and goats). In: Smith BP (ed) Large Animal Internal Medicine, 5th edn. Elsevier, St. Louis, pp 584–617
Yaeger M, Holler LD (2007) Bacterial causes of bovine infertility and abortion. In: Youngquist RS (ed) Current therapy in large animal theriogenology. 2 WB Saunders, Philadelphia; pp 389–399
Yarnall M, Widders PR, Corbeil LB (1988) Isolation and characterization of Fc receptors from Haemophilus somnus. Scand J Immunol 28:129–137
Zekarias B, Mattoo S, Worby C, Lehmann J, Rosenbusch RF, Corbeil LB (2010) Histophilus somni IbpA DR2/Fic in virulence and immunoprotection at the natural host alveolar epithelial barrier. Infect Immun 78:1850–1858
Zekarias B, O’Toole D, Lehmann J, Corbeil LB (2011) Histophilus somni IbpA Fic cytotoxin is conserved in disease strains and most carrier strains from cattle, sheep and bison. Vet Microbiol 149:177–185
Acknowledgements
We are grateful to Drs. Lynnette Corbeil and Ted Clark for critical reviews on this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
O’Toole, D., Sondgeroth, K.S. (2015). Histophilosis as a Natural Disease. In: Inzana, T. (eds) Histophilus somni. Current Topics in Microbiology and Immunology, vol 396. Springer, Cham. https://doi.org/10.1007/82_2015_5008
Download citation
DOI: https://doi.org/10.1007/82_2015_5008
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29554-1
Online ISBN: 978-3-319-29556-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)