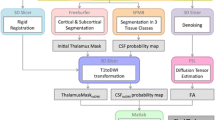
Abstract
Recent work shows that diffusion tensor imaging (DTI) can help resolving thalamic nuclei based on the characteristic fiber orientation of the corticothalamic/thalamocortical striations within each nucleus. In this paper we describe a novel segmentation method based on spectral clustering. We use Markovian relaxation to handle spatial information in a natural way, and we explicitly minimize the normalized cut criteria of the spectral clustering for a better optimization. Using this modified spectral clustering algorithm, we can resolve the organization of the thalamic nuclei into groups and subgroups solely based on the voxel affinity matrix, avoiding the need for explicitly defined cluster centers. The identification of nuclear subdivisions can facilitate localization of functional activation and pathology to individual nuclear subgroups.
Chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
References
Behrens, T.E., Johansen-Berg, H., Woolrich, M.W., Smith, S.M., Wheeler-Kingshott, C.A., Boulby, P.A., Barker, G.J., Sillery, E.L., Sheehan, K., Ciccarelli, O., Thompson, A.J., Brady, J.M., Matthews, P.M.: Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 6(7), 750–757 (2003)
Wiegell, M.R., Tuch, D.S., Larsson, H.B., Wedeen, V.J.: Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. Neuroimage 19, 391–401 (2003)
Beaulieu, C.: The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 15(7-8), 435–455 (2002)
Jonasson, L., Hagmann, P., Richero Wilson, C., Bresson, X., Pollo, C., Meuli, R., Thiran, J.P.: Coupled, region based level sets for segmentation of the thalamus and its subnuclei in DT-MRI. In: ISMRM, vol. 731 (2005)
Shi, J., Malik, J.: Normalized cuts and image segmentation. PAMI 22(8), 888–905 (2000)
Higham, D., Kibble, M.: A unified view of spectral clustering. Mathematics Research Report, 1–17 (2004)
Kannan, R., Vempala, S., Vetta, A.: On clusterings: Good, bad and spectral. J ACM 51(3), 497–515 (2004)
Weiss, Y.: Segmentation using eigenvectors: A unifying view. In: ICCV, vol. 975 (1999)
Jones, D.K., Horsfield, M.A., Simmons, A.: Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. MRM 42(3), 515–525 (1999)
Alexander, D.C., Gee, J.C., Bajcsy, R.: Similarity measures for matching diffusion tensor images. In: BMVC (1999)
Wang, Z., Vemuri, B.C.: An affine invariant tensor dissimilarity measure and its applications to tensor-valued image segmentation. CVPR (1), 228–233 (2004)
Tishby, N., Slonim, N.: Data clustering by markovian relaxation and the information bottleneck method. In: NIPS, pp. 640–646 (2000)
Reese, T.G., Heid, O., Weisskoff, R.M., Wedeen, V.J.: Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. MRM 49(1), 177–182 (2003)
Jenkinson, M., Bannister, P., Brady, M., Smith, S.: Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2), 825–841 (2002)
Basser, P.J., Mattiello, J., LeBihan, D.: Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103(3), 247–254 (1994)
Pierpaoli, C., Basser, P.J.: Toward a quantitative assessment of diffusion anisotropy. MRM 36(6), 893–906 (1996)
Mazziotta, J.C., Toga, A.W., Evans, A., Fox, P., Lancaster, J.: A probabilistic atlas of the human brain: theory and rationale for its development. The international consortium for brain mapping (icbm). Neuroimage 2(2), 89–101 (1995)
Dice, L.R.: Measures of the amount of ecologic association between species. Ecology 26, 297–302 (1945)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2006 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Ziyan, U., Tuch, D., Westin, CF. (2006). Segmentation of Thalamic Nuclei from DTI Using Spectral Clustering. In: Larsen, R., Nielsen, M., Sporring, J. (eds) Medical Image Computing and Computer-Assisted Intervention – MICCAI 2006. MICCAI 2006. Lecture Notes in Computer Science, vol 4191. Springer, Berlin, Heidelberg. https://doi.org/10.1007/11866763_99
Download citation
DOI: https://doi.org/10.1007/11866763_99
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-44727-6
Online ISBN: 978-3-540-44728-3
eBook Packages: Computer ScienceComputer Science (R0)