Abstract
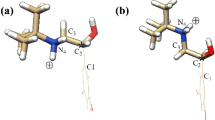
Separation of stereoisomers of organic compounds is an important and challenge task for chemists. Cyclodextrins and their derivatives have been widely used in chromatography for this application. Experimental results indicated that substituents on the hydroxyl groups of cyclodextrin affect the efficiency of the chiral separation of β-butyrolactone. The understanding of the interactions contributed to the chiral recognition of cyclodextrin would help us predict the separation capability of a specific pair of cyclodextrin and chiral compound. Thus, the cyclodextrin substituent effect on the chiral recognition should be systematically investigated. In this study, Hartree Fock method with 3-21G basis set and density functional theory B3LYP with 6-31G* basis set were applied to determine the chiral recognition of a chiral model, β- butyrolactone, by β-cyclodextrin and its derivatives. Both methods predicted comparable values of chiral recognition of β-cyclodextrin derivatives. We found that methoxyl substitution on the wider rim of cyclodextrin (secondary hydroxyl groups) give the most effective chiral separation (ΔΔE=18.2 kcal/mol in favor of R-isomer) followed by substitution on the narrow rim (ΔΔE=9.5 kcal/mol in favor of S-isomer) while substitution on both side give the worst recognition (ΔΔE=3.2 kcal/mol in favor of S-isomer). This suggests that β-cyclodextrin with substitution only on the wider rim give the best chiral selectivity. By replacing methyl group with chiral hydroxypropyl group, we found that the chiral selectivity is reduced (ΔΔE=6.4 and 8.4 kcal/mol respectively for R- and S-form of hydroxypropyl group). This implies that the bulky group causes the reduction of the chiral selectivity.
Chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
References
Li, S., Purdy, W.C.: Cyclodextrins and Their Applications in Analytical Chemistry. Chem. Rev. 92, 1457–1470 (1992)
Lipkowitz, K.B., Pearl, G., Coner, B., Peterson, M.A.: Explanation of Where and How Enantioselective Binding Takes Place on Permethylated β-Cyclodextrin, a Chiral Stationary Phase used in Gas Chromatography. J. Am. Chem. Soc. 119, 600–610 (1997)
Armstrong, D.W., Li, W., Chang, C.-D., Pitha, J.: Polar-liquid, Derivatized Cyclodextrin Stationary Phases for the Capillary Gas Chromatography Separation of Enantiomers. Anal. Chem. 62, 914–923 (1990)
Schurig, V., Nowotny, H.-P.: Gas Chromatographic Separation of Enantiomers on Cyclodextrin Derivatives. Angew. Chem. Int. Ed. Engl. 29, 939–957 (1990)
Armstrong, D.W., Ward, T.J., Armstrong, R.D., Beesley, T.E.: Separation of Drug Stereoisomers by the Formation of β-Cyclodextrin Inclusion Complexes. Science 232, 1132–1135 (1986)
Shitangkoon, A., Vigh, G.: Gas Chromatographic Enantiomer Separations using Chloroacylpentyl Cyclodextrins. In: Abstracts of the 24th Congress on Science and Technology of Thailand, Bangkok, pp. 218–219 (1998)
Parasuk, W., Longwan, N., Tasanakosol, W.: Enantiomer Recognition of of β-Butyrolactone by Cyclodextrin. In: The fifth Annual National Symposium on Computational Science and Engineering, Bangkok, pp. 187–193 (2001)
Gaussian 98, Revision A9, Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery Jr., J.A., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Baboul, A.G., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M. W., Andres, J.L., Gonzalez, C., Head-Gordon, M., Replogle, E.S., Pople, J.A.: Gaussian, Inc., Pittsburgh, PA (1998)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2006 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Parasuk, W., Parasuk, V. (2006). Ab initio Study of Chiral Recognition of β-Butyrolactone by Cyclodextrins. In: Alexandrov, V.N., van Albada, G.D., Sloot, P.M.A., Dongarra, J. (eds) Computational Science – ICCS 2006. ICCS 2006. Lecture Notes in Computer Science, vol 3993. Springer, Berlin, Heidelberg. https://doi.org/10.1007/11758532_20
Download citation
DOI: https://doi.org/10.1007/11758532_20
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-34383-7
Online ISBN: 978-3-540-34384-4
eBook Packages: Computer ScienceComputer Science (R0)