Abstract
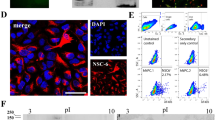
A surface marker specific for cardiac progenitors (CPs) allows the robust isolation of CPs, effectively circumventing the necessity of genetic modification. Recently, we have reported that glial cell line–derived neurotrophic factor receptor alpha 2 (Gfra2), which is anchored to the plasma membrane via Glycosylphosphatidylinositol, specifically marks CPs of both the first and second heart fields within the early mouse embryo [1], identified by single-cell expression profiling on mouse embryonic CPs (Fig. 56.1) [2, 3].
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
A surface marker specific for cardiac progenitors (CPs) allows the robust isolation of CPs, effectively circumventing the necessity of genetic modification. Recently, we have reported that glial cell line–derived neurotrophic factor receptor alpha 2 (Gfra2), which is anchored to the plasma membrane via Glycosylphosphatidylinositol, specifically marks CPs of both the first and second heart fields within the early mouse embryo [1], identified by single-cell expression profiling on mouse embryonic CPs (Fig. 56.1) [2, 3].
-
GFRA2 facilitates the isolation of CPs by fluorescence-activated cell sorting from differentiating mouse and human pluripotent stem cells. If utilizing pan-mesoderm marker PDGFRA and hemangioblastic marker KDR in conjunction with GFRA2, multipotent CPs giving rise to cardiomyocytes, smooth muscle cells and endothelial cells are identified as GFRA2+/PDGFRA+/KDR+ whereas CPs already committed only to cardiomyocytes are identified as GFRA2+/PDGFRA+/KDRneg.
-
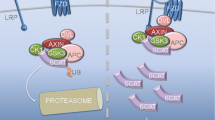
In loss-of-function of Gfra2, the functional redundancy of GFRA1 compensated for the cardiac GFRA2 signal in the mutant mouse embryos. Compound mutant of Gfra1/2 showed non-compaction cardiomyopathy due to the down-regulation of NOTCH signal in the developing heart. Cardiac GFRA2 signal is distinct from the canonical pathway that depends on the RET tyrosine kinase and its established ligands.
GFRA2 is a specific marker for CPs which governs myocardial compaction. Upper case: A signal transduction via GFRA1/2 is upstream of NOTCH signal that is indispensable for the myocardial compaction process. Lower case: GFRA2 is a specific surface antigen to identify CPs from differentiating pluripotent stem cells in mice and humans. This illustration is cited from Ishida, H et al. Cell Rep. 2016 Jul 26;16(4):1026–38, and is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA
Collectively our findings establish a platform for investigating the biology of CPs as a foundation for the future development of CP transplantation therapies for treating heart failure.
This work was supported by MRC New Investigator Research Grant G0900105 and MRC Research Grant MR/J007625/1 to K.Y., Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad and JSPS (Japan Society for the Promotion of Science) Postdoctoral Fellowship for Research Abroad to H.I. We thank Ruchaya PJ at Kings College London for the support in writing.
References
Ishida H, Saba R, Kokkinopoulos I, et al. GFRA2 identifies cardiac progenitors and mediates cardiomyocyte differentiation in a RET-independent signaling pathway. Cell Rep. 2016;16(4):1026–38.
Brouilette S, Kuersten S, Mein C, et al. A simple and novel method for RNA-seq library preparation of single cell cDNA analysis by hyperactive Tn5 transposase. Dev Dyn. 2012;241(10):1584–90.
Kokkinopoulos I, Ishida H, Saba R, et al. Single-cell expression profiling reveals a dynamic state of cardiac precursor cells in the early mouse embryo. PLoS One. 2015;10(10):e0140831.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this paper
Cite this paper
Ishida, H., Miyagawa, S., Ozono, K., Suzuki, K., Sawa, Y., Yashiro, K. (2020). A Neurotrophic Factor Receptor GFRA2, a Specific Surface Antigen for Cardiac Progenitor Cells, Regulates the Process of Myocardial Compaction. In: Nakanishi, T., Baldwin, H., Fineman, J., Yamagishi, H. (eds) Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension. Springer, Singapore. https://doi.org/10.1007/978-981-15-1185-1_56
Download citation
DOI: https://doi.org/10.1007/978-981-15-1185-1_56
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1184-4
Online ISBN: 978-981-15-1185-1
eBook Packages: MedicineMedicine (R0)