Abstract
Structural heart disease is the most common congenital anomaly, affecting almost 1% of live births [1]. Approximately 25% of newborns with congenital heart disease (CHD) require surgical or trans-catheter intervention in the first year of life [2]. Artificial materials such as polytetrafluoroethylene are used for the surgical repair of CHD, but these materials lack growth potential and lead to stenosis according to patient’s growth. Therefore, biological tissue-engineered blood vessels are desired for pediatric patients. Recently, we fabricated implantable “scaffold-free” grafts consisting of rat vascular smooth muscle cells by periodic hydrostatic pressure (PHP) [3]. Here we aimed to fabricate implantable human grafts and examine the molecular response to PHP.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Structural heart disease is the most common congenital anomaly, affecting almost 1% of live births [1]. Approximately 25% of newborns with congenital heart disease (CHD) require surgical or trans-catheter intervention in the first year of life [2]. Artificial materials such as polytetrafluoroethylene are used for the surgical repair of CHD, but these materials lack growth potential and lead to stenosis according to patient’s growth. Therefore, biological tissue-engineered blood vessels are desired for pediatric patients. Recently, we fabricated implantable “scaffold-free” grafts consisting of rat vascular smooth muscle cells by periodic hydrostatic pressure (PHP) [3]. Here we aimed to fabricate implantable human grafts and examine the molecular response to PHP.
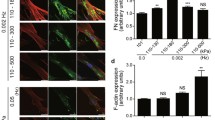
We seeded human umbilical arterial smooth muscle cells (hUASMCs) on a culture disk to make the first cell layer. Twenty-four hours after seeding, cells were exposed to PHP for 24 h, and then cells for the next layer were seeded on the top of the first layer, followed by repeating the same procedure to construct ten layered cell sheets. The multi-layered construct exhibited high elasticity (Fig. 46.1) and was successfully implanted at the aorta of nude rat. Echocardiography confirmed patency, and histological analysis demonstrated complete endothelialization.
We previously demonstrated that PHP promoted actin polymerization and fibronectin fibrillogenesis [3]. We further investigated the molecular response to PHP. hUASMCs were exposed to PHP and subjected to a microarray analysis. A microarray analysis revealed PHP-response genes, which are related to angiogenesis and stabilization of fibronectin. These genes were increased in a pressure-dependent manner, and co-localized with focal adhesion. These mechanoresponse molecules may contribute to construct the multi-layered cell sheets.
References
Van Der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7.
Simeone RM, Oster ME, Cassell CH, et al. Pediatric inpatient hospital resource use for congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2014;100:934–9433.
Yokoyama U, Tonooka Y, Koretake R, et al. Arterial graft with elastic layer structure grown from cells. Sci Rep. 2017;7:140.
Acknowledgment
This work was supported by JSPS KAKENHI (U.Y., JP17K19403, JP16H05358; M.K., JP15H05761) and AMED (Y.I., 66890007).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this paper
Cite this paper
Saito, J. et al. (2020). Fabrication of Implantable Human Arterial Graft by Periodic Hydrostatic Pressure. In: Nakanishi, T., Baldwin, H., Fineman, J., Yamagishi, H. (eds) Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension. Springer, Singapore. https://doi.org/10.1007/978-981-15-1185-1_46
Download citation
DOI: https://doi.org/10.1007/978-981-15-1185-1_46
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1184-4
Online ISBN: 978-981-15-1185-1
eBook Packages: MedicineMedicine (R0)