Abstract
Inositol triphosphate receptor (IP3R) is an intracellular Ca2+ release channel located on the membrane of the sarco/endoplasmic reticulum (SR/ER), a major intracellular storage site for Ca2+. There are three subtypes of IP3R (IP3R1, 2, and 3), each of which is known to have versatile roles in the pathophysiology of many organs [1].
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Inositol trisphosphate receptor
- Calcium signaling
- Cardiovascular system
- Pulmonary arterial hypertension
Inositol triphosphate receptor (IP3R) is an intracellular Ca2+ release channel located on the membrane of the sarco/endoplasmic reticulum (SR/ER), a major intracellular storage site for Ca2+. There are three subtypes of IP3R (IP3R1, 2, and 3), each of which is known to have versatile roles in the pathophysiology of many organs [1].
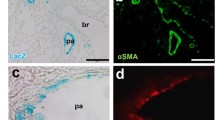
In the cardiovascular system, all subtypes of IP3Rs are expressed from embryonic to postnatal periods. We previously reported that IP3R1-IP3R2 or IP3R1-IP3R3 double-knockout (DKO) mice died around embryonic day (E) 11.5 due to disorder of cardiovascular development (Fig. 13.1). IP3R1-IP3R2 DKO mice showed developmental defects of the myocardium and endocardial cushion, resulting from the disturbance of calcineurin/NFAT signaling [2]. IP3R1-IP3R3 DKO mice demonstrated hypoplasia of the outflow tract and the right ventricle of embryonic heart with reduced expression levels of molecular markers for the second heart field [3]. In addition, IP3R1-IP3R3 DKO embryos exhibited defects in the vasculature of the labyrinth layers of the placenta, allantois, and yolk sac. Therefore, this suggests the essential redundant roles of endothelial IP3R1 and 3 for angiogenesis during embryonic and extra-embryonic vascular development [4].
Recently, we found the specific expression of IP3R in the pulmonary artery smooth muscle cells (PASMCs) of murine lungs. Although there are some reports that implicated IP3R1 in vasoconstriction or remodeling in vascular smooth muscle cells [5], the precise role of IP3Rs in PASMCs remains unknown. Since intracellular Ca2+ signaling in PASMCs is a major regulator for pulmonary vasoconstriction and remodeling, its dysregulation leads to the progression of pulmonary arterial hypertension (PAH). It was reported that store-operated Ca2+ (SOC) entry was upregulated in PASMCs with PAH patients. Our results suggest that intracellular Ca2+ depletion through Ca2+ release by IP3R from the SR/ER may lead to the opening of SOC channels, a mechanism potentially involved in the pathophysiology of PAH (Fig. 13.1) [6].
References
Mikoshiba K. Role of IP3 receptor signaling in cell functions and diseases. Adv Biol Regul. 2015;57:217–27.
Uchida K, Aramaki M, Nakazawa M, et al. Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis. PLoS One. 2010;5(9):e12500.
Nakazawa M, Uchida K, Aramaki M, et al. Inositol 1,4,5-trisphosphate receptors are essential for the development of the second heart field. J Mol Cell Cardiol. 2011;51(1):58e66.
Uchida K, Nakazawa M, Yamagishi C, et al. Type 1 and 3 inositol trisphosphate receptors are required for extra-embryonic vascular development. Dev Biol. 2016;418(1):89–97.
Narayanam D, Adebiyi A, Jaggar JH, et al. Inositol trisphosphate receptors in smooth muscle cells. Am J Physiol Heart Circ Physiol. 2012;302:H2190–210.
Shibata A, Uchida K, Kodo K, et al. Type 2 inositol 1,4,5-trisphosphate receptor inhibits the progression of pulmonary arterial hypertension via calcium signaling and apoptosis. Heart and Vessels. 2019;34(4):724–734.
Acknowledgment
This work was supported by Acterion Academia Prize 2015 to A.S. and JSPS KAKENHI Grant Numbers JP19390288 to H.Y. and JP26461619 to K.U.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this paper
Cite this paper
Shibata, A., Uchida, K., Mikoshiba, K., Yamagishi, H. (2020). Ca2+ Signal Through Inositol Trisphosphate Receptors for Cardiovascular Development and Pathophysiology of Pulmonary Arterial Hypertension. In: Nakanishi, T., Baldwin, H., Fineman, J., Yamagishi, H. (eds) Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension. Springer, Singapore. https://doi.org/10.1007/978-981-15-1185-1_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-1185-1_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1184-4
Online ISBN: 978-981-15-1185-1
eBook Packages: MedicineMedicine (R0)