Abstract
Animal assays represent an important stage between in vitro studies and human clinical applications. These models are crucial for biomedical research and regenerative medicine studies, as these offer precious information for systematically assessing the efficacy and risks of recently created biomaterials, medical devices, drugs, and therapeutic modalities prior to initiation of human clinical trials. Therefore, selecting a suitable experimental model for tissue engineering purposes is essential to establish valid conclusions. However, it remains important to be conscious of the advantages and limitations of the various small and large animal models frequently used for biomedical research as well as the different challenges encountered in extrapolating data obtained from animal studies and the risks of misinterpretation. This chapter discusses the various small animal model strategies used for osteochondral defect repair. Particular emphasis will be placed on analyzing the materials and strategies used in each model.
Similar content being viewed by others
Keywords
- Small animal models
- Scaffolds
- Biomaterials
- Stem cells
- Growth factors
- Osteochondral regeneration strategies
1 Introduction
The use of animal models for investigation is both a long-standing practice in biological research and medicine and a common matter of discussion in our societies. Animal models are currently used in biomedical research for the following motives:
-
(i)
Similarities to Human. The notable physiological and anatomical similarities between humans and animals, principally mammals, have encouraged researchers to explore a large range of mechanisms and consider novel therapies in animal models before applying their findings to humans. For example, chimpanzees and mice share about 99% and 98% of DNA with humans, respectively [1, 2]. Then, animals have the trend to be affected by different human worrying problems and represent good models for the study of human diseases.
-
(ii)
Feasibility . The management of animal models is relatively easy since different factors can be controlled from the composition of food intake to temperature and lighting. Therefore, compared to human studies, there are less environmental variations. Moreover, the animal lifespan is shorter than humans. Hence, they represent good models, as they can be studied over their entire life cycle or even through several generations [3, 4].
-
(iii)
Drug Safety . Preclinical toxicity testing, pharmacokinetics, and pharmacodynamic profiles of drugs can be investigated on animal models before use in humans. It remains important to evaluate the effectiveness of a drug as potential treatment on animals prior testing on humans. Drug safety profiles need to be established in order to protect the animals, human, and environment. Nevertheless, not all results acquired on animals can be directly translated to humans.
The use of animal models for biological research is restricted by the presence of confounding variables; limited accessibility of imaging for observation, throughput, and usability; and differences between human and animal biology [5]. These points are emphasized by those who refute any value to animal research. Moreover, the place of the animals in our modern society is frequently debated, namely, the right to use animals to benefit human purposes, with the risk that animals could be harmed. These aspects lead regularly in confusing opinions, which made the citizens and politicians to have an unclear picture of the problems. This has been the case during the evaluation of the European Citizen Initiative “Stop Vivisection ” recently presented to the European Commission [6]. Despite that, animal studies remain essential to fill the gap between in vitro experimentation and human clinical trials.
Tissue engineering and regenerative medicine (TERM) are innovative research areas dealing with the potential of natural signaling pathways combined with the components of the organism to induce repair and regeneration of organs and tissues. Basically, the principal constituents of a tissue engineering approach are (i) cells, (ii) bioactive signals as growth factors or bioreactors, and (iii) biomaterial scaffolds which act as template for tissue formation [7]. There is a growing demand for new biomaterials to replace damaged osteoarticular tissue. Therefore, orthopedic applications represent one of the main market of tissue engineering [8]. The International Cartilage Repair Society (ICRS) developed a five-grade cartilage lesion classification score system based on the macroscopic evaluation and the depth of the cartilage defect [9]. In ICRS Grade 0, the cartilage is normal. Grade 1 is divided into 1a, which includes cartilage lesions with a cartilage softening with or without superficial fissures, and 1b, which includes also superficial lesions, with the presence of fissures and cracks. Grade 2 is when cartilage lesion is deeper, extending to less than 50% of the cartilage thickness and with fraying. For classifying a cartilage lesion as a Grade 3, the cartilage injury has to be deeper than 50% of the cartilage thickness as well as down to the calcified layer. Grade 4 lesion is characterized by a complete loss of cartilage thickness and exposure of the underlying bone. In the ICRS classification, osteochondral defect corresponds to the worst case of cartilage lesion (Grade 4). Osteochondral defect management and repair represent a significant challenge in orthopedic surgery because it simultaneously affects both articular cartilage and the underlying subchondral bone. Then, the cartilage, bone, and the cartilage-bone interface have to be taken into account on the development of new strategies to repair an osteochondral defect. Recently, TERM approach emerged as a potential alternative to the current clinical palliative treatments for osteochondral defect repair, because this approach can be efficiently used to regenerate the cartilage, bone, and the cartilage-bone interface.
Because the choice of the appropriate animal model is fundamental to establish pertinent conclusions, the factors that will allow it must be identified and well understood. Before choosing the ideal animal model , it remains crucial to identify correctly the problem that has to be solved in order to obtain the right answer to the right question. Thus, the animal species to be used as well as the experimental design to be established will clearly depend upon the question asked. Animal models used in preclinical studies for osteoarticular tissue engineering goals cannot accurately reproduce the human biomechanical conditions. A preclinical study for bone and cartilage repair may be conducted in large animal models as sheep, goat, or horse. The time for recovery and the dimension of the defect should be enough and sufficient in order to obtain the evidence and allow a robust analysis. Small animal models are crucial in “proof-of-concept ” studies where theories are verified and results acquired in vitro are applied in vivo. Small animal models are frequently used to study the pathophysiology and pathogenesis of the disease process. These smaller models are faster, low-cost, easy to handle and house, and easier to implement and study than the large models. They are currently used as the first screening tool for new drugs and treatment development which then warrants further testing in large animal models before clinical trials. But important limitations in translational studies are identified as (i) the limited volume of bone and cartilage defects, (ii) the less thickness of the cartilage, and (iii) the high degree of flexion of those small animals and consequent partial weight-bearing condition, which are important drawbacks when compared with human conditions [10, 11]. Moreover, the drugs, which demonstrated to be efficient in small animal studies, may not be translatable to humans with the same effectiveness [12]. One of the reasons for this might be the well-known difference of anatomy, histology, and physiology between these animals and humans.
The present chapter will focus on the use of small animal models for the development of new strategies for osteochondral defect (Grade 4 of ICRS classification).
2 Small Animal Model Strategies for Osteochondral Repair
2.1 Mouse
Before applying new product on tissue engineering purposes , initial studies are required to evaluate important issues such as biocompatibility, degradation, and biofunctionality. This evaluation is firstly achieved through the surgical implantation of the product in ectopic subcutaneous sites. These studies are typically performed on small animal species such as mice. These small animal models have some benefits: (i) expenses are low; (ii) large groups of animals can be used; (iii) homogeneous response of strains reduces individual deviations commonly observed in large animal models; (iv) advanced imaging techniques are available such as microCT and bioluminescence imaging ; (v) a variety of genetic modifications are commercially available; and (vi) the use of immune-deficient strains allows studies of human cells or grafts without immune response implication.
Different animal models are currently used in research on restoration of osteochondral lesions including medium- (rabbits and dogs) [13,14,15,16,17] and large-sized (sheep and horses) [18,19,20,21,22,23] animals. However, the use of rodent models (mice and rats) to study osteochondral (OC) lesions is limited, despite the benefits previously described. The main concern regarding these models is their high rate of spontaneous repair after osteochondral defect induction. Despite that, and in order to better understand the cartilage repair process, an osteochondral defect model in mice has been established. Through a small (~0.5–1 cm) medial parapatellar skin incision, the joint capsule was opened and the patella dislocated laterally to expose the trochlear groove articular surface. The full thickness lesion was made in the cartilage with 21–27 G needles using a circular motion (0.4–0.5 mm diameter) until reaching the subchondral bone. Invasion of the subchondral bone was confirmed by the presence of blood resulting from removal of the needle [24,25,26,27,28]. This surgical protocol has recently been applied to evaluate the potential of an injectable cellularized PEG-based scaffold [29] and a 3D alginate-Gelfoam complexes [30] on cartilage repair. The data obtained in both studies provide proof of principle that the resultant structures possess great capacity for articular cartilage repair using tissue engineering approach.
This OC defect procedure allowed the development of a murine model of spontaneous cartilage regeneration. However, from these studies, it remains obvious that spontaneous healing capacity is clearly dependent on mouse age and strains. The spontaneous cartilage recovery is not the only way in sustaining the importance of the mouse strains used in cartilage recovery applications. Recently, Mak et al. [31] have evaluated the impact of intra-articular injections of synovial mesenchymal stem cells (MSCs) , isolated from two different strains (C57BL6 and MRL strains) on cartilage repair using the same mouse injury model. They demonstrated that intra-articular injection of these synovial MSCs, isolated from MRL or C57BL6 mice, protects against the joint deterioration that would normally result after a surgically induced focal cartilage defect, although the mechanism of protection does appear to be different between the two strains of mice [31]. Instead the existence of a spontaneous recovery in mice model.
Then, the strain of the mouse showed to have some importance when studying approaches to improve cartilage and bone repair.
Nowadays, an innovative approach aims to analyze genetic and biomolecular mechanisms underlying cartilage repair. For this reason, the use of genetically modified animals represents a powerful tool to investigate the biological mechanisms involved. Mice offer robust benefits for mechanistic in vivo studies due to the accessibility to athymic, transgenic, and knockout strains. Athymic mice, which have a limited cellular immune response, allow initial in vivo study of allogenic and xenogeneic cartilage repair approaches [32,33,34,35,36]. Genetically modified mice, including transgenic and knockout models, are currently used to study the effects of a particular gene or protein on bone and cartilage repair and regeneration in different musculoskeletal diseases [37,38,39].
2.2 Guinea Pig
Guinea pigs (Cavia porcellus or Cavia cobaya) had a special place in research. This rodent is considered as a suitable model of human skeletal growth pattern because its epiphyses fuse as growth is completed [40]. However, it presented many disadvantages, namely, the fact that growth plate fusion occurs several months after bone growth stops and that guinea pig presents various alignment of the knees, which results in an increased load on medial compartment [41]. Therefore a reduced number of studies have used this animal model for osteochondral repair strategies. Kaar et al. [40] have evaluated the impact of this model on cartilage full thickness defect and concluded that despite the regeneration occurred in all cases, the level of tissue restoration was variable and the degree of repair was independent of the age. Actually, and mostly due to the increased use of genetically engineered mice and rats for specific disease models, the usage of guinea pig in research declined. Furthermore, guinea pigs demonstrated spontaneous cartilage degeneration [42], which, associated with age-related osteophyte formation, subchondral bone changes, and synovitis, made these animals a popular model for the study of osteoarthritis [41, 43].
2.3 Rat
Small animal models have been explored in order to address the challenge for osteochondral repair [44, 45]. The use of rats as osteochondral defect model seemed very attractive in order to provide proof-of-concept data. Rat model display some advantages: (i) economically rats are relatively low cost and easy to care of; and (ii) clinically they are more relevant than the mouse model based on their articular cartilage which presents typically also a zonal structure mimicking the one observed in human joints [46]. And as for mice, immune-deficient models are also available. However, articular cartilage is thinner, and defects are much smaller compared with humans; moreover, most defects cannot be set without penetrating the subchondral bone plate. Therefore, the rat model, as well as mice model, seems suitable only for preliminary in vivo assays and not for preclinical studies, but there is a constant requirement to better understand the biology of osteochondral defects. Different approaches have been applied on rat models to evaluate osteochondral defects restoration. Joint surface of rat knee demonstrated some regenerative ability. The major and growing concerns in osteochondral repair remained to evaluate the normal progression of spontaneous osteochondral healing during time, not only regarding the altered area but also in the cartilage surrounding the defect. Therefore, it remained fundamental to define a critical size osteochondral defect model and to establish the subchondral bone plate advancement toward the join surface [47]. Katagiri et al. defined a critical size osteochondral defect as 1.4 mm in diameter in rat and showed that the subchondral bone plate advancement happened quickly [48]. Moreover, they showed that the articular cartilage close to the osteochondral defect presented expression of Interleukin 1 beta (IL1β) , fibroblast growth factor 2 (FGF2) , and a disturbed FGF receptor 1/FGF receptor 3 balance, resulting in a catabolic activity which potentially could be responsible of an early osteoarthritic disease process.
Instead it is well described that a large osteochondral defect does not repair itself with original cartilage and leads to osteoarthritis; other approaches have been evaluated in order to induce the repair of osteochondral defect. Scaffold-free cell-based strategies have been tested. The transplantation of autologous chondrocytes organized in sheets has been showed to promote the repair mechanism of osteochondral defect [49] compared to synovium cells, described to have the highest potential for both proliferation and chondrogenesis [50]. Another approach was the use of cartilage-like tissue, generated ectopically by muscle-derived cells or amnion-derived cells using bone morphogenetic protein-2, which showed to be effective in repairing articular cartilage defects in rats [51, 52]. However, problems have been reported such as the dedifferentiation of cells with passaging [53]. Therefore, a strategy that mobilizes the endogenous pool of mesenchymal stem cells (MSCs) would offer a cheaper and less invasive alternative. MSCs are widely used as scaffold-free cell strategy for osteochondral defects regeneration [53,54,55,56,57]. Moreover, Yamaguchi demonstrated that exercise could efficiently promote cartilage repair after an MSC intra-articular injection [58]. As with all cell-based strategies, there are significant logistic and operational challenges associated with proper handling and cell storage required to maintain cell viability and vitality. Therefore, in view of all these issues, cell-free-based approaches have been tested. The administration of the myelostimulant granulocyte-colony stimulating factor (G-CSF) [59], a cytokine that serves as a growth factor for the hematopoietic stem cells, or exosome [60] (Fig. 19.1), a cell-secreted nano-sized vesicles present in the MSC secretome, has demonstrated potential for cartilage repair. Both strategies could overcome the impeding restrictions of current cell-based therapies.
In vivo cartilage repair at 6- and 12-week post-surgery. (Reprinted with permission [60]. Copyright © 2016, Elsevier)
Another therapeutic strategy for osteochondral repair is based on the implantation of scaffolds. Since rat model also offers a cost-effective means for in vivo evaluation of degradation characteristics and safety profile of new biodegradable scaffolds and polymers, cell-free and cell-seeded scaffolds approaches have been investigated. Regarding the acellular scaffold-based strategies, the impact of different biomaterials and structures has been evaluated on cartilage and bone repair . Ferretti et al. used the rat osteochondral defect model to support the use of genipin crosslinked polyethylene glycol hydrogels as an innovative delivery system to control in vivo release of growth factors for improving articular cartilage repair [61]. Nanofiber scaffold, composed of poly(vinyl alcohol) or chondroitin sulfate, has enhanced the endogenous repair process without exogenous cells [62]. The use of cell-free multilayered silk fibroin-based scaffolds, combined or not with TGFb2 and BMP-2 growth factors, has shown to possess an inherent ability to attract endogenous, joint-resident cells capable of differentially differentiating down the osteochondral lineages [63]. Nogami et al. [64] developed a cell-free scaffold composed of human amniotic mesenchymal (HAM) cell-derived extracellular matrix and polylactic-co-glycolic acid. They demonstrated that the implantation of this cell-free scaffold, in rat model of osteochondral defect, promoted in-growth of endogenous cells and resulted in good cartilage repair [64]. More recently the administration of absorbable gelatin sponges, combined with insulin-like growth factor-1 or hyaluronic acid, in rat knee has showed to be efficient in the repair of osteochondral lesions [65]. The potential of cellular scaffolds on osteochondral defect repair in rat model has also been investigated. Within those assays, both cell types and scaffold materials have been studied. Dahlin et al. evaluated the impact of the ability of cocultures of articular chondrocytes and MSCs to repair articular cartilage in osteochondral defects [66]. For that purpose, bovine articular chondrocytes and rat MSCs were seeded separately or in coculture onto electrospun poly(ε-caprolactone) scaffolds and implanted in the defect. The authors demonstrated the potential for the use of cocultures of articular chondrocytes and MSCs for the in vivo repair of cartilage defects [66]. Moreover, the implantation of autologous chondrocyte, cultured in media supplemented with recombinant acid ceramidase and seeded on a biphasic material containing a collagen I top layer and a porous collagen III bottom layer (Bio-Gide), has enhanced cartilage repair in a rat osteochondral defect model [67]. All these studies supported the importance of designing tissue-engineered scaffolds that mimic the physical and biological components of extracellular matrix to produce ideal tissue repair in vivo. Overall, small rodents are attractive models for cartilage research due to the accessibility of immune-deficient and transgenic animals, as well as cheaper to house and purchase. Nevertheless, their translational potential remains limited due to their small joint size and tiny cartilage. In the context of bone and cartilage repair and regeneration, rodent models are most useful for in vivo mechanistic studies, feasibility studies , and preliminary testing of new therapy strategies.
2.4 Rabbit
The rabbit model provides a more appropriate small animal model for the assessment of osteochondral defect repair as they have larger joints and are a sufficient size for easy surgical procedures. Moreover they presented a bone plate thickness of 0.4–0.5 mm and a cartilage thickness of 0.25–0.75 mm [68, 69]. As for the previously described models, rabbits are easy to handle and low cost to maintain in-house. However, this model presents some disadvantages, i.e., an increased intrinsic healing due to increased cell density, different load characteristics on the join, and the difficulty to achieve a consistent partial thickness. In all studies that will be cited thereafter, the creation of an osteochondral defect was always based on the same protocol. The rabbits were anesthetized and, through a longitudinal parapatellar incision, the patella was laterally dislocated. All visible bleeding was carefully cauterized. With the knee joint maximally flexed, an osteochondral defect of 3–5 mm in diameter and 2–3 mm deep was created in the load-bearing region of the medial condyle. All debris were removed from the defect with a curette and the edges cleaned with sharp scalpel blade. After, the patella was relocated and the wound sutured in layers [70, 71]. Moreover, the age of the rabbit at the time of the surgery remains important. A histological and radiographic study of the closure of the distal femur, proximal tibia, and proximal fibula demonstrated the New Zealand white rabbits are skeletally mature between 19 and 24 weeks old [72].
Different tissue engineering (TE) strategies have been developed to address osteochondral defect. These approaches are mainly applied for restoration/regeneration of the tissues and based on the use of cells, scaffolds, and growth factors alone or combined. Cell-based approach is one of the current osteochondral repair option. This approach is increasingly explored to deliver biological substitution of the injured tissue, either by injection of chondrocytes or implantation of specific grafts. However, it is limited by the number of cells available for isolation and by the uncontrolled phenotypic alterations in those cells. As such, stem cells have been investigated as cell sources for cartilage and bone engineering due to their well-established ability to generate cartilage-like and bone-like tissues under the appropriate culture conditions. As alternative cell-based approach, the use of platelet-rich plasma (PRP) for the treatment of numerous types of orthopedic disorders, including chondral and osteochondral injuries, has increased recently. PRP is a plasma fraction containing a high concentration of platelets and is rich in many growth factors (GF) . These GF take part in the natural process of tissue healing and homeostasis. They present the capability to stimulate cell proliferation, mesenchymal stem cell chemotaxis, and cell differentiation . Nevertheless, the use of PRP in preclinical and clinical studies, in chondral injuries, remains controversial [73].
Recently reported rabbit preclinical studies for the treatment of OC lesions using different scaffold-free strategies are summarized in Table 19.1 (Fig. 19.2).
Macroscopic and microscopic findings at 4- and 12-week posttreatment. (Reprinted with permission [82]. Copyright © 2017)
Current approaches for articular OC repair are centered on the use of hydrogels and scaffolds providing a suitable three-dimensional (3D) environment supporting the growth of cartilaginous and bone repair tissues. These 3D structures are often critical, both in vitro and in vivo, to summarizing the in vivo milieu and allowing cells to modulate their own microenvironment. The ideal scaffolds for OC tissue engineering must to be based on the following basic requirements: porous, biocompatible , biodegradable, and appropriate for cell attachment, proliferation, and differentiation. Therefore the biomaterial is one of the key design factors to be considered in scaffold- or hydrogel-based OC tissue engineering. Different biomaterials are currently used including naturally or synthetically derived polymers. Some rabbit preclinical studies for the treatment of OC lesions using different hydrogel and scaffold-based strategies are summarized in Table 19.2 (Fig. 19.3).
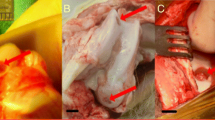
“(a - f) Histological analysis of the explants from rabbit OCD. (a, b) H&E and Masson’s trichrome staining’s of the longitudinal section of the explants, respectively. (c, d) H&E and Masson’s trichrome staining’s of the cross-section of the explants in the silk-nanoCaP layer, respectively. (e, f) H&E staining and Masson’s trichrome staining’s of the longitudinal section of the defect, respectively. Neocartilage formation in the silk layer of the bilayered scaffolds is indicated by black arrows, and indicates new subchondral bone formation inside the silk-nanoCaP layer of the bilayered scaffolds is indicated by the white arrows. (Reprinted with permission [100]. Copyright © 2015, Elsevier)
3 Conclusions
Over the previous few years, progress has been realized to reinforce the use of tissue engineering strategies in preclinical studies and clinical assays aiming the regeneration of OC lesions. In preclinical studies, the main approaches involve the improvement of new biomaterials used for the development of biocompatible scaffolds/hydrogels combined or not with growth factors and/or cells. Grafts, from allogenic or autologous origin, or arthroplasty already proved their possibilities in cartilage repair. Despite this numerous therapeutic proposals for the chondral and osteochondral lesions, it remains difficult to agree on the best treatment to be applied. Before clinical trials, those strategies have to demonstrate their potential during preclinical studies in animal models. Animal studies are essential to establish a proof-of-concept, which will be based on biological responses, degradation time, and dose response of the implanted materials. However, and in order to evaluate the potential of new regenerative strategies on OC defects, small animal models, which include mouse, guinea pig, rat, and rabbit, might not be the most suitable models, since large animal models (e.g., pig, sheep, goat, or horse) more closely resemble to the human tissue.
References
Mural RJ et al (2002) A comparison of whole-genome shotgun-derived mouse chromosome 16 and the human genome. Science 296(5573):1661
Chimpanzee Sequencing and Analysis Consortium (2005) Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437(7055):69–87
Erickson ZT, Falkenberg EA, Metz GA (2014) Lifespan psychomotor behaviour profiles of multigenerational prenatal stress and artificial food dye effects in rats. PLoS One 9(6):e92132
Franklin TB et al (2010) Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 68(5):408–415
Shanks N, Greek R, Greek J (2009) Are animal models predictive for humans? Philos Ethics Humanit Med 4:2
Wang X, Ye JD, Wang Y (2007) Influence of a novel radiopacifier on the properties of an injectable calcium phosphate cement. Acta Biomater 3:757
O’Brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Mater Today 14(3):88–95
PR Newswire (18 Dec 2016) Tissue Engineering – Global Market Outlook (2016–2022).
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A(Suppl 2):58–69
Vilela CA et al (2015) Cartilage repair using hydrogels: a critical review of in vivo experimental designs. ACS Biomater Sci Eng 1(9):726–739
McCoy AM (2015) Animal models of osteoarthritis. Vet Pathol 52(5):803–818
Pelletier J-P et al (2010) Experimental models of osteoarthritis usefulness in the development of disease-modifying osteoarthritis drugs/agents. Therapy 7(6):621–634
Kim K et al (2013) Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J Control Release 168(2):166–178
Madry H et al (2013) Cartilage constructs engineered from chondrocytes overexpressing IGF-I improve the repair of osteochondral defects in a rabbit model. Eur Cell Mater 25:229–247
Lv YM, Yu QS (2015) Repair of articular osteochondral defects of the knee joint using a composite lamellar scaffold. Bone Joint Res 4(4):56–64
Iamaguti LS et al (2012) Reparação de defeitos osteocondrais de cães com implante de cultura de condrócitos homólogos e membrana biossintética de celulose: avaliação clínica, ultrassonográfica e macroscópica. Arq Bras Med Vet Zootec 64:1483–1490
Deng T et al (2012) Construction of tissue-engineered osteochondral composites and repair of large joint defects in rabbit. J Tissue Eng Regen Med 8(7):546–556
Cokelaere S, Malda J, van Weeren R (2016) Cartilage defect repair in horses: current strategies and recent developments in regenerative medicine of the equine joint with emphasis on the surgical approach. Vet J 214:61–71
Schleicher I et al (2013) Biphasic scaffolds for repair of deep osteochondral defects in a sheep model. J Surg Res 183(1):184–192
Rautiainen J et al (2013) Osteochondral repair: evaluation with sweep imaging with Fourier transform in an equine model. Radiology 269(1):113–121
Pilichi S et al (2014) Treatment with embryonic stem-like cells into osteochondral defects in sheep femoral condyles. BMC Vet Res 10:301
Desjardin C et al (2014) Omics technologies provide new insights into the molecular physiopathology of equine osteochondrosis. BMC Genomics 15(1):947
Orth P et al (2013) Improved repair of chondral and osteochondral defects in the ovine trochlea compared with the medial condyle. J Orthop Res 31(11):1772–1779
Eltawil NM et al (2009) A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthr Cartil 17(6):695–704
Fitzgerald J (2017) Enhanced cartilage repair in “healer” mice-new leads in the search for better clinical options for cartilage repair. Semin Cell Dev Biol 62:78–85
Fitzgerald J et al (2008) Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthr Cartil 16(11):1319–1326
Matsuoka M et al (2015) An articular cartilage repair model in common C57Bl/6 mice. Tissue Eng Part C Methods 21(8):767–772
Rai MF et al (2012) Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum 64(7):2300–2310
Wang J et al (2017) Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering. Biomaterials 120:11–21
Wang P et al (2016) Flavonoid compound icariin activates hypoxia inducible factor-1alpha in chondrocytes and promotes articular cartilage repair. PLoS One 11(2):e0148372
Mak J et al (2016) Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci Rep 6:23076
Jin G-Z et al (2014) Biphasic nanofibrous constructs with seeded cell layers for osteochondral repair. Tissue Eng Part C Methods 20(11):895–904
Sartori M et al (2017) A new bi-layered scaffold for osteochondral tissue regeneration: in vitro and in vivo preclinical investigations. Mater Sci Eng C Mater Biol Appl 70(Pt 1):101–111
Shalumon K et al (2016) Microsphere-based hierarchically juxtapositioned biphasic scaffolds prepared from poly(lactic-co-glycolic acid) and nanohydroxyapatite for osteochondral tissue engineering. Polymers 8(12):429
Sheehy EJ et al (2013) Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater 9(3):5484–5492
Yan L-P et al (2015) Current concepts and challenges in osteochondral tissue engineering and regenerative medicine. ACS Biomater Sci Eng 1(4):183–200
Li S et al (2017) A conditional knockout mouse model reveals a critical role of PKD1 in osteoblast differentiation and bone development. Sci Rep 7:40505
Hu K, Olsen BR (2016) Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest 126(2):509–526
Oh H, Chun CH, Chun JS (2012) Dkk-1 expression in chondrocytes inhibits experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum 64(8):2568–2578
Kaar TK, Fraher JP, Brady MP (1998) A quantitative study of articular repair in the guinea pig. Clin Orthop Relat Res 346:228–243
Kraus VB et al (2010) The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthr Cartil 18(Suppl 3):S35–S52
Bendele AM, Hulma JF (1988) Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum 31(4):561–565
Vázquez-Portalatín N et al (2015) Accuracy of ultrasound-guided intra-articular injections in guinea pig knees. Bone Joint Res 4:1–5
Chu CR, Szczodry M, Bruno S (2010) Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev 16(1):105–115
Gregory MH et al (2012) A review of translational animal models for knee osteoarthritis. Arthritis 2012:764621
Ahern BJ et al (2009) Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthr Cartil 17(6):705–713
Orth P, Madry H (2015) Advancement of the subchondral bone plate in translational models of osteochondral repair: implications for tissue engineering approaches. Tissue Eng Part B Rev 21(6):504–520
Katagiri H, Mendes LF, Luyten FP (2017) Definition of a critical size osteochondral knee defect and its negative effect on the surrounding articular cartilage in the rat. Osteoarthr Cartil 25(9):1531–1540
Shimizu R et al (2015) Repair mechanism of osteochondral defect promoted by bioengineered chondrocyte sheet. Tissue Eng Part A 21(5–6):1131–1141
Yoshimura H et al (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327(3):449–462
Nawata M et al (2005) Use of bone morphogenetic protein 2 and diffusion chambers to engineer cartilage tissue for the repair of defects in articular cartilage. Arthritis Rheum 52(1):155–163
Wei JP et al (2009) Human amniotic mesenchymal cells differentiate into chondrocytes. Cloning Stem Cells 11(1):19–26
Lee JM et al (2012) In vivo tracking of mesenchymal stem cells using fluorescent nanoparticles in an osteochondral repair model. Mol Ther 20(7):1434–1442
Chijimatsu R et al (2017) Characterization of mesenchymal stem cell-like cells derived from human iPSCs via neural crest development and their application for osteochondral repair. Stem Cells Int 2017:1960965
Itokazu M et al (2016) Transplantation of scaffold-free cartilage-like cell-sheets made from human bone marrow mesenchymal stem cells for cartilage repair: a preclinical study. Cartilage 7(4):361–372
Muttigi MS et al (2017) Matrilin-3 co-delivery with adipose-derived mesenchymal stem cells promotes articular cartilage regeneration in a rat osteochondral defect model. J Tissue Eng Regen Med 2017;1–9. https://doi.org/10.1002/term.2485
Oshima Y et al (2005) Behavior of transplanted bone marrow-derived GFP mesenchymal cells in osteochondral defect as a simulation of autologous transplantation. J Histochem Cytochem 53(2):207–216
Yamaguchi S et al (2016) The effect of exercise on the early stages of mesenchymal stromal cell-induced cartilage repair in a rat osteochondral defect model. PLoS One 11(3):e0151580
Okano T et al (2014) Systemic administration of granulocyte colony-stimulating factor for osteochondral defect repair in a rat experimental model. Cartilage 5(2):107–113
Zhang S et al (2016) Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil 24(12):2135–2140
Ferretti M et al (2006) Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng 12(9):2657–2663
Coburn JM et al (2012) Bioinspired nanofibers support chondrogenesis for articular cartilage repair. PNAS 109(25):10012–10017
Saha S et al (2013) Osteochondral tissue engineering in vivo: a comparative study using layered silk fibroin scaffolds from mulberry and nonmulberry silkworms. PLoS One 8(11):e80004
Nogami M et al (2016) A human amnion-derived extracellular matrix-coated cell-free scaffold for cartilage repair: in vitro and in vivo studies. Tissue Eng Part A 22(7–8):680–688
Alemdar C et al (2016) Effect of insulin-like growth factor-1 and hyaluronic acid in experimentally produced osteochondral defects in rats. Indian J Orthop 50(4):414–420
Dahlin RL et al (2014) Articular chondrocytes and mesenchymal stem cells seeded on biodegradable scaffolds for the repair of cartilage in a rat osteochondral defect model. Biomaterials 35(26):7460–7469
Frohbergh ME et al (2016) Acid ceramidase treatment enhances the outcome of autologous chondrocyte implantation in a rat osteochondral defect model. Osteoarthr Cartil 24(4):752–762
ASTM F2451-05 (2010) Standard guide for in vivo assessment of implantable devices intended to repair or regenerate articular cartilage. ASTM International, West Conshohocken, PA
Chevrier A et al (2015) Interspecies comparison of subchondral bone properties important for cartilage repair. J Orthop Res 33(1):63–70
Qiu YS et al (2003) Observations of subchondral plate advancement during osteochondral repair: a histomorphometric and mechanical study in the rabbit femoral condyle. Osteoarthr Cartil 11(11):810–820
Gao J et al (2002) Repair of osteochondral defect with tissue-engineered two-phase composite material of injectable calcium phosphate and hyaluronan sponge. Tissue Eng 8(5):827–837
Kaweblum M et al (1994) Histological and radiographic determination of the age of physeal closure of the distal femur, proximal tibia, and proximal fibula of the New Zealand white rabbit. J Orthop Res 12(5):747–749
Kon E et al (2011) Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc 19(4):516–527
Mahmoud EE et al (2017) Role of mesenchymal stem cells densities when injected as suspension in joints with osteochondral defects. Cartilage https://doi.org/10.1177/1947603517708333
Ishihara K et al (2014) Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells. J Orthop Surg Res 9:98
Liu S et al (2017) Repair of osteochondral defects using human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in a rabbit model. Biomed Res Int 2017:8760383
Dashtdar H et al (2011) A preliminary study comparing the use of allogenic chondrogenic pre-differentiated and undifferentiated mesenchymal stem cells for the repair of full thickness articular cartilage defects in rabbits. J Orthop Res 29(9):1336–1342
Mahmoud EE et al (2016) Cell magnetic targeting system for repair of severe chronic osteochondral defect in a rabbit model. Cell Transplant 25(6):1073–1083
Li H et al (2016) Osteochondral repair with synovial membrane-derived mesenchymal stem cells. Mol Med Rep 13(3):2071–2077
Mehrabani D et al (2015) The healing effect of adipose-derived mesenchymal stem cells in full-thickness femoral articular cartilage defects of rabbit. Int J Organ Transplant Med 6(4):165–175
Du D et al (2015) Repairing osteochondral defects of critical size using multiple costal grafts: an experimental study. Cartilage 6(4):241–251
Sasaki T et al (2017) The effect of systemic administration of G-CSF on a full-thickness cartilage defect in a rabbit model MSC proliferation as presumed mechanism. Bone Joint Res 6:123–131
Wu CC et al (2017) Intra-articular injection of platelet-rich fibrin releasates in combination with bone marrow-derived mesenchymal stem cells in the treatment of articular cartilage defects: an in vivo study in rabbits. J Biomed Mater Res B Appl Biomater 105(6):1536–1543
Maruyama M et al (2017) Comparison of the effects of osteochondral autograft transplantation with platelet-rich plasma or platelet-rich fibrin on osteochondral defects in a rabbit model. Am J Sports Med 45(14):3280–3288
Boakye LA et al (2015) Platelet-rich plasma increases transforming growth factor-beta1 expression at graft-host interface following autologous osteochondral transplantation in a rabbit model. World J Orthop 6(11):961–969
Altan E et al (2014) The effect of platelet-rich plasma on osteochondral defects treated with mosaicplasty. Int Orthop 38(6):1321–1328
Smyth NA et al (2016) Platelet-rich plasma may improve osteochondral donor site healing in a rabbit model. Cartilage 7(1):104–111
Bahmanpour S et al (2016) Effects of platelet-rich plasma & platelet-rich fibrin with and without stromal cell-derived factor-1 on repairing full-thickness cartilage defects in knees of rabbits. Iran J Med Sci 41(6):507–517
Danieli MV et al (2014) Treatment of osteochondral injuries with platelet gel. Clinics 69(10):694–698
Gugjoo MB et al (2017) Mesenchymal stem cells with IGF-1 and TGF- beta1 in laminin gel for osteochondral defects in rabbits. Biomed Pharmacother 93:1165–1174
Han F et al (2015) Photocrosslinked layered gelatin-chitosan hydrogel with graded compositions for osteochondral defect repair. J Mater Sci Mater Med 26(4):160
Chen P et al (2015) Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials 39:114–123
Mazaki T et al (2014) A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci Rep 4:4457
Wang CC et al (2016) Expandable scaffold improves integration of tissue-engineered cartilage: an in vivo study in a rabbit model. Tissue Eng Part A 22(11–12):873–884
Zhang W et al (2013) The promotion of osteochondral repair by combined intra-articular injection of parathyroid hormone-related protein and implantation of a bi-layer collagen-silk scaffold. Biomaterials 34(25):6046–6057
Duan P et al (2014) The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J Biomed Mater Res A 102(1):180–192
Vayas R et al (2017) Evaluation of the effectiveness of a bMSC and BMP-2 polymeric trilayer system in cartilage repair. Biomed Mater 12(4):045001
Han L et al (2017) Biohybrid methacrylated gelatin/polyacrylamide hydrogels for cartilage repair. J Mater Chem B 5:731
Lu S et al (2014) Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 35(31):8829–8839
Yan LP et al (2015) Bilayered silk/silk-nanoCaP scaffolds for osteochondral tissue engineering: in vitro and in vivo assessment of biological performance. Acta Biomater 12:227–241
Naskar D et al (2017) Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials 136:67–85
Ruan SQ et al (2017) Preparation of a biphase composite scaffold and its application in tissue engineering for femoral osteochondral defects in rabbits. Int Orthop 41(9):1899–1908
Shimomura K et al (2017) Comparison of 2 different formulations of artificial bone for a hybrid implant with a tissue-engineered construct derived from synovial mesenchymal stem cells: a study using a rabbit osteochondral defect model. Am J Sports Med 45(3):666–675
Żylińska B et al (2017) Osteochondral repair using porous three-dimensional nanocomposite scaffolds in a rabbit model. In Vivo 31(5):895–903
Du Y et al (2017) Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 137:37–48
Wada S et al (2016) Hydroxyapatite-coated double network hydrogel directly bondable to the bone: biological and biomechanical evaluations of the bonding property in an osteochondral defect. Acta Biomater 44:125–134
Higa K et al (2017) Effects of osteochondral defect size on cartilage regeneration using a double-network hydrogel. BMC Musculoskelet Disord 18(1):210
Wang C et al (2017) Cartilage oligomeric matrix protein improves in vivo cartilage regeneration and compression modulus by enhancing matrix assembly and synthesis. Colloids Surf B Biointerfaces 159:518–526
Acknowledgments
Alain da Silva Morais acknowledges ERC-2012-ADG 20120216–321266 (ComplexiTE) for his postdoc scholarship. The research leading to this work has received funding from the Portuguese Foundation for Science and Technology for the funds provided under the program Investigador FCT 2012 and 2015 (IF/00423/2012 and IF/01285/2015).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
da Silva Morais, A., Oliveira, J.M., Reis, R.L. (2018). Small Animal Models. In: Oliveira, J., Pina, S., Reis, R., San Roman, J. (eds) Osteochondral Tissue Engineering. Advances in Experimental Medicine and Biology, vol 1059. Springer, Cham. https://doi.org/10.1007/978-3-319-76735-2_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-76735-2_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76734-5
Online ISBN: 978-3-319-76735-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)